Abstract
Expression of activated telomerase and subversion of the p16/pRb pathway is sufficient and essential for the in vitro immortalization of primary keratinocytes. Most cancers - including cervical carcinoma - over-express hTERT, the catalytic domain of the telomerase complex.
Only a limited set of viruses within the Alphapapillomavirus genus are oncogenic. The viral functions responsible for this distinction are not well understood. The human papillomavirus type 16 E6 protein activates the hTERT promoter.
We used a luciferase-based assay to test the ability of 29 viral types, representing all current species within the Alphapapillomavirus genus, to activate the hTERT promoter. We show that oncogenic types specifically activate the hTERT promoter, while non-oncogenic types do not. Statistical analysis supports the notion that activation of the hTERT promoter is uniquely associated with oncogenic types, independent of evolutionary relationships. This finding begins to shed light on the viral phenotypes correlated with oncogenic potential.
The Papillomaviridae comprise a family of viruses that infect most vertebrate species, while showing a high species specificity. More than 150 HPV types have been described, and among them, about 30–40 types infect the genital tract (Bernard et al., 2010; de Villiers et al., 2004). Based on nucleotide similarity across the major structural gene (L1), the PVs are divided into 29 genera, and these genera are further divided into numbered species (Bernard et al., 2010). Above the species level, the HPV types cluster into three main genera, roughly corresponding to preferred tissue tropism. The Alphapapillomavirus genus (alpha-PVs) contains all the viral types that have been associated with anogenital cancer in humans. Alpha-HPVs are categorized as ‘low-risk’ or ‘high-risk’ types, according to their epidemiological association with benign or pre-cancerous and cancerous lesions, respectively (Bouvard et al., 2009; IARC, 2012). Within the alpha-PV genus, the HR-clade is monophyletic (Schiffman et al.), suggesting that oncogenesis is an apomorphy (an acquired phenotype) associated with this clade of the viral tree. Nevertheless, only a subset of types within this HR-clade actually have oncogenic potential (oncogenic types, OTs) (Bouvard et al., 2009; IARC, 2012). It is, to date, not known which of the viral phenotypes/activities are also associated with oncogenesis. Traditionally, comparisons of HPV16 and HPV6 have been used to address these questions (reviewed in Klingelhutz and Roman, 2012). However, differences in viral tropism and evolutionary history confound this paradigm (Van Doorslaer and Burk, 2010).
In vitro assays have shown that disruption of the Rb/p16 pathway and induction of telomerase activity are sufficient to immortalize human epithelial cells (Garbe et al., 1999; Kiyono et al., 1998; Lundberg et al., 2002). The E7 protein successfully interacts with and degrades pRb and the other members of the pocket family of proteins, thereby uncoupling the cells’ control of E2F from normal mitogenic stimuli (Boyer et al., 1996; Helt and Galloway, 2001). In addition, it has been demonstrated that HPV16 E6 binds to the hTERT promoter and induces its expression (Gewin and Galloway, 2001; Oh et al., 2001; Veldman et al., 2001; Veldman et al., 2003) resulting in functional activation of the telomerase complex. The proximal hTERT promoter contains two E-boxes. These E-boxes are canonical binding sites for c-myc, a strong activator of the hTERT promoter (Takakura et al., 1999). It was shown that HPV16 E6 and c-myc cooperatively activate the hTERT promoter in vivo (Veldman et al., 2003). The ubiquitin ligase E6AP appears to play a key role in the activation of the hTERT promoter (Gewin et al., 2004; James et al., 2006; Liu et al., 2005). Moreover, it has been shown that induction of telomerase is separable from p53 degradation and that telomerase activation rather than p53 degradation is critical for immortalization of epithelial cells by HPV16 (Kiyono et al., 1998; Klingelhutz et al., 1996), although this view is not universally accepted (McMurray and McCance, 2004). The consequence of perturbations in the telomerase pathway during immortalization of primary keratinocytes prompted us to analyze the importance of hTERT activation in conjunction with available epidemiological and evolutionary data. Using this approach, a viral phenotype/activity is correlated with carcinogenicity when only epidemiologically established oncogenic types exhibit the phenotype studied. By analyzing the results of biochemical assays in this manner, our lab has previously suggested that the degradation of p53 (Fu et al., 2010) and PDZ containing proteins (Van Doorslaer et al., in preparation) by the E6 protein was a conserved phenotype of the high-risk clade; however, at least for human alpha-PV infections, the degradation of these targets is not strictly associated with cancer. In the present study we analyzed the E6 proteins from 29 distinct viruses representing the known evolutionary spectra of the alpha-PVs (Bernard et al., 2010; de Villiers et al., 2004). Epidemiological evidence was used to classify viruses according to risk (Bouvard et al., 2009; IARC, 2012; Schiffman et al., 2009). hTERT promoter activation was tested using a previously described luciferase based assay (Veldman et al., 2001). In this assay, the hTERT minimal promoter is cloned upstream of the luciferase reporter gene. Activation of the promoter results in increased luciferase expression. To calibrate the assay, we measured the effects of c-myc which is a strong activator of the hTERT promoter (reviewed in Cerni, 2000). As shown in figure 1, c-myc activated the hTERT promoter about 3.5-fold (compare top two rows). Based on the activity of c-myc, we set the cut-off value for activation of the hTERT promoter at three-fold activity over that driven by the pQCXIN control vector (vertical dotted line on figure 1). To demonstrate that the activation by c-myc was specific, we used a construct in which both E-boxes (essential for c-myc binding) were mutated (Veldman et al., 2001). When using this construct no activation over background was observed (data not shown).
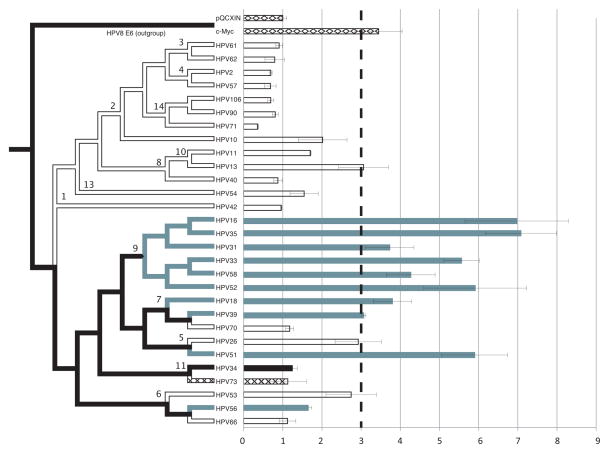
Figure 1. hTERT promoter regulation by Alphapapillomavirus E6 proteins.
Left panel –phylogenetic tree- The nucleotide sequences for the E6 proteins of the types included in this study were downloaded from GenBank and translated into amino-acid sequences. The sequences were aligned using MAFFT (linsi) (Katoh et al., 2002) as implemented in Seaview (Gouy et al., 2010). HPV8 was used as the out-group to root the tree. The amino-acid sequence alignment was reverse-translated into a nucleotide alignment in the Seaview package (Gouy et al., 2010). The Bayesian tree shown in figure 1 was constructed using MrBayes (Ronquist and Huelsenbeck, 2003). GTR+I+G was selected as the evolutionary model based on Modeltest (Posada and Crandall, 1998). Two chains of 10,000,000 generations were run and the first 10,000 generations were discarded. Chain convergence was checked using AWTY (Nylander et al., 2008). Numbers at the nodes indicate the viral species within the Alphapapillomavirus genus. Epidemiological carcinogenicity was extrapolated from two recent reviews (Bouvard et al., 2009; IARC, 2012; Schiffman et al., 2009), and is indicated using colors. Grey branches indicate oncogenicity; white = not oncogenic; X-ed = potentially oncogenic; black = insufficient evidence).
U2OS cells express wild type p53 and pRb. These cells do not use the telomerase complex for the maintenance of telomere length (i.e. they use the ALT mechanism). Preliminary analysis showed that these cells had low background levels of hTERT promoter activity. While these cells were derived from a human osteosarcoma, it is unlikely that the choice of cell line significantly influenced the results of the presented analysis. These U2OS were obtained from the ATCC and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Mediatech, Manassas, VA, USA) containing 10% fetal bovine serum (Gemini bioproducts, West Sacramento, CA, USA). 100,000 U2OS cells were plated into 24-well plates and transfected using lipofectamine LTX (Invitrogen, Carlsbad, CA, USA) according to the manufacturers’ instructions. 100 ng pQCXIN based vector was mixed with 400 ng pGL3B-255wt (Veldman et al., 2001) in a total of 800 ng including carrier DNA (pQCXIN empty vector). 24 hours post transfection the cells were lysed in 100 ul Passive Lysis Buffer (Promega, Madison, WI, USA) and assayed using firefly luciferase (Dual luciferase Reporter assay system, Promega) on a BMG FLUOstar Optima plate reader. Total protein concentration was measured using the BCA Protein assay kit (Thermo scientific, Waltham, MA, USA) and was used to normalize the luciferase signal. The fold changes over empty vector (=background) are shown. The graph represents the mean signal of three independent experiments each performed in triplicate (±SEM). The dotted line represents the level above which the promoter was considered to be activated.
The E6 proteins from 29 viral types (Fu et al., 2010) were tested for their ability to activate the hTERT promoter as measured by luciferase activity. The tested types represent all human species groups (alpha 1–11, and 13–14; species 12 contains PVs infecting non-human primates) in the Alphapapillomavirus genus, with an over-representation of types within the HR-clade (alpha-5, -6, -7 and -9). The Bayesian phylogenetic tree in figure 1 shows the evolutionary relationships between all tested types. In addition, the coloring of the branches (oncogenic = grey, Not oncogenic = white) indicates the epidemiological risk as classified by the International Agency for Research on Cancer (Bouvard et al., 2009; IARC, 2012). The figure shows that the majority of types associated with cancer significantly increased luciferase signals, suggesting upregulated hTERT promoter activity. Since hTERT levels are the limiting cellular factor for enzymatic activity, this suggests activation of the hTERT complex. The only exception to this pattern was the E6 from HPV56, which did not increase the luciferase signal. Of note, none of the NOTs (e.g. HPV34, HPV70) in the HR-clade augmented the hTERT promoter activity greater than threefold above the control. HPV26 and HPV53 are not considered oncogenic viruses, although they are embedded deep within the HR-clade. While these viruses show a certain level of upregulation of the hTERT promoter, this activation did not reach the a-priori defined cut-off. These results indicate a strong correlation between hTERT promotor activation and cervix cancer risk. To confirm that hTERT activation did not just appear to be correlated merely because it was inherited from a common ancestor, we used a z-test to formally test whether hTERT activation was associated with viral classification based on epidemiology (OT vs NOT) or on phylogeny (HR vs. LR-clades). 94% (17/18) of viruses able to activate the hTERT promoter belonged to the OT group, vs. 61% (11/18) belonging to the HR-clade. The test had a z-value of 2.3708 with a p=0.018. The statistical analysis supports the notion that activation of the hTERT promotor is associated with oncogenic classification.
The clinical significance of telomerase upregulation during cervical carcinogenesis has been described in multiple studies (Anderson et al., 1997; Branca et al., 2006; Frost et al., 2000; Gorham et al., 1997; Iwasaka et al., 1998; Pao et al., 1997; Zhang et al., 2002; Zhang et al., 1999). Telomerase activity parallels an increase in the grade of cervical intraepithelial neoplasia, suggesting an important role during the oncogenic process. Immuno-histochemical staining showed that hTERT activity was significantly associated with the presence of HR-HPV DNA in the cell (Branca et al., 2006) confirming a link with viral gene expression. In addition to these studies, the importance of hTERT activity has been confirmed in cell culture. Telomerase activity was shown to be essential for the in vitro transformation of primary keratinocytes (Garbe et al., 1999; Kiyono et al., 1998; Lundberg et al., 2002). It has been observed that while HPV positive cells have induced telomerase activity, the telomeres of E6-transduced cells do not increase in length during in vitro passaging (Stoppler et al., 1997). Non-canonical functions independent of telomere-lengthening by the telomerase enzyme might explain these observation (Liu et al., 2009).
In the present study we show that in addition to alpha-species 9 (HPV16 and HPV16-related types), HPV18, HPV39 and HPV51 upregulate the hTERT promoter.
The types tested in this study are responsible for ~95% of all cervical cancers worldwide. The observation that all these types upregulate telomerase activity supports the importance of hTERT activation during HPV induced oncogenesis. HPV56 was the only oncogenic type that was unable to transcriptionally regulate the hTERT promoter in these experiments. However, the epidemiological evidence supporting the oncogenicity of HPV56 is equivocal (Schiffman et al., 2009).
Since HPVs infect epithelial cells that undergo terminal differentiation, all HPV types need to subvert the hosts’ control over the cell cycle. Our previous work on p53 suggested that earlier steps in the transformation process (e.g., apoptosis inhibition through p53 degradation) are shared by all members of the HR-clade irrespective of oncogenic risk (Fu et al., 2010). It is feasible that the extended survival afforded through M2 crisis avoidance initially provides the virus with an ideal environment for its vegetative life cycle. However, the extended expression of both oncogenes (i.e., E6 and E7) likely results in chromosomal instability, and genetic and epigenetic changes in the host cell. This can ultimately result in the malignant transformation of such persistently infected cells. Since loss of cellular differentiation is not compatible with a productive viral infection, it is likely that this process represents ‘collateral damage’ of infection with a handful of viruses that evolved this phenotype in order to complete their lifecycle in a specific biological niche.
In conclusion, in the present study, we hypothesized that if hTERT upregulation is a key function in cervical carcinogenesis, oncogenic viral types should uniquely possess this activity. To test this hypothesis, we assayed the effects of 29 E6 proteins isolated from HPV types representing all species within the alpha-PV genus. This approach allowed us to demonstrate that hTERT promoter activity was associated with oncogenic risk, and discordant to evolutionary relationships.
Acknowledgments
The pGL3B-255wt luciferase plasmid was a kind gift of Dr. Schlegel. C-Myc was sub-cloned into a pQCXIN background from a plasmid kindly provided by Dr. Denise Galloway. This work was supported in part by the Einstein-Montefiore Center for AIDS funded by the NIH (AI-51519) and the Einstein Cancer Research Center (P30CA013330) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson S, Shera K, Ihle J, Billman L, Goff B, Greer B, Tamimi H, McDougall J, Klingelhutz A. Telomerase activation in cervical cancer. Am J Pathol. 1997;151:25–31. [PMC free article] [PubMed] [Google Scholar]
- Bernard HU, Burk RD, Chen Z, van Doorslaer K, Zur Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- Branca M, Giorgi C, Ciotti M, Santini D, Di BL, Costa S, Benedetto A, Bonifacio D, Di BP, Paba P, Accardi L, Mariani L, Ruutu M, Syrjanen S, Favalli C, Syrjanen K. Upregulation of telomerase (hTERT) is related to the grade of cervical intraepithelial neoplasia, but is not an independent predictor of high-risk human papillomavirus, virus persistence, or disease outcome in cervical cancer. Diagn Cytopathol. 2006;34:739–748. doi: 10.1002/dc.20554. [DOI] [PubMed] [Google Scholar]
- Cerni C. Telomeres, telomerase, and myc. An update. Mutat Res. 2000;462:31–47. doi: 10.1016/s1383-5742(99)00091-5. [DOI] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Frost M, Bobak JB, Gianani R, Kim N, Weinrich S, Spalding DC, Cass LG, Thompson LC, Enomoto T, Uribe-Lopez D, Shroyer KR. Localization of telomerase hTERT protein and hTR in benign mucosa, dysplasia, and squamous cell carcinoma of the cervix. Am J Clin Pathol. 2000;114:726–734. doi: 10.1309/XWFE-ARMN-HG2D-AJYV. [DOI] [PubMed] [Google Scholar]
- Fu L, Van Doorslaer K, Chen Z, Ristriani T, Masson M, Trave G, Burk RD. Degradation of p53 by Human Alphapapillomavirus E6 Proteins Shows a Stronger Correlation with Phylogeny than Oncogenicity. PLoS ONE. 2010;5:e12816. doi: 10.1371/journal.pone.0012816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe J, Wong M, Wigington D, Yaswen P, Stampfer MR. Viral oncogenes accelerate conversion to immortality of cultured conditionally immortal human mammary epithelial cells. Oncogene. 1999;18:2169–2180. doi: 10.1038/sj.onc.1202523. [DOI] [PubMed] [Google Scholar]
- Gewin L, Galloway DA. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J Virol. 2001;75:7198–7201. doi: 10.1128/JVI.75.15.7198-7201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewin L, Myers H, Kiyono T, Galloway DA. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004;18:2269–2282. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham H, Yoshida K, Sugino T, Marsh G, Manek S, Charnock M, Tarin D, Goodison S. Telomerase activity in human gynaecological malignancies. J Clin Pathol. 1997;50:501–504. doi: 10.1136/jcp.50.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Helt AM, Galloway DA. Destabilization of the retinoblastoma tumor suppressor by human papillomavirus type 16 E7 is not sufficient to overcome cell cycle arrest in human keratinocytes. J Virol. 2001;75:6737–6747. doi: 10.1128/JVI.75.15.6737-6747.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. A Review of Human Carcinogens. 100B. Biological Agents; 2012. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- Iwasaka T, Zheng PS, Yokoyama M, Fukuda K, Nakao Y, Sugimori H. Telomerase activation in cervical neoplasia. Obstet Gynecol. 1998;91:260–262. doi: 10.1016/s0029-7844(97)00595-4. [DOI] [PubMed] [Google Scholar]
- James MA, Lee JH, Klingelhutz AJ. HPV16-E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300. Int J Cancer. 2006;119:1878–1885. doi: 10.1002/ijc.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/pl6INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- Klingelhutz AJ, Roman A. Cellular transformation by human papillomaviruses: Lessons learned by comparing high- and low-risk viruses. Virology. 2012;424:77–98. doi: 10.1016/j.virol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dakic A, Zhang Y, Dai Y, Chen R, Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc Natl Acad Sci U S A. 2009;106:18780–18785. doi: 10.1073/pnas.0906357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yuan H, Fu B, Disbrow GL, Apolinario T, Tomaic V, Kelley ML, Baker CC, Huibregtse J, Schlegel R. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J Biol Chem. 2005;280:10807–10816. doi: 10.1074/jbc.M410343200. [DOI] [PubMed] [Google Scholar]
- Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, Fleming MD, Olsen JC, Miller SW, Weinberg RA, Hahn WC. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene. 2002;21:4577–4586. doi: 10.1038/sj.onc.1205550. [DOI] [PubMed] [Google Scholar]
- McMurray HR, McCance DJ. Degradation of p53, not telomerase activation, by E6 is required for bypass of crisis and immortalization by human papillomavirus type 16 E6/E7. J Virol. 2004;78:5698–5706. doi: 10.1128/JVI.78.11.5698-5706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander JA, Wilgenbusch JC, Warren DL, Swofford DL. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
- Oh ST, Kyo S, Laimins LA. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J Virol. 2001;75:5559–5566. doi: 10.1128/JVI.75.12.5559-5566.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao CC, Tseng CJ, Lin CY, Yang FP, Hor JJ, Yao DS, Hsueh S, Hsueh S. Differential expression of telomerase activity in human cervical cancer and cervical intraepithelial neoplasia lesions. J Clin Pathol. 1997;15:1932–1937. doi: 10.1200/JCO.1997.15.5.1932. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M, Herrero R, DeSalle R, Hildesheim A, Wacholder S, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Hutchinson M, Wright TC, Solomon D, Chen Z, Schussler J, Castle PE, Burk RD. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Stoppler H, Hartmann DP, Sherman L, Schlegel R. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J Biol Chem. 1997;272:13332–13337. doi: 10.1074/jbc.272.20.13332. [DOI] [PubMed] [Google Scholar]
- Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- Van Doorslaer K, Burk RD. Evolution of Human Papillomavirus Carcinogenicity. Advances in Virus Research. 2010;77:41–62. doi: 10.1016/B978-0-12-385034-8.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75:4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman T, Liu X, Yuan H, Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc Natl Acad Sci U S A. 2003;100:8211–8216. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zheng C, Hou M, Lindvall C, Wallin KL, Angstrom T, Yang X, Hellstrom AC, Blennow E, Bjorkholm M, Zetterberg A, Gruber A, Xu D. Amplification of the telomerase reverse transcriptase (hTERT) gene in cervical carcinomas. Genes Chromosomes Cancer. 2002;34:269–275. doi: 10.1002/gcc.10071. [DOI] [PubMed] [Google Scholar]
- Zhang DK, Ngan HY, Cheng RY, Cheung AN, Liu SS, Tsao SW. Clinical significance of telomerase activation and telomeric restriction fragment (TRF) in cervical cancer. Eur J Cancer. 1999;35:154–160. doi: 10.1016/s0959-8049(98)00303-7. [DOI] [PubMed] [Google Scholar]