Abstract
The primary function of the mammalian lung is to facilitate diffusion of oxygen to venous blood and to ventilate carbon dioxide produced by catabolic reactions within cells. However, it is also responsible for a variety of other important functions, including host defense and production of vasoactive agents to regulate not only systemic blood pressure, but also water, electrolyte and acid-base balance. Caveolin-1 is highly expressed in the majority of cell types in the lung, including epithelial, endothelial, smooth muscle, connective tissue cells, and alveolar macrophages. Deletion of caveolin-1 in these cells results in major functional aberrations, suggesting that caveolin-1 may be crucial to lung homeostasis and development. Furthermore, generation of mutant mice that under-express caveolin-1 results in severe functional distortion with phenotypes covering practically the entire spectrum of known lung diseases, including pulmonary hypertension, fibrosis, increased endothelial permeability, and immune defects. In this Chapter, we outline the current state of knowledge regarding caveolin-1-dependent regulation of pulmonary cell functions and discuss recent research findings on the role of caveolin-1 in various pulmonary disease states, including obstructive and fibrotic pulmonary vascular and inflammatory diseases.
INTRODUCTION
The primary function of the lung is to transfer oxygen from the atmosphere to the blood and carbon dioxide from the blood to the atmosphere, a process known as “gas exchange.” In order to facilitate efficient gas exchange, with each heartbeat blood is pumped from the right ventricle into the pulmonary artery and through the pulmonary vascular system where the vast surface area of alveolar epithelial cells are in close contact with the extensive blood capillary network.1 Alveolar epithelial and capillary endothelial cells are in close contact with each other, forming an exquisitely thin barrier2,3 such that gas transit time between blood and the airspace is minimal. An intricate system of branched tapering bronchi from the trachea to the numerous alveoli conducts air to the distal lung airspaces.4 Bronchial and vascular smooth muscle cell layers regulate vascular and bronchial diameter, thus optimally matching alveolar airflow, or ventilation, to blood flow, or perfusion.5 In addition to gas exchange, the lung is constantly producing mucous, which traps inhaled particles, and pulmonary surfactant, which lowers alveolar surface tension, thus preventing alveolar collapse.6 Due to its position at the interface between the environment and the body’s interior, the lung possesses all the necessary tools to mount an innate and adaptive immune response. Finally, the lung synthesizes mediators important to the homeostasis of the organism, including angiotensin II, an important regulator of blood pressure. These diverse functions must be performed by different populations of highly specialized cells. In this Chapter, we present the current state of knowledge on the role of caveolin-1 and caveolin-2 protein expression and function in the various cell types of the lung.7 We also summarize research findings linking caveolin expression to the pathogenesis of pulmonary disease.
CAVEOLIN IN LUNG DEVELOPMENT
Caveolin family protein members, caveolin-1 and caveolin-2, are co-expressed primarily in endothelial cells and Type I epithelial cells in the adult lung where they function both as structural and signaling proteins due to their localization in specialized plasma membrane domains—caveolae, enriched in cholesterol, sphingolipids, ceramides and other lipids.8 Importantly, caveolae also contain heterotrimeric G proteins, G-protein coupled receptors, Src family kinases, receptor tyrosine kinases, and other signaling molecules as well as enzymes.9,10 The presence of these signaling molecules in caveolae places caveolin at the crossroad of multiple signaling pathways. Therefore, it is not surprising that caveolin modulates the activity of various receptors and enzymes or that loss of caveolin-1 expression leads to cellular transformation, implying that caveolin-1 may also function as a tumor suppressor protein.11
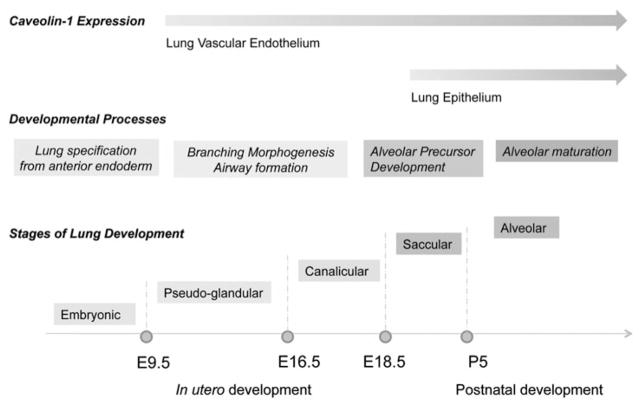
Expression of caveolins is differentially regulated in various cell types of the developing and adult lung. Lung development begins at embryonic day E9.5 in mice and at around 28 days in humans, when 2 lung buds appear within the endoderm-derived embryonic foregut.12 At around the same time, primitive hemangioblasts located in the mesoderm surrounding the foregut begin differentiating and give rise to a vascular plexus, which later becomes the peripheral lung vasculature. However, the mechanism by which pulmonary blood vessels develop is not clear.5,13 Williams and associates showed that mRNA of both caveolin-1α and caveolin-1β isoforms were readily detected at E12.5.14 At the protein level, expression of caveolin-1α was detected exclusively in endothelial cells of the developing lung (Fig. 1). Postnatally, caveolin-1α was not detected in the lung epithelium until mice were >2 months of age and was observed only in vessels and in Type I epithelial cells.14 Although the study did not address the expression pattern of caveolin-1β during development, there is some evidence that caveolin-1β is expressed in fetal lung epithelium.15 Thus, it is tempting to speculate that this finding may relate to the observed delay in lung development in caveolin-1 knockout mice.16,17 Caveolin-1 null mice lack expression of both caveolin-1 isoforms (α and β) as well as caveolin-2, which is apparently unstable in the absence of caveolin-1.16 Lung abnormalities in knockout animals are fully realized at 4–5 months of age and are represented by alveolar wall thickening, hypercellularity, and extensive remodeling of pulmonary vessels.16,18 Interestingly, the caveolin-1 knockout lung phenotype develops at the same time that caveolin-1α expression reaches its highest levels in the alveolar epithelium.
Figure 1.
Expression of caveolin-1 in pulmonary epithelium and endothelium during mouse lung development. Caveolin-1 and -2 expression begins at day E10 in pulmonary endothelial cells and E19 in alveolar epithelial cells. The temporal and cell-specific regulation of caveolin expression in the lung may play an important role in the mechanisms which govern branching morphogenesis and alveolar maturation during lung development.
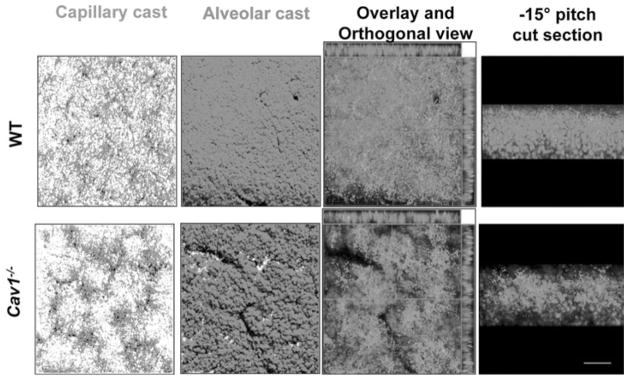
Although studies in global knockout mice provide important information about protein function, they do not discriminate between the various cell types of the lung and thus many questions regarding the role of caveolin-1 in lung development remain unanswered. Still, some insights can be gained from studies of caveolin-1 role in morphogenesis of other organs and tissues.19,20 The study by Yang and colleagues reports that caveolin-1 deficient mice demonstrate abnormal epithelial differentiation and/or hyperplasia in pancreas, prostate, uterus and breast as well as hypercellularity in lung and the exocrine pancreas.20 The authors propose that “loss of caveolin-1 function in stromal cells of various organs directly leads to a disorganized stromal compartment that, in turn, indirectly promotes abnormal growth and differentiation of adjacent epithelium.”20 Lung epithelial morphogenesis from specification of ventral foregut endoderm to formation of alveolar septa is driven by epithelial-mesenchymal interactions.20 Stromal cells such as fibroblasts and endothelial cells present morphogenetic signals to developing epithelium. Interestingly, caveolin-1 expression is detected early in development in the lung endothelium suggesting that it may be important for development of mesoderm and its proper inductive function. Caveolin-1 knockout lungs display hypercellularity, enlarged airspaces, and nonhomogenous filling of capillaries (Fig. 2). The abnormal architecture of vasculature and alveolar compartment can be attributed to inefficient signaling from mesenchymal cells, which in the absence of caveolin-1 remain in an under-differentiated proliferative state.
Figure 2.
Architectural changes of vascular and alveolar compartments in Cav1−/− lungs. Vascular and alveolar casts were generated by chilling lungs after perfusion of fluorescent beads in a solution containing 0.3% agarose via pulmonary cannula and trachea, respectively. 3D rendering of thick lung section confocal z-stack images shows nonhomogeneous filling of capillaries and enlarged airspaces in Cav1−/− compared to wild type (WT) lungs. Reproduced with permission from Maniatis NA, Am J Physiol 2008; 294(5):L865–873.18 © 2008, the American Physiological Society. A color version of this image is available online at www.landesbioscience.com/curie.
Recent research from different groups suggests involvement of caveolin-1 in proliferation and differentiation of cells in the fetal and adult lung. In the lung, caveolin-1 is considered to be a marker of Type I cell differentiation.21 It was reported that Type II cells cultured in vitro exhibit loss of surfactant protein C (SP-C) expression concomitant with an increase in caveolin-1 expression.22 Contrary to the long prevailing view that Type II cells do not express caveolin-1, a recent study demonstrated presence of caveolin-1 expression in embryonic E19 rat Type II cells.23 Moreover, this study reports that caveolin-1 participates in stretch-induced ERK activation and SP-C mRNA expression in isolated Type II cells, suggesting a potential role of caveolin-1 in mechanotransduction during fetal lung development.23
There is also a growing interest in the involvement of caveolin-1 in Wnt/β-catenin signaling pathways which regulates development and pathogenesis in vertebrates and invertebrates.24 In brief, the secreted glycoprotein Wnt binds to Frizzled receptors initiating a cascade which results in displacement of the multifunctional kinase GSK-3β from the APC/Axin/GSK-3β complex.24 In the absence of Wnt signaling, APC/Axin/ GSK-3β ubiquitinates β-catenin and targets it for degradation by the proteasome. Upon Wnt binding, GSK-3β is recruited away from the degradation complex which allows for stabilization of β-catenin protein, nuclear import, recruitment to LEF/TCF DNA-binding factors, and activation of transcription. Wnt target genes include c-myc, Cyclin D, and other genes involved in proliferation and differentiation.25 Involvement of caveolin-1 in regulating Wnt/β-catenin/Lef-1 signaling was first shown a decade ago.26 It was found that caveolin-1 efficiently inhibits β-catenin/Lef-1 responsive transcription, possibly by recruiting β-catenin to caveolae. Based on the fact that β-catenin interacts with E-cadherin as a component of intercellular adherens junction adhesion complexes, the authors proposed that recruitment of β-catenin to the membrane reinforces cell-cell adhesion while reducing transcription of Wnt responsive genes.26 This attractive model places caveolin-1 as a regulator of cancer-associated gene expression as well as intercellular adhesion impairment which is another characteristic feature of malignant transformation. A later study from the same group demonstrated that mammary gland epithelial cells isolated from caveolin-1 knockout mice exhibit hyperactive Wnt/β-catenin signaling, which is likely to be responsible for mammary gland hyperplasia observed in caveolin-1 knockout animals.26 As mentioned above, the Wnt/β-catenin pathway is known to regulate cell fate decisions and differentiation during development and in the adult organism. The role of β-catenin in differentiation of lung epithelium has been documented.27 When an activated form of β-catenin was expressed in respiratory epithelial cells of fetal lung, air space enlargement, epithelial cell dysplasia, and appearance of atypical Type II-like cells in the airways were observed postnatally.27 The role of caveoin-1 in respiratory epithelial differentiation in vivo remains to be tested.
Caveolin-1 is thought to function as a negative regulator of many other signaling cascades involved in proliferation and oncogenesis. For example, caveolin-1 inactivates members of PI3-kinase/Akt-, receptor tyrosine kinase- and MAP kinase-pathways. The general inhibitory action of caveolin-1 on protein kinases is thought to be mediated through a specific sequence, the caveolin scaffolding domain.28
CAVEOLIN AND THE ALVEOLAR EPITHELIUM
The alveolar epithelium of higher vertebrates, birds and mammals, which occupies more than 99% of the lung surface area,29 is formed by Type I and II cells.21,30 Alveolar Type I (ATI) cells are squamous epithelial cells that comprise roughly 8% of total lung cells but cover 90% of the lung surface area31 and whose main function is gas exchange. ATI cells contain sparse intracellular organelles, including Golgi apparatus, endoplasmic reticulum, and mitochondria.32 They also contain abundant cell membrane invaginations and vesicular structures and, according to estimates, 70% of the ATI cell plasmalemmal surface area is contained in such invaginations, which are distributed both on the luminal and interstitial side of the membrane.32,33 In fact, the density of vesicles per unit volume in ATI cells, at least in some species, matches that of adjacent endothelial cells34 implying a role of ATI cells in fluid and macromolecular transport processes and host defense.
Recent studies provide evidence for an important role of ATI cells in regulating lung fluid homeostasis. ATI cells express Na+ channels and Na+/K+ ATPase pumps, which control Na+ transport across the alveolo-capillary membrane.35,36 As Na+ moves across cell membranes, water passively follows. ATI cell permeability to water in response to osmotic gradients is the highest of any mammalian cell, indicating that this cell type is a major pathway for water transport between the airspace and the endovascular compartment.37 Water permeability seems to be particularly high in plasmalemmal vesicles of ATI cells, presumably due to the presence of water channels, most notably aquaporin-537
In ultrastructural studies, a large fraction of the aforementioned vesicles in ATI cells were identified as caveolae.38 Using immunolabeling with anticaveolin-1 antibodies in rat lungs, Newman et al identified caveolin-1 in membrane invaginations of ATI cells as well as free vesicles in the thin cytoplasmic extensions, which lack Golgi.38 Caveolin-negative invaginations were also found in ATI cells, which, aside from technical limitations leading to incomplete antibody binding, could indicate the presence of a morphologically and functionally heterogeneous population of these membranous structures in these cells. However, electron-dense structures consistent with clathrin-coated pits have not been described in these cells and it is likely that the overwhelming majority of membrane invaginations and plasmalemmal vesicles in ATI cells (as well as endothelial cells) are of the noncoated type, i.e., caveolae.39,40 A number of other workers reported caveolin-1 expression in ATI cells,29,41 and thus caveolin-1 seems to be restricted to this cell type in the alveolar epithelium. Interestingly, ATII cells, which are primarily responsible for producing, secreting and recycling surfactant,6,42 have no caveolae and weak caveolin-1 expression.22,38,43,44 It has been implied that a certain threshold of caveolin-1 expression may be required for caveolae to be formed.39 Caveolin-1 expression seems to coincide with the process of differentiation into the ATI phenotype in vitro.43 In addition, caveolin-1 negatively regulates mechanical stretch-induced differentiation of fetal ATII cells into mature surfactant-producing cells by controlling the Extracellular-Regulated Kinase 1/2 pathway.23
ATI cells predominantly express the β-isoform of caveolin-1 lacking the 31-amino acid N-terminal sequence, whereas endothelial cells primarily express the full length α-isoform.45 Given that the β-isoform does not contain the tyrosine residue at position 14, a substrate of Src kinase, it would appear that this important regulatory mechanism of caveolin-1 function may not apply to ATI cells.
Since the alveolar epithelium is a highly absorptive surface for water, it would be attractive to speculate that caveolae function as facilitators of lung water and protein homeostasis under physiological conditions and regulate permeability, thereby contributing to lung edema formation and clearance under pathological conditions. By substantially increasing the plasma membrane surface area, caveolar invaginations could provide space for water channel and Na+ pump insertion into the membrane as well as removal of proteins from the alveolar space through the process of transcytosis. This notion is supported by the observation that the protein concentration in the alveolar lining fluid is substantially lower than in the plasma and rises during an acute inflammatory insult.46–48 A number of experimental studies to address the issue of transcytotic protein removal from the airspace have been conducted.49–52 The results indicate that transcytosis can account for albumin transport through epithelial cells at low concentrations. At high concentrations, such as those encountered during acute lung injury (ALI), soluble protein removal seems to take place overwhelmingly via a passive process, most likely occurring via intercellular junctions.51 Thus, at present, the role of epithelial caveolae in lung edema clearance, in particular with regard to lung injury, is unclear.
CAVEOLIN AND THE PULMONARY ENDOTHELIUM
As outlined above, the lung’s main function is to ensure oxygen saturation of venous blood and carbon dioxide elimination. The relatively unimpeded passage of the entire cardiac output through the lung is facilitated by a variety of factors, including: (a) an extensive vascular network to accommodate blood flow at rest and during increased flow requirements (during exertion) by mechanisms of vasodilation and vascular recruitment; (b) the production of vasodilators, which help regulate vascular tone and reduce flow resistance; (c)the production of biomolecules, which increase hemofluidity and reduce blood viscosity and propensity for clot development. Nitric oxide (NO) and prostacyclin are the most important of these mediators synthesized primarily by the pulmonary endothelium. In addition to its synthetic function, the pulmonary endothelium forms a semi-permeable membrane, which separates and also connects the interstitium with the intravascular space by controlling fluid, solute and macromolecule exchange across the capillary barrier. Thus, the endothelium not only regulates tissue homeostasis, it also determines lung fluid balance. In pathologic states of increased endothelial permeability, including ALI and the Acute Respiratory Distress Syndrome (ARDS),53 fluid filtration across the endothelium may exceed the clearance capacity of the lymphatic vessel system resulting in pulmonary edema, impairment of oxygen diffusion, and hypoxemic respiratory failure. 54–56
Under normal conditions, the endothelium restricts movement of macromolecules and fluid to the perivascular space, allowing passage of only a very small amount of protein, including albumin, the most abundant plasma protein. According to recent evidence, caveolae are important carriers of albumin, thus regulating tissue oncotic pressure and trans-endothelial permeability.57–60 Binding of albumin to albumin-binding protein gp60 leads to gp60 clustering and association with caveolin-1 on the surface of endothelial cells.52,61–63 The GTP-binding protein Gi is activated downstream of gp60 and, specifically, the Gβγ subunit of Gi triggers a series of signaling events leading to activation of pp60c-Src tyrosine kinase (Src) activation and signaling.62,64,65 This seems to be a critical step in the endocytic process, as activated Src phosphorylates downstream target proteins dynamin-264 and caveolin-1,61,64,66 resulting in dynamin oligomerization into circular structures surrounding the neck of caveolae.67 Scission (“pinching off”) of caveolar invaginations and formation of endocytic vesicles is thought to be accomplished by the constrictive action of activated dynamin-2 rings.67 This mechanism, which accounts for the overwhelming majority of albumin exchange across the vascular wall, is at the same time coupled to constitutive production of NO by lung endothelial cells by a mechanism involving Src, phosphoinositide-3-kinase and Akt activation.68
In addition to protein-permeability regulation, caveolar structures are also permeable to water. This was initially ascribed to the presence of aquaporin-1 water channels in caveolae,69 although this was not confirmed by a subsequent study.70 More robust evidence has been provided in support of caveolar regulation of inter-endothelial junctional integrity. This action is primarily related to control of endothelial NO synthase (eNOS) function and NO release. In mice lacking caveolin-1, extravascular accumulation of protein is increased, which would appear counter-intuitive, given that these mice also lack caveolae.71,72 However, it was demonstrated by electron microscopy that microvascular endothelial cells from caveolin-1 knockout lungs also have a higher percentage of “open” inter-endothelial junctions which can be “closed,” thus restoring junctional integrity and barrier function, by eNOS inhibition. Thus, these data indicate that excess eNOS-derived NO may be responsible for inter-endothelial junction instability in the absence of caveolin-171,72
Even though the role of caveolin-1 in pulmonary endothelial homeostasis has been well documented, its involvement in endothelial pathophysiology is considerably less well understood. One important question is whether transcellular protein permeability, the main route of protein influx in the lung under normal conditions, can be accelerated and contribute to protein-rich lung edema formation during pathologic conditions, the main characteristic of ALI and ARDS. This concept has been addressed using in vitro and in vivo models of neutrophil-and oxidant-induced lung injury. Sun et al73 and Hu et al74 showed that caveolin-1 is essential for mediating transcellular albumin shuttling across the capillary endothelium in response to both types of insults, introducing a new paradigm in the mechanistic understanding of endothelial permeability regulation in pathologic conditions such as inflammatory hyperpermeability.73,74
ROLE OF CAVEOLIN IN PULMONARY ARTERIAL HYPERTENSION
Pulmonary arterial hypertension, defined as mean pulmonary arterial pressure above 25 mm Hg at rest, is a rare disorder affecting young women more commonly than men. The underlying defect is occlusion of medium- and small-sized arteries in the pulmonary circulation caused by thickening of the endothelial layer, known as the “intima,” and the surrounding smooth muscle layer, or “media.” The resulting obstruction leads to increased resistance of the pulmonary vascular network to the flow of blood, causing right heart strain and, ultimately, right heart failure and death.75,76
Key to disease pathogenesis is considered to be the dysregulated hyper-proliferation of the pulmonary endothelium in response to noxious stimuli, including shear stress, reactive oxygen species, autoimmune processes, and toxins.77 Consequently, the disease is associated with scleroderma and other connective tissue diseases, blood dyscrasias-including sickle cell disease and thalassemia, congenital cardiac defects-including ventricular septal defect, HIV infection, portal hypertension, and use of anorexigenic drugs.78 The disease may also be heritable, which is commonly associated with gene mutations in members of the transforming growth factor (TGF) receptor super-family including bone morphogenetic protein receptor-2 and activin receptor-like kinase Type 1.78 Finally, in some cases, no apparent cause is found.78 A common feature to the above heterogeneous patient groups is the histological appearance of vaso-occlussive defects, thrombosis, and neo-vascularization, collectively known as “plexiform” lesions. Specifically, the “plexiform” lesion is a glomeruloid structure of proliferating endothelial cells forming neo-vessels.79 Plexiform lesions are frequently located distal to bifurcation sites of small pulmonary artery branches.80 The fact that PAH cases share common histological features despite different etiologies supports the notion that the disease constitutes a uniform reaction of the pulmonary endothelium to a variety of insults. This response comprises de-regulated angiogenic proliferation of apoptosis-resistant cells expressing endothelial markers, which replace the damaged normal endothelium.77,81 These cells are, at least in some cases, monoclonal in origin and express anti-apoptotic factors and endothelial cell growth factor receptors while lacking certain tumor suppressor genes.79,80 Thus, they share characteristics of neoplastic cells.
Ultrastructurally, endothelial cells observed in lungs from patients with pulmonary hypertension are rich in endoplasmic reticulum, Golgi apparatus, and Weibel-Palade bodies.82 In advanced plexiform lesions, they contain fibrillar structures and, at least in one report, no caveolae.83,84 Using microarray analysis of lung tissue samples from patients with PAH, Geraci et al reported reduced caveolin-1 mRNA levels compared to controls.85 The same group further investigated caveolin-1 expression in human lungs and found that, although caveolin-1 levels in the whole lung homogenate were not different between the PAH and controls groups, caveolin-1 was very weakly expressed in the endothelium of plexiform lesions and pathological pulmonary arteries.86 These studies demonstrated that the abnormal endothelium in lungs from PAH patients contains low caveolin-1 levels, although the significance of this finding is only now beginning to emerge.
The role of caveolin-1 in PAH has recently been addressed in a series of experimental studies. Reduction in caveolin-1 expression has been demonstrated in two rat models of PAH, one using the classic PAH inducing agent monocrotaline,87 and the other using a recently developed method of pharmacologic VEGF receptor-2 antagonism and hypoxia.86 In the study by Matthew et al rats exposed to monocrotaline showed a reduction in lung caveolin-1 expression as early as 48 hours post-insult. This was associated with activation of the transcription factor STAT3 and DNA synthesis in lung tissue and cultured endothelial cells treated with monocrotaline pyrrole, consistent with the function of caveolin-1 as a negative regulator of cell growth. Moreover, incubation of cultured endothelial cells with monocrotaline pyrrole led to sequestration of eNOS within the cytoplasm and away from the cell membrane, its normal site of action, implying that caveolin-1 down-regulation could lead to reduced NO bioavailability and possibly, reactive oxygen species production by eNOS, thus fueling the development of PAH.88 The findings of Matthew et al were recapitulated by Jasmin et al who in addition, treated mice with the caveolin-1 scaffold domain peptide which was able to attenuate the development of PAH along with activation of STAT3 and induction of cyclin D1 and D3 expression.89 Similarly, in the model presented by Achcar et al, caveolin-1 and -2 protein expression was reduced in the vascular lesions of experimental animals.
An important development in the field was the observation that caveolin-1 knockout mice developed PAH and right ventricular hypertrophy.90 This may be attributable to increased pulmonary vascular resistance arising from an abnormally developed vascular system.18 Serum nitrate levels in these mice are increased18,90 but so is oxidative and nitrosative stress,91,92 possibly the result of eNOS uncoupling. This seems to be a critical issue in the pathophysiology of the caveolin-1 knockout as pharmacologic eNOS blockade in these mice reduced oxidant production and reversed pulmonary hypertension.92,93 These observations were confirmed by Zhao et al with the aid of eNOS/caveolin-1 double knockout mice in which it was shown that eNOS dis-inhibition in the absence of caveolin-1 was associated with protein kinase G inactivation, which was sufficient to explain the pulmonary vascular phenotype in these mice.17,94 These studies support the concept that caveolin-1 regulation of eNOS activity is crucial to endothelial homeostasis in the lung. Down-regulation of caveolin-1 and associated eNOS dysfunction could be an important factor in PAH pathogenesis.
ROLE OF CAVEOLIN IN PULMONARY FIBROSIS
Pulmonary fibrosis, the progressive replacement of functional lung parenchyma by scar tissue, may represent the final stage of a variety of disorders, including interstitial lung diseases (idiopathic pulmonary fibrosis-IPF, nonspecific interstitial pneumonitis), collagen/vascular diseases-most notably scleroderma, occupational exposures (silicosis, asbestosis), and less frequently Acute Respiratory Distress Syndrome. Common features in these disorders are the over-production of collagen and extracellular matrix by activated fibroblasts and myofibroblasts, which seems to be driven by TGF-β.95–99 As pointed out in a detailed review by Del Galdo et al,100 caveolin-1 seems to be a critical regulator in TGF-β signaling and thus implicated in the pathogenesis of pulmonary fibrosis.
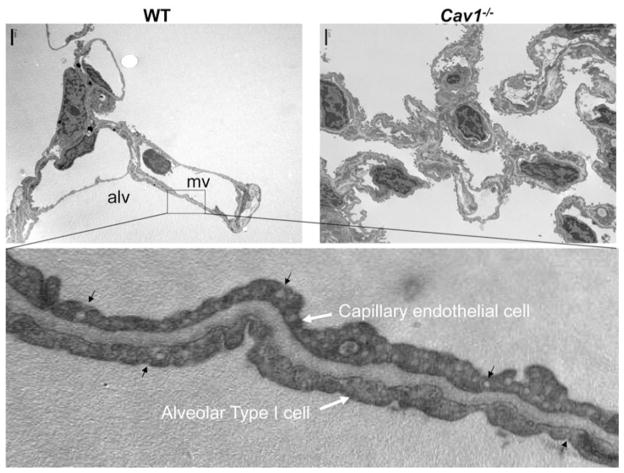
Studies in the caveolin-1 knockout mouse have paved the way for research on the involvement of this protein in fibrotic disorders. Surprisingly, genetic deletion of caveolin-1 in mice was not lethal, although this did result in marked abnormalities in the lung microachitecture. Probably the most prominent finding was the loss of the typical two-layer pattern of the alveolar septa consisting of endothelial and adjacent epithelial cells in favor of a multi-layered, hypercellular appearance resulting in thickened alveolar walls (Fig. 3)11,101 Areas of increased collagen and reticulin fibers as well as α-smooth muscle actin-expressing cells, most likely myofibroblasts, were also found and these alterations were associated with reduced lung compliance.16,18,101,102 Moreover, embryonic fibroblasts from these mice proliferated in culture at double the rate of control fibroblasts, indicating that caveolin-1 was required for cell-cycle control in these cells and that, in the absence of caveolin-1, fibroblast hyperproliferation and enhanced collagen production could be expected.16 TGF-β expression was increased in these mice as well, which resulted in augmented SMAD signaling.102,103 Interestingly, rescue of caveolin-1 expression selectively in the endothelium partially reduced the levels of TGF-β and collagen I and III, indicating that the fibrosis may be sustained, at least in part, by the endothelium.
Figure 3.
Ultrastructure of alveolar septa in wild type and Cav1−/− lungs. Note absence of caveolae, hypercellularity and septal thickening of Cav1−/− lung (top right) and abundance of caveolae in thin alveolar, and capillary gas exchange cellular membranes of WT mouse lung (top left and bottom). Images courtesy of Oleg Chaga, PhD., University of Illinois-Chicago.
On the basis of these data, expression patterns of caveolin-1 were explored in experimental animal models offibrosis as well as in human cells and lung tissue. Kasper et al described in 1998 the down-regulation of caveolin-1 in ATI cells of mouse lungs rendered fibrotic by irradiation.41 Similar findings were obtained using the bleomycin fibrosis model in mice.104 Furthermore, caveolin-1 was down-regulated in fibroblasts obtained from patients with IPF and scleroderma-induced lung fibrosis.105,106 Similarly, caveolin-1 was under-expressed in lung tissues from patients with the above disorders.104,106,107 In this context, Odajima et al described the loss of caveolin-1 in the metaplastic bronchial epithelium that lines the areas of scarring in fibrotic lungs.104 These workers argued that loss of caveolin-1 may be associated with abnormal re-epithelialization in lung fibrosis. Interestingly, in at least two studies, treatment of mice exposed to bleomycin with the caveolin-1 scaffold domain peptide was enough to ameliorate fibrosis, suggesting that this part of the protein, which is known to inhibit the activity of various kinases and eNOS, somehow regulates pulmonary responses to pro-fibrotic stimuli.106,108
Mechanisms by which caveolin-1 regulates fibroblast function and how this is perturbed in fibrosis include caveolar trafficking of the TGF-β receptor and control of cell signaling processes via the scaffold domain. TGF-β signals by binding and forming a complex with its cell surface receptors, TGF-β RI and II, which possess Ser/Thr kinase activity.109 Upon receptor activation, the complex is endocytosed via clathrin-, caveolin-1, and early endosome antigen-1 (EEA1)-positive vesicles.70,109–112 Endocytosis via clathrin-coated pits and EEA-1 positive vesicles can enhance TGF-β signaling by bringing the receptors in close proximity with SMAD effector molecules.109 Conversely, endocytosis via caveolae seems to facilitate ubiquitinylation and degradation of the receptors, thus turning off signaling. It follows therefore, that in conditions in which caveolin-1 is depleted, TGF-β activity would be dis-inhibited. However, TGF-β may also trigger SMAD-independent pathways, including Mitogen-Activated Kinases (MAPK). In this respect, the p42/44 MAPK pathway, a known activator of collagen synthesis, is upregulated in lung fibroblasts from scleroderma patients or following down-regulation of caveolin-1 with small-interfering RNA. 105 Similarly, the c-Jun terminal kinase pathway (JNK) is also activated in the absence of caveolin-1 and may be suppressed by administering the scaffold peptide.106,108 These results highlight the importance of caveolin-1 in TGF-β regulation on multiple levels and provide important insights into the mechanisms of TGF-β-induced fibrogenesis. As there is no known therapy for these diseases, caveolin-1-regulated pathways could provide a novel and viable therapeutic approach.
ROLE OF CAVEOLIN IN OBSTRUCTIVE AIRWAY DISEASES
Asthma, one of the most common respiratory diseases, is a condition of episodic cough and dyspnea caused by reversible peripheral airway obstruction. In many instances the disease is allergic in etiology, although other factors including airway irritant substances and emotional or physical stress may trigger asthma attacks. Chronic inflammation of the bronchial mucosa with mucous gland and bronchial smooth muscle hypertrophy and hyperplasia is invariably found in biopsies from asthmatic patients and thus airflow limitations in asthma can be due to airway lumen obliteration by mucous plugs and reduced airway caliber due to bronchoconstriction.113 In many patients with chronic asthma, airway remodeling, characterized by increased muscle mass and peribronchial fibrosis, has been observed.113,114 Although the functional consequences and long-term prognosis of this process are still debated, it is becoming increasingly clear that it may lead to a sustained state of airway narrowing. Moreover, contraction of an abnormally thickened airway muscle layer produces greater lumen narrowing and is more effective at occluding peripheral airways than muscle layers of normal thickness, thus increasing symptom severity during episodic asthma attacks.113 A host of inflammatory and mitogenic mediators drive bronchial cell responses in asthma, including interleukin-4, -5, -6, -17, and -33, TGF-β, TNF-α, VEGF and others.115
Depending on the extracellular environment, airway smooth muscle cells are able to shift between a more differentiated, contractile state, and a synthetic state (“phenotypic plasticity”).116 Cells in the contractile state are elongated and express muscarinic M3 cell-surface receptors, which, in conjunction with the presence of cytoskeletal and contractile-apparatus proteins (α-, γ-smooth-muscle actin, myosin heavy chain, calponin, h-caldesmon, SM22, desmin, smoothelin), render them responsive to the length-shortening effects of acetylcholine.116–118 Transition to this phenotype is triggered by stimuli including TGF-β, laminin and insulin.119–122 Conversely, in the presence of platelet-derived growth factor (PDGF), fibronectin, and fetal bovine serum, the synthetic phenotype is favored, characterized by a marked capacity to hyperproliferate and synthesize extracellular matrix components.116,119,123 Caveolae formation seems to be a feature of the differentiated state, and thus it is not surprising that in synthetic cells, caveolin-1, despite being expressed, is mislocalized in a perinuclear distribution away from the membrane.124
Given its multiplicity of functions ranging from cell-cycle control to signal transduction regulation, caveolin-1 has been the subject of scrutiny in the field of smooth muscle pathophysiology. Regarding cell signaling control, it appears that caveolin-1 modulates key mechanisms underlying smooth muscle differentiation. This process depends on expression of a specific set of genes under the control of the transcription factor serum response factor (SRF) and is triggered by ligand binding to cell surface receptors, such as the M3 muscarinic receptor, TGF-β, and TNF-α. Communication of surface receptors with the nucleus is facilitated by signaling intermediates, which include the RhoA/Rho kinase pathway, PI3-kinase, and the SMAD system. Although caveolin-1 has no apparent signaling function per se, its contribution in this context lies in coupling and co-ordinating cell signaling networks downstream of cell surface receptors. Importantly, while the RhoA/Rho kinase pathway seems to require caveolin-1 for its activation, SMADs and PI3-kinase are inhibited.125–128 Thus, caveolin-1 is not only required for smooth muscle cell differentiation, but also for smooth muscle contraction in response to agonists.116
With respect to caveolin-1 and cell division, it was shown that incubation of airway smooth muscle cells with PDGF in vitro resulted in accelerated lysosomal degradation of caveolin-1, accompanied by a marked reduction in caveolae and cell proliferation.123 In this context, siRNA-mediated caveolin-1 gene-silencing activates the p42/44 MAP-kinase pathway and increases cell proliferation.7 Interestingly, the same group reported later that p42/44 MAP kinases were not found within caveolar structures, indicating that in the cell types examined, the inhibitory effect of caveolin-1 on p42/44 MAP-kinase was indirect.129 In line with these in vitro findings, modest caveolin-1 down-regulation was demonstrated in an asthma mouse model, predominantly in lung fibroblasts.130
From the above presented studies, it is becoming increasingly evident that caveolae are central organelles for the processing of environmental signals which control airway smooth muscle function and differentiation. Accordingly, caveolin-1 and caveolae appear to participate in the pathogenetic mechanisms of bronchial smooth muscle thickening and hypercontractility, which are the anatomic basis of asthma symptoms. To what extent these insights will translate into treatment interventions remains to be determined.
CAVEOLIN CONTROL OF THE INFLAMMATORY RESPONSE
A unique feature of the lung and the gut is that they are in direct contact with the external environment and yet lack the benefits of a protective layer of skin. The lung is the organism’s first line of defense against inhaled threats and must possess the armamentarium to perform this task. Tools available to deal with these threats include structures capable of trapping and removing particulate matter such as nasal hair, airway mucous, ciliated airway epithelium, and cough reflexes; antibodies secreted in epithelial lining fluid; and a highly developed cellular and humoral immune system capable of antigen recognition, phagocytosis, and production of antibodies specific to the invading agents. In recent years, it has become apparent that caveolin-1 is not only involved in maintaining homeostasis in healthy tissues but is also involved in disease pathogenesis, with particular respect to infection and inflammation. Work in this field has been motivated by observations that caveolae may serve as entry routes for several micro-organisms, including viruses,131 bacteria132 or even immune cells.133 In addition, caveolin-1 expression may be induced by endotoxin in an NF-κB-dependent manner.134 Thus, caveolin-1 may be an important factor regulating cellular responses to pathogen invasion and stress in general.
A seminal event in ALI pathogenesis is the breakdown of the pulmonary endothelial and epithelial barrier and the subsequent leakage of plasma fluid in the airspace. As outlined above, an important determinant of microvascular permeability in the lung is eNOS-derived NO which is under the control of caveolin-1. In response to vasoactive agents, including Platelet Activating Factor (PAF) or thrombin, microvascular permeability increases in various vascularbeds62,135 associated with Ca2+-mediated eNOS activation by Ca2+-dependent calmodulin.136 Caveolin-1 via its scaffold domain9 may compete with calmodulin for binding to eNOS and thus block eNOS-mediated permeability increases.135,137,138 This effect could potentially be exploited therapeutically, depending on the availability of nontoxic and effective carrier systems for intracellular delivery of the scaffold domain peptide.
An important function of caveolin-1 in innate immunity lies in the regulation of cellular signaling events which drive the pulmonary response to such insults as invasion of micro-organisms, physical or chemical injury, or systemic inflammatory states. Challenge of caveolin-knockout mice with endotoxin showed that these mice were resistant to LPS-induced mortality, systemic cytokine response, and acute lung injury compared to WT mice.139,140 The protection of caveolin-1 knockout mice was abrogated upon administration of an eNOS inhibitor, in line with the established finding that caveolin-1 is an endogenous inhibitor of eNOS.
Insight into the significance of caveolin-1 in immune function was gained from experiments involving exposure of knockout mice to live micro-organisms. In the first of these studies, Medina et al infected mice with Salmonella typhimurium.141 These workers noted that caveolin-1 knockout mice exhibited higher mortality rates and bacterial numbers than caveolin-1-expressing mice.141 Interestingly, levels of several pro- and anti-inflammatory cytokines, as well as blood nitrate, were higher in knockout mice, consistent with an excessive inflammatory response. In later work by the same group, mice were challenged with the parasite Trypanosoma Cruzi.142 A striking feature of this experiment was that levels of major cytokines, including TNF-α, IL-6 and IFN-γ failed to rise in knockout mice to the extent noted in WT mice, despite similar degrees of tissue invasion by the pathogen.142 In cultured peritoneal macrophages infected by the parasite, reduction in the levels of inflammatory cytokines was documented in cells from knockout mice. Using the cecal ligation and puncture model, which produces peritonitis and sepsis, Feng et al143 reported an exuberant production of TNF-α and IL-6 in caveolin-1 knockout mice compared to WT controls. However, this was associated with impaired ability to clear bacteria, increased thymocyte apoptosis, and mortality.
The above studies using different invading pathogens underscore the importance of caveolin-1 in the generation of the innate immune response. This issue has been dealt with by a number of mechanistic studies. A general conclusion to be drawn from these reports, which will subsequently be discussed in more detail, is that caveolin-1 is not only required for proper function of immune cells (primarily neutrophils and macrophages, since lymphocytes do not express caveolin-1), but also for the activation of lung endothelial and possibly epithelial cells in the context of inflammation. However, emerging data also point to a role of caveolin-1 in limiting the magnitude of the inflammatory response by interfering with Toll-like receptor-4 (TLR-4) and NF-κB signal transduction pathways. In in vitro assays, Li et al observed decreased phagocytotic ability in cultured primary peritoneal macrophages isolated from caveolin-1 knockout mice compared to WT.144 This finding was correlated with increased numbers of apoptotic cells found in the thymus of irradiated knockout mice, which resulted from failure of macrophages to clear apoptotic cells.144 Hu et al focused on the other important cellular component of the innate immune system, the neutrophil. Using in vitro stimulated peripheral blood neutrophils isolated from mice, they observed that the oxidative burst was attenuated in neutrophils lacking caveolin-1, and that caveolin-1 knockout neutrophils also demonstrated reduced migratory capacity and adhesiveness to fibrinogen-coated surfaces. 145 Activation of Rac1 and Rac2 in these neutrophils following challenge with Formyl-Methionyl-Leucyl-Phenylalanine (fMLP) was also attenuated in the absence of caveolin-1145 These functional deficits in neutrophils from caveolin-1 knockout mice were associated with an inability to cause microvascular barrier disruption and edema in ex vivo lung preparations perfused with fMLP or PAF-stimulated neutrophils.145
By generating mice deficient in both caveolin-1 and eNOS, Mirza et al showed that eNOS derepression due to lack of caveolin-1 expression could result in failure of endotoxin to activate transcription factor NF-κB, thus eliminating the cellular inflammatory response.140 This effect was mediated by excessive nitration, leading to reduced activity of interleukin-1-receptor-associated-kinase-(IRAK)-4. This work uncovered an important molecular mechanism to explain how fine-tuning of eNOS function by caveolin-1 may regulate the NF-κB pathway activation status. Another mechanism by which caveolin-1 and caveolae may activate the inflammatory response is the endocytosis of the IL-1 receptor, which is a mandatory step in order for the cytokine to activate gene transcription via NF-κB.146 On a similar note, caveolin-1 seems to be instrumental in the mechanism of up-regulation of inflammatory cytokine MCP-1147 by environmental toxins as well as for the pro-inflammatory effects of TNF-α on endothelial cells.148
Conversely, caveolin-1 may exert inhibitory effects on the inflammatory cascade, as demonstrated by Wang et al in the case of TLR-4.149 This group demonstrated direct binding of caveolin-1 to TLR-4 in murine macrophages and showed that this binding was inhibitory with respect to the ability of endotoxin to stimulate inflammatory cytokine production via TLR-4 stimulation.149 They further observed that caveolin-1-mediated recruitment of Heme oxygenase-1 into lipid rafts was able to suppress TLR-4 signaling via carbon monoxide production, indicating that caveolin-1 exerts control on TLR-4 downstream events via multiple pathways.
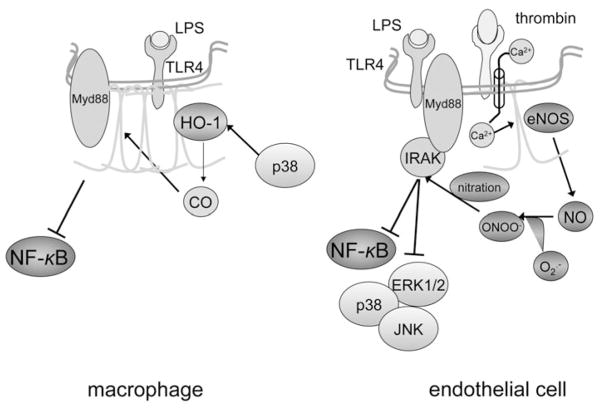
Summarizing the above studies, it would seem that caveolin-1 and caveolae, besides being a portal of entry for intracellular invasion of micro-organisms, are also central orchestrators of the innate immune system defensive strategy against these pathogens (Fig. 4). This function may be attributed to the modulation of key signaling pathways including NF-κB, TNF-α, IL-1 and TLR-4, either due to direct binding of their components to caveolin-1 or through other mediators, including NO and CO.
Figure 4.
Modulation of Toll-like-receptor-dependent signaling pathways by caveolin-1. Data obtained primarily on macrophages (left panel) favor a suppressive function of caveolin-1 on Toll-like receptor-4 (TLR-4) signaling. This is accomplished on several levels, starting by physical association with the receptor within caveolae, which presumably diminishes binding to adaptor protein MyD88, a required step for propagation of the TLR-4 signal upon docking of lipopolysaccharide (LPS). Activation of heme oxygenase-derived CO production by p38, which is expected to occur following TLR-4 ligation by LPS, is an additional mechanism to turn off the TLR-4 signal (adapted from Wang et al 2009). A seemingly opposing function of caveolin-1 has been advocated in knockdown experiments in endothelial cells (right panel). Excessive free radical (nitric oxide-NO and superoxide-O2−) production due to lack of caveolin-1-mediated endothelial NO synthase (eNOS) inhibition may lead to nitration of interleukin-1-associated receptor kinase (IRAK)-4, an important intermediate between TLR-4 and its effector molecules nuclear factor-κ (NF-κB) and mitogen-activated kinases (p38, ERK1/2, JNK). Hypothetically, this mechanism could take effect in situations in which eNOS is uncoupled from caveolin-1, as may occur in the presence of vasoactive mediators including thrombin. In this case, dual activation of eNOS due to Ca++ influx and dissociation from caveolin-1 could result in production of large amounts of free radicals and tyrosine nitration via the reactive metabolite peroxynitrite (ONOO−). A color version of this image is available online at www.landesbioscience.com/curie.
ROLE OF CAVEOLIN IN LUNG TUMORIGENESIS
Lung cancer is the most common cancer-related cause of death in both men and women in the United States. The most common histologic types of lung cancer are small cell lung carcinoma (SCLC) and nonsmall cell lung carcinoma (NSCLC). SCLC is derived from pulmonary neuroendocrine cells, which are relatively small, grow in diffuse patterns, and express neuroendocrine markers including synaptophysin, chromogranin, CD56, and TTF-1.150 These tumors are often metastatic at the time of clinical presentation and are responsive to treatment with chemotherapy but invariably relapse, accounting for the very high 5-year mortality rate seen in patients with these tumors. NSCLC are most frequently comprised of, in descending order of frequency, adeno-, squamous-, and large cell-carcinomas.151 These tumors grow locally and metastasize relatively later in the disease course. When detected at an early stage, they can be cured by surgical resection alone or followed by chemotherapy.152 At later stages of more extensive local or metastatic spread, the treatment usually includes conventional chemotherapy with or without VEGF or EGF inhibitors, radiation, and symptomatic measures,151 while surgery is much less commonly performed.153
Of importance to the pathogenesis of lung cancer is the effect of carcinogens found in cigarette smoke on the bronchial epithelium.152 These substances induce premalignant cellular alterations of varying degrees across large areas of bronchial epithelium exposed to cigarette smoke (the ‘field cancerization effect’), thus creating the substrate of malignant transformation.152 Point mutations or chromosomal alterations may result in deletions of tumor suppressor genes and/or activation of proto-oncogenes, leading to malignant transformation and clonal expansion.152
Consistent with the first description of caveolin-1 as a substrate of v-Src, a tyrosine kinase product of the v-src oncogene10,15–56 which is able to induce avian sarcomas, caveolin-1 has been extensively investigated for its role in cell cycle control and cancer development. Initial reports described caveolin-1 downregulation in cells transformed by various oncogenes, including v-abl and H-ras.157 Conversely, downregulation of caveolin-1 expression resulted in dis-inhibition of the p42/44 MAP-kinase pathway, loss of anchorage-dependent cell growth, and tumorigenesis in mice.15 In addition, a number of signaling proteins relevant to tumorigenesis, including the EGF receptor, H-ras, and p42/44 MAPK were found to be physically associated with and inhibited by caveolin-1.15,158 Moreover, caveolin-1 was found to suppress levels of Cyclin D1 which is involved in malignant cell transformation and also to reduce tumor vessel leakiness by regulating eNOS-derived NO levels. 159,160
On the basis of these and other data, researchers quantified caveolin-1 expression levels in various human cancers. One of the earliest publications was by Racine et al who reported decreased caveolin-1 expression in a variety of human lung cancer cell lines.161 Using DNA microarrays, Wilkman et al reported reduced mRNA transcripts for caveolin-1 and -2 in human lung adenocarcinomas compared to normal lung tissue.162 The same group later presented similar findings corroborated by immunohistochemical techniques.163 In apparent contrast to these data was the report by Sunaga et al who examined cell lines derived from a large number of cancer patients. They showed that caveolin-1 was upregulated in nonsmall cell cancers and down-regulated in small cell cancers.164 Similarly, Ho et al showed that caveolin-1 was down-regulated in regionally limited adenocarcinomas, whereas it was upregulated in cases presenting with lymph node metastasis. The authors argued that caveolin-1 could be a factor associated with enhanced metastatic potential in cancer cells.165 These findings were corroborated by Zhang et al who observed enhanced caveolin-1 expression in more advanced lesions.126 In the same context, Yeh et al studied the properties of a small-cell cancer cell line in vitro. They found that baseline caveolin-1 expression was low and that re-introduction of caveolin-1 into the cells was associated with decelerated growth rate but enhanced metastatic potential.166 Of note, a relationship between phosphorylated caveolin-1 and activation of the RhoA pathway was detected in colon cancer cells, which appears to enhance cell motility and the ability of tumor cells to metastasize.167 Finally, in the same context, Bonuccelli et al showed in breast cancer cells that the P132L caveolin-1 mutation is associated with a differential gene expression profile which favors cancer invasiveness and propensity to extramammary spread.168
An interesting twist to the story came in a series of observations in which caveolin-1 expression in cancer cells was monitored following chemotherapy.169,170 These studies showed that exposure of cultured lung cancer cells to various chemotherapeutics was associated with a strong induction in caveolin-1 expression. Since most studies looking at caveolin-1 expression levels do not report the chemotherapy exposure of donor subjects, the effect of chemotherapy on caveolin-1 expression level cannot be assessed.
In summary, while the majority of in vitro mechanistic data support a role of caveolin-1 as a cell cycle regulator and tumor suppressor, it seems possible that it may also be instrumental in increasing metastatic potential by promoting cell motility. The observation that caveolin-1 may be induced by cytotoxic chemotherapy implies that it could be involved in tumor cell resistance to drugs and emergence of relapse. These findings underscore the biological diversity of human tumors and the multiplicity of caveolin’s biological functions.
CONCLUSION
Data from in vitro, animal, and human studies conducted over the past 20 years have revealed, unexpectedly, abnormalities in caveolin-1 expression and caveolin-1-related pathways in a variety of lung diseases and experimental models. However, our understanding of how caveolin-1 is involved in these disease processes is rudimentary, owing in part to the complex phenotype of the global caveolin-1 knockout mouse. Cell-type specific caveolin-1 deletion will further our insights into the many biological roles of this important protein. In addition, search for caveolin-1 mutations and polymorphisms, such as P132L152 which predicts poor prognosis of breast cancer, may be fruitful in determining the roles of caveolin-1 in lung disease pathogenesis.
References
- 1.West JB, Watson RR, Fu Z. The human lung: did evolution get it wrong? Eur Respir J. 2007;29(1):11–17. doi: 10.1183/09031936.00133306. [DOI] [PubMed] [Google Scholar]
- 2.West JB. Thoughts on the pulmonary blood-gas barrier. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L501–L513. doi: 10.1152/ajplung.00117.2003. [DOI] [PubMed] [Google Scholar]
- 3.Gehr P, Bachofen M, Weibel ER. The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respir Physiol. 1978;32(2):121–140. doi: 10.1016/0034-5687(78)90104-4. [DOI] [PubMed] [Google Scholar]
- 4.Phillips CG, Kaye SR. On the asymmetry of bifurcations in the bronchial tree. Respir Physiol. 1997;107(1):85–98. doi: 10.1016/s0034-5687(96)02506-6. [DOI] [PubMed] [Google Scholar]
- 5.Stevens T, Phan S, Frid MG, et al. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc. 2008;5(7):783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med. 2010;61:105–119. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosens R, Mutawe M, Martin S, et al. Caveolae and caveolins in the respiratory system. Curr Mol Med. 2008;8(8):741–753. doi: 10.2174/156652408786733720. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 9.Couet J, Li S, Okamoto T, et al. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272(10):6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271(46):29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razani B, Lisanti MP. Caveolin-deficient mice: insights into caveolar function human disease. J Clin Invest. 2001;108(11):1553–1561. doi: 10.1172/JCI14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 18(1):8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz MA, Caldwell L, Cafasso D, et al. Emerging pulmonary vasculature lacks fate specification. Am J Physiol Lung Cell Mol Physiol. 2009;296(1):L71–L81. doi: 10.1152/ajplung.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez MI, Pollack L, Millien G, et al. The alpha-isoform of caveolin-1 is a marker of vasculogenesis in early lung development. J Histochem Cytochem. 2002;50(1):33–42. doi: 10.1177/002215540205000104. [DOI] [PubMed] [Google Scholar]
- 15.Engelman JA, Zhang X, Galbiati F, et al. Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer disease, and muscular dystrophy. Am J Hum Genet. 1998;63(6):1578–1587. doi: 10.1086/302172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razani B, Engelman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276(41):38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 17.Zhao YY, Zhao YD, Mirza MK, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119(7):2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maniatis NA, Shinin V, Schraufnagel DE, et al. Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1−/− mice. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L865– L873. doi: 10.1152/ajplung.00079.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikol DD, Scherer SS, Duckett SJ, et al. Schwann cell caveolin-1 expression increases during myelination and decreases after axotomy. Glia. 2002;38(3):191–199. doi: 10.1002/glia.10063. [DOI] [PubMed] [Google Scholar]
- 20.Yang G, Timme TL, Naruishi K, et al. Mice with cav-1 gene disruption have benign stromal lesions and compromised epithelial differentiation. Exp Mol Pathol. 2008;84(2):131–140. doi: 10.1016/j.yexmp.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Williams MC. Alveolar typeI cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs S, Hollins AJ, Laue M, et al. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res. 2003;311(1):31–45. doi: 10.1007/s00441-002-0653-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Maciejewski BS, Drouillard D, et al. A role for caveolin-1 in mechanotransduction of fetal type II epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2010 doi: 10.1152/ajplung.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 25.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 26.Galbiati F, Volonte D, Brown AM, et al. Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by recruiting beta-catenin to caveolae membrane domains. J Biol Chem. 2000;275(30):23368–23377. doi: 10.1074/jbc.M002020200. [DOI] [PubMed] [Google Scholar]
- 27.Mucenski ML, Nation JM, Thitoff AR, et al. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L971–L979. doi: 10.1152/ajplung.00172.2005. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto T, Schlegel A, Scherer PE, et al. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273(10):5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 29.Dahlin K, Mager EM, Allen L, et al. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31(3):309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 30.Maina JN. Fundamental structural aspects and features in the bioengineering of the gas exchangers: comparative perspectives. Adv Anat Embryol Cell Biol. 2002;163:III–XII. 1–108. doi: 10.1007/978-3-642-55917-4. [DOI] [PubMed] [Google Scholar]
- 31.Gold WA, Murray JF, Nadel JA, editors. Procedures in Respiratory Medicine. Philadelphia: Saunders; 2002. [Google Scholar]
- 32.Weibel ER. Morphological basis of alveolar-capillary gas exchange. Physiol Rev. 1973;53(2):419–495. doi: 10.1152/physrev.1973.53.2.419. [DOI] [PubMed] [Google Scholar]
- 33.Gil J. Number and distribution of plasmalemmal vesicles in the lung. Fed Proc. 1983;42(8):2414–2418. [PubMed] [Google Scholar]
- 34.Gil J, Silage DA, McNiff JM. Distribution of vesicles in cells of air-blood barrier in the rabbit. J Appl Physiol. 198l;50(2):334–340. doi: 10.1152/jappl.1981.50.2.334. [DOI] [PubMed] [Google Scholar]
- 35.Borok Z, Liebler JM, Lubman RL, et al. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L599–L608. doi: 10.1152/ajplung.00130.2000. [DOI] [PubMed] [Google Scholar]
- 36.Johnson MD, Widdicombe JH, Allen L, et al. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci U S A. 2002;99(4):1966–1971. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobbs LG, Gonzalez R, Matthay MA, et al. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci U S A. 1998;95(6):2991–2996. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman GR, Campbell L, von Ruhland C, et al. Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium: implications for alveolar epithelial type I cell function. Cell Tissue Res. 1999;295(1):111–120. doi: 10.1007/s004410051217. [DOI] [PubMed] [Google Scholar]
- 39.Gumbleton M. Caveolae as potential macromolecule trafficking compartments within alveolar epithelium. Adv Drug Deliv Rev. 2001;49(3):281–300. doi: 10.1016/s0169-409x(01)00142-9. [DOI] [PubMed] [Google Scholar]
- 40.Atwal OS, Viel L, Minhas KJ. An uptake of cationized ferritin by alveolar type I cells in airway- instilled goat lung: distribution of anionic sites on the epithelial surface. J Submicrosc Cytol Pathol. 1990;22(3):425–432. [PubMed] [Google Scholar]
- 41.Kasper M, Reimann T, Hempel U, et al. Loss of caveolin expression in type I pneumocytes as an indicator of subcellular alterations during lung fibrogenesis. Histochem Cell Biol. 1998;109(1):41–48. doi: 10.1007/s004180050200. [DOI] [PubMed] [Google Scholar]
- 42.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L259–L271. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 43.Campbell L, Hollins AJ, Al-Eid A, et al. Caveolin-1 expression and caveolae biogenesis during cell transdifferentiation in lung alveolar epithelial primary cultures. Biochem Biophys Res Commun. 1999;262(3):744–751. doi: 10.1006/bbrc.1999.1280. [DOI] [PubMed] [Google Scholar]
- 44.Bignon J, Jaurand MC, Pinchon MC, et al. Immunoelectron microscopic and immunochemical demonstrations of serum proteins in the alveolar lining material of the rat lung. Am Rev Respir Dis. 1976;113(2):109–120. doi: 10.1164/arrd.1976.113.2.109. [DOI] [PubMed] [Google Scholar]
- 45.Kogo H, Aiba T, Fujimoto T. Cell type-specific occurrence of caveolin-1 alpha and -1 beta in the lung caused by expression of distinct mRNAs. J Biol Chem. 2004;279(24):25574–25581. doi: 10.1074/jbc.M310807200. [DOI] [PubMed] [Google Scholar]
- 46.Peterson BT, Idell S, MacArthur C, et al. A modified bronchoalveolar lavage procedure that allows measurement of lung epithelial lining fluid volume. Am Rev Respir Dis. 1990;141(2):314–320. doi: 10.1164/ajrccm/141.2.314. [DOI] [PubMed] [Google Scholar]
- 47.Peterson BT, Griffith DE, Tate RW, et al. Single-cycle bronchoalveolar lavage to determine solute concentrations in epithelial lining fluid. Am Rev Respir Dis. 1993;147(5):1216–1222. doi: 10.1164/ajrccm/147.5.1216. [DOI] [PubMed] [Google Scholar]
- 48.Pusch R, Kleen M, Habler O, et al. Biochemical and cellular composition of alveolar epithelial lining fluid in anesthetized healthy lambs. Eur J Med Res. 1997;2(12):499–505. [PubMed] [Google Scholar]
- 49.Hastings RH, Wright JR, Albertine KH, et al. Effect of endocytosis inhibitors on alveolar clearance of albumin, immunoglobulin G, and SP-A in rabbits. Am J Physiol. 1994;266(5 Pt 1):L544–L552. doi: 10.1152/ajplung.1994.266.5.L544. [DOI] [PubMed] [Google Scholar]
- 50.Hastings RH, Folkesson HG, Petersen V, et al. Cellular uptake of albumin from lungs of anesthetized rabbits. Am J Physiol. 1995;269(4 Pt 1):L453–L462. doi: 10.1152/ajplung.1995.269.4.L453. [DOI] [PubMed] [Google Scholar]
- 51.Hastings RH, Folkesson HG, Matthay MA. Mechanisms of alveolar protein clearance in the intact lung. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L679–L689. doi: 10.1152/ajplung.00205.2003. [DOI] [PubMed] [Google Scholar]
- 52.John TA, Vogel SM, Minshall RD, et al. Evidence for the role of alveolar epithelial gp60 in active transalveolar albumin transport in the rat lung. J Physiol. 200l;533(Pt 2):547–559. doi: 10.1111/j.1469-7793.2001.0547a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 54.Orfanos SE, Mavrommati I, Korovesi I, et al. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med. 2004;30(9):1702–1714. doi: 10.1007/s00134-004-2370-x. [DOI] [PubMed] [Google Scholar]
- 55.Maniatis NA, Orfanos SE. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14(1):22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 56.Maniatis NA, Kotanidou A, Catravas JD, et al. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol. 2008;49(4–6):119–133. doi: 10.1016/j.vph.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2007;293(4):L823–L842. doi: 10.1152/ajplung.00436.2006. [DOI] [PubMed] [Google Scholar]
- 58.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 59.Minshall RD, Malik AB. Transport across the endothelium: regulation of endothelial permeability. Handb Exp Pharmacol. 2006;(176 Pt 1):107–144. doi: 10.1007/3-540-32967-6_4. [DOI] [PubMed] [Google Scholar]
- 60.Schnitzer JE, Allard J, Oh P. NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia. Am J Physiol. 1995;268(1 Pt 2):H48–H55. doi: 10.1152/ajpheart.1995.268.1.H48. [DOI] [PubMed] [Google Scholar]
- 61.Tiruppathi C, Song W, Bergenfeldt M, et al. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272(41):25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 62.Minshall RD, Tiruppathi C, Vogel SM, et al. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150(5):1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.John TA, Vogel SM, Tiruppathi C, et al. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L187–L196. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 64.Shajahan AN, Timblin BK, Sandoval R, et al. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279(19):20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 65.Shajahan AN, Tiruppathi C, Smrcka AV, et al. Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279(46):48055–48062. doi: 10.1074/jbc.M405837200. [DOI] [PubMed] [Google Scholar]
- 66.Minshall RD, Tiruppathi C, Vogel SM, et al. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol. 2002;117(2):105–112. doi: 10.1007/s00418-001-0367-x. [DOI] [PubMed] [Google Scholar]
- 67.Parkar NS, Akpa BS, Nitsche LC, et al. Vesicle formation and endocytosis: function, machinery, mechanisms, and modeling. Antioxid Redox Signal. 2009;11(6):1301–1312. doi: 10.1089/ars.2008.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maniatis NA, Brovkovych V, Allen SE, et al. Novel mechanism of endothelial nitric oxide synthase activation mediated by caveolae internalization in endothelial cells. Circ Res. 2006;99(8):870–877. doi: 10.1161/01.RES.0000245187.08026.47. [DOI] [PubMed] [Google Scholar]
- 69.Schnitzer JE, Oh P. Aquaporin-1 in plasma membrane and caveolae provides mercury-sensitive water channels across lung endothelium. Am J Physiol. 1996;270(1 Pt 2):H416–H422. doi: 10.1152/ajpheart.1996.270.1.H416. [DOI] [PubMed] [Google Scholar]
- 70.Zhang XL, Topley N, Ito T, et al. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta 1 signaling. J Biol Chem. 2005;280(13):12239–12245. doi: 10.1074/jbc.M413284200. [DOI] [PubMed] [Google Scholar]
- 71.Schubert W, Frank PG, Woodman SE, et al. Microvascular hyperpermeability in caveolin-1 (−/−) knock -out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277(42):40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 72.Miyawaki-Shimizu K, Predescu D, Shimizu J, et al. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290(2):L405–L413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- 73.Sun Y, Hu G, Zhang X, et al. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res. 2009;105(7):676–685. 615. doi: 10.1161/CIRCRESAHA.109.201673. following 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu G, Vogel SM, Schwartz DE, et al. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102(12):e120–e131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith P, Heath D, Yacoub M, et al. The ultrastructure of plexogenic pulmonary arteriopathy. J Pathol. 1990;160(2):111–121. doi: 10.1002/path.1711600204. [DOI] [PubMed] [Google Scholar]
- 76.Dorfmuller P, Humbert M, Capron F, et al. Pathology and aspects of pathogenesis in pulmonary arterial hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20(1):9–19. [PubMed] [Google Scholar]
- 77.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res. 2009;10:95. doi: 10.1186/1465-9921-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Tuder RM, Groves B, Badesch DB, et al. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144(2):275–285. [PMC free article] [PubMed] [Google Scholar]
- 80.Cool CD, Groshong SD, Oakey J, et al. Pulmonary hypertension: cellular and molecular mechanisms. Chest. 2005;128(6 Suppl):565S–57lS. doi: 10.1378/chest.128.6_suppl.565S. [DOI] [PubMed] [Google Scholar]
- 81.Sakao S, Taraseviciene-Stewart L, Lee JD, et al. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19(9):1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 82.Sehgal PB, Mukhopadhyay S. Dysfunctional intracellular trafficking in the pathobiology of pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2007;37(1):31–37. doi: 10.1165/rcmb.2007-0066TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith P, Heath D. Electron microscopy of the plexiform lesion. Thorax. 1979;34(2):177–186. doi: 10.1136/thx.34.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heath D, Smith P, Gosney J, et al. The pathology of the early and late stages of primary pulmonary hypertension. Br Heart J. 1987;58(3):204–213. doi: 10.1136/hrt.58.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geraci MW, Moore M, Gesell T, et al. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88(6):555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 86.Achcar RO, Demura Y, Rai PR, et al. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest. 2006;129(3):696–705. doi: 10.1378/chest.129.3.696. [DOI] [PubMed] [Google Scholar]
- 87.Mathew R, Huang J, Shah M, et al. Disruption of endothelial-cell caveolin-1 alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation. 2004;110(11):1499–1506. doi: 10.1161/01.CIR.0000141576.39579.23. [DOI] [PubMed] [Google Scholar]
- 88.Mukhopadhyay S, Xu F, Sehgal PB. Aberrant cytoplasmic sequestration of eNOS in endothelial cells after monocrotaline, hypoxia, and senescence: live-cell caveolar and cytoplasmic NO imaging. Am J Physiol Heart Circ Physiol. 2007;292(3):H1373–H1389. doi: 10.1152/ajpheart.00990.2006. [DOI] [PubMed] [Google Scholar]
- 89.Jasmin JF, Mercier I, Dupuis J, et al. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006;114(9):912–920. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 90.Zhao YY, Liu Y, Stan RV, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002;99(17):11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wunderlich C, Schober K, Lange SA, et al. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340(2):702–708. doi: 10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 92.Wunderlich C, Schober K, Schmeisser A, et al. The adverse cardiopulmonary phenotype of caveolin-1 deficient mice is mediated by a dysfunctional endothelium. J Mol Cell Cardiol. 2008;44(5):938–947. doi: 10.1016/j.yjmcc.2008.02.275. [DOI] [PubMed] [Google Scholar]
- 93.Wunderlich C, Schmeisser A, Heerwagen C, et al. Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm Pharmacol Ther. 2008;21(3):507–515. doi: 10.1016/j.pupt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Zhao YY, Malik AB. A novel insight into the mechanism of pulmonary hypertension involving caveolin-1 deficiency and endothelial nitric oxide synthase activation. Trends Cardiovasc Med. 2009;19(7):238–242. doi: 10.1016/j.tcm.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khalil N, O’Connor RN, Flanders KC, et al. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996;14(2):131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- 96.Khalil N, O’Connor RN, Unruh HW, et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathicpulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5(2):155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 97.Khalil N, O’Connor RN, Flanders KC, et al. Regulation of type II alveolar epithelial cell proliferation by TGF-beta during bleomycin-induced lung injury in rats. Am J Physiol. 1994;267(5 Pt 1):L498–L507. doi: 10.1152/ajplung.1994.267.5.L498. [DOI] [PubMed] [Google Scholar]
- 98.Verma S, Slutsky AS. Idiopathic pulmonary fibrosis—new insights. N Engl J Med. 2007;356(13):1370–1372. doi: 10.1056/NEJMcibr070490. [DOI] [PubMed] [Google Scholar]
- 99.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-beta receptor internalization and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20(6):713–719. doi: 10.1097/bor.0b013e3283103d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 102.Le Saux O, Teeters K, Miyasato S, et al. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am J Physiol Lung Cell Mol Physiol. 2008;295(6):L1007–L1017. doi: 10.1152/ajplung.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murata T, Lin MI, Huang Y, et al. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204(10):2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Odajima N, Betsuyaku T, Nasuhara Y, et al. Loss of caveolin-1 in bronchiolization in lung fibrosis. J Histochem Cytochem. 2007;55(9):899–909. doi: 10.1369/jhc.7A7203.2007. [DOI] [PubMed] [Google Scholar]
- 105.Tourkina E, Gooz P, Pannu J, et al. Opposing effects of protein kinase Calpha and protein kinase Cepsilon on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J Biol Chem. 2005;280(14):13879–13887. doi: 10.1074/jbc.M412551200. [DOI] [PubMed] [Google Scholar]
- 106.Wang XM, Zhang Y, Kim HP, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203(13):2895–2906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Del Galdo F, Sotgia F, de Almeida CJ, et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008;58(9):2854–2865. doi: 10.1002/art.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tourkina E, Richard M, Gooz P, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L843–L861. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 109.Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19(1):58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 110.Hayes S, Chawla A, Corvera S. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J Cell Biol. 2002;158(7):1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Di Guglielmo GM, Le Roy C, Goodfellow AF, et al. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5(5):410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 112.Runyan CE, Schnaper HW, Poncelet AC. The role of internalization in transforming growth factor beta1-induced Smad2 association with Smad anchor for receptor activation (SARA) and Smad2-dependent signaling in human mesangial cells. J Biol Chem. 2005;280(9):8300–8308. doi: 10.1074/jbc.M407939200. [DOI] [PubMed] [Google Scholar]
- 113.Bousquet J, Jeffery PK, Busse WW, et al. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 114.Camoretti-Mercado B. Targeting the airway smooth muscle for asthma treatment. Transl Res. 2009;154(4):165–174. doi: 10.1016/j.trsl.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murphy DM, O’Byrne PM. Recent advances in the pathophysiology of asthma. Chest. 2010;137(6):1417–1426. doi: 10.1378/chest.09-1895. [DOI] [PubMed] [Google Scholar]
- 116.Halayko AJ, Tran T, Gosens R. Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proc Am Thorac Soc. 2008;5(1):80–88. doi: 10.1513/pats.200705-057VS. [DOI] [PubMed] [Google Scholar]
- 117.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75(3):487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 118.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 119.Hirst SJ, Twort CH, Lee TH. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol. 2000;23(3):335–344. doi: 10.1165/ajrcmb.23.3.3990. [DOI] [PubMed] [Google Scholar]
- 120.Dekkers BG, Schaafsma D, Tran T, et al. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2009;41(4):494–504. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 121.Dekkers BG, Schaafsma D, Nelemans SA, et al. Extracellular matrix proteins differentially regulate airway smooth muscle phenotype and function. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1405–L1413. doi: 10.1152/ajplung.00331.2006. [DOI] [PubMed] [Google Scholar]
- 122.Schaafsma D, McNeill KD, Stelmack GL, et al. Insulin increases the expression of contractile phenotypic markers in airway smooth muscle. Am J Physiol Cell Physiol. 2007;293(1):C429–C439. doi: 10.1152/ajpcell.00502.2006. [DOI] [PubMed] [Google Scholar]
- 123.Peterson TE, Guicciardi ME, Gulati R, et al. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol. 2003;23(9):1521–1527. doi: 10.1161/01.ATV.0000081743.35125.05. [DOI] [PubMed] [Google Scholar]
- 124.Thyberg J, Roy J, Tran PK, et al. Expression of caveolae on the surface of rat arterial smooth muscle cells is dependent on the phenotypic state of the cells. Lab Invest. 1997;77(1):93–101. [PubMed] [Google Scholar]
- 125.Zhang Y, Peng F, Gao B, et al. Mechanical Strain-Induced RhoA Activation Requires NADPH Oxidase-Mediated ROS Generation in Caveolae. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2908. [DOI] [PubMed] [Google Scholar]
- 126.Peng F, Zhang B, Wu D, et al. TGFbeta- induced RhoA activation and fibronectin production in mesangial cells require caveolae. Am J Physiol Renal Physiol. 2008;295(1):F153–F164. doi: 10.1152/ajprenal.00419.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Somara S, Gilmont RR, Martens JR, et al. Ectopic expression of caveolin-1 restores physiological contractile response of aged colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G240–G249. doi: 10.1152/ajpgi.00064.2007. [DOI] [PubMed] [Google Scholar]
- 128.Hunter I, Nixon GF. Spatial compartmentalization of tumor necrosis factor (TNF) receptor 1-dependent signaling pathways in human airway smooth muscle cells. Lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kappaB and MAPK pathways. J Biol Chem. 2006;281(45):34705–34715. doi: 10.1074/jbc.M605738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gosens R, Dueck G, Gerthoffer WT, et al. p42/p44 MAP kinase activation is localized to caveolae-free membrane domains in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1163–L1172. doi: 10.1152/ajplung.00471.2006. [DOI] [PubMed] [Google Scholar]
- 130.Le Saux CJ, Teeters K, Miyasato SK, et al. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling. J Biol Chem. 2008;283(9):5760–5768. doi: 10.1074/jbc.M701572200. [DOI] [PubMed] [Google Scholar]
- 131.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 132.Zaas DW, Duncan MJ, Li G, et al. Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J Biol Chem. 2005;280(6):4864–4872. doi: 10.1074/jbc.M411702200. [DOI] [PubMed] [Google Scholar]
- 133.Millan J, Hewlett L, Glyn M, et al. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8(2):113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 134.Tiruppathi C, Shimizu J, Miyawaki-Shimizu K, et al. Role of NF-kappaB-dependent caveolin-1 expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J Biol Chem. 2008;283(7):4210–4218. doi: 10.1074/jbc.M703153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hatakeyama T, Pappas PJ, Hobson RW, 2nd, et al. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol. 2006;574(Pt 1):275–281. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117(Pt 12):2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 137.Zhou X, He P. Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules. Cardiovasc Res. 87(2):340–347. doi: 10.1093/cvr/cvq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bucci M, Gratton JP, Rudic RD, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6(12):1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 139.Garrean S, Gao XP, Brovkovych V, et al. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177(7):4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 140.Mirza MK, Yuan J, Gao XP, et al. Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am J Pathol. 176(5):2344–2351. doi: 10.2353/ajpath.2010.091088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Medina FA, de Almeida CJ, Dew E, et al. Caveolin-1-deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74(12):6665–6674. doi: 10.1128/IAI.00949-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Medina FA, Cohen AW, de Almeida CJ, et al. Immune dysfunction in caveolin-1 null mice following infection with Trypanosoma cruzi (Tulahuen strain) Microbes Infect. 2007;9(3):325–333. doi: 10.1016/j.micinf.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feng H, Guo L, Song Z, et al. Caveolin-1 protects against sepsis by modulating inflammatory response, alleviating bacterial burden, and suppressing thymocyte apoptosis. J Biol Chem. 285(33):25154–25160. doi: 10.1074/jbc.M110.116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J, Scherl A, Medina F, et al. Impaired phagocytosis in caveolin-1 deficient macrophages. Cell Cycle. 2005;4(11):1599–1607. doi: 10.4161/cc.4.11.2117. [DOI] [PubMed] [Google Scholar]
- 145.Hu G, Ye RD, Dinauer MC, et al. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L178–L186. doi: 10.1152/ajplung.00263.2007. [DOI] [PubMed] [Google Scholar]
- 146.Oakley FD, Smith RL, Engelhardt JF. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J Biol Chem. 2009;284(48):33255–33264. doi: 10.1074/jbc.M109.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Majkova Z, Smart E, Toborek M, et al. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237(1):1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang L, Lim EJ, Toborek M, et al. The role of fatty acids and caveolin-1 in tumor necrosis factor alpha-induced endothelial cell activation. Metabolism. 2008;57(10):1328–1339. doi: 10.1016/j.metabol.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang XM, Kim HP, Nakahira K, et al. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182(6):3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 150.Schwartz AM, Henson DE. Diagnostic surgical pathology in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):78S–93S. doi: 10.1378/chest.07-1350. [DOI] [PubMed] [Google Scholar]
- 151.Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6(2):233–241. doi: 10.1513/pats.200809-110LC. [DOI] [PubMed] [Google Scholar]
- 152.Masson RJ, Broaddus VC, Murray JF, et al. Murray and Nadel’s Textbook of Respiratory Medicine. 4. Philadelphia: Saunders; 2005. [Google Scholar]
- 153.Jett JR, Schild SE, Keith RL, et al. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):266S–276S. doi: 10.1378/chest.07-1380. [DOI] [PubMed] [Google Scholar]
- 154.Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264(34):20163–20166. [PubMed] [Google Scholar]
- 155.Glenney JR, Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A. 1992;89(21):10517–10521. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989;108(6):2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci U S A. 1995;92(5):1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.An SS, Bai TR, Bates JH, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29(5):834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hulit J, Bash T, Fu M, et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 2000;275(28):21203–21209. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- 160.Gratton JP, Lin MI, Yu J, et al. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4(1):31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 161.Racine C, Belanger M, Hirabayashi H, et al. Reduction of caveolin 1 gene expression in lung carcinoma cell lines. Biochem Biophys Res Commun. 1999;255(3):580–586. doi: 10.1006/bbrc.1999.0236. [DOI] [PubMed] [Google Scholar]
- 162.Wikman H, Kettunen E, Seppanen JK, et al. Identification of differentially expressed genes in pulmonary adenocarcinoma by using cDNA array. Oncogene. 2002;21(37):5804–5813. doi: 10.1038/sj.onc.1205726. [DOI] [PubMed] [Google Scholar]
- 163.Wikman H, Seppanen JK, Sarhadi VK, et al. Caveolins as tumour markers in lung cancer detected by combined use of cDNA and tissue microarrays. J Pathol. 2004;203(1):584–593. doi: 10.1002/path.1552. [DOI] [PubMed] [Google Scholar]
- 164.Sunaga N, Miyajima K, Suzuki M, et al. Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res. 2004;64(12):4277–4285. doi: 10.1158/0008-5472.CAN-03-3941. [DOI] [PubMed] [Google Scholar]
- 165.Ho CC, Huang PH, Huang HY, et al. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol. 2002;161(5):1647–1656. doi: 10.1016/S0002-9440(10)64442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Huang J, Kaminski PM, Edwards JG, et al. Pyrrolidine dithiocarbamate restores endothelial cell membrane integrity and attenuates monocrotaline-induced pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1250–L1259. doi: 10.1152/ajplung.00069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Joshi B, Strugnell SS, Goetz JG, et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68(20):8210–8220. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 168.Bonuccelli G, Casimiro MC, Sotgia F, et al. Caveolin-1 (P132L), a common breast cancer mutation, confers mammary cell invasiveness and defines a novel stem cell/metastasis-associated gene signature. Am J Pathol. 2009;174(5):1650–1662. doi: 10.2353/ajpath.2009.080648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Belanger MM, Gaudreau M, Roussel E, et al. Role of caveolin-1 in etoposide resistance development in A549 lung cancer cells. Cancer Biol Ther. 2004;3(10):954–959. doi: 10.4161/cbt.3.10.1112. [DOI] [PubMed] [Google Scholar]
- 170.Belanger MM, Roussel E, Couet J. Up-regulation of caveolin expression by cytotoxic agents in drug-sensitive cancer cells. Anticancer Drugs. 2003;14(4):281–287. doi: 10.1097/00001813-200304000-00005. [DOI] [PubMed] [Google Scholar]