Abstract
TNFRSF4 (OX40, CD134) and TNFRSF25 are costimulatory receptors that influence CD4+ and CD8+ T cell responses to cognate-antigen. Independently, these receptors have been described to stimulate overlapping functions including enhanced proliferation and activation for both regulatory (CD4+FoxP3+, Treg) and conventional (CD4+FoxP3- or CD8+FoxP3-) T cells. To determine the relative functionality of TNFRSF4 and TNFRSF25 in T cell immunity, the activity of TNFRSF4 and TNFRS25 agonistic antibodies was compared in the context of both traditional protein/adjuvant (ovalbumin/alum) and CD8+ specific heat shock protein based (gp96-Ig) vaccine approaches. These studies demonstrate that both TNFRSF4 and TNFRSF25 independently and additively costimulate vaccine induced CD8+ T cell proliferation following both primary and secondary antigen challenge. In contrast, the activity of TNFRSF4 and TNFRSF25 were observed to be divergent in the costimulation of CD4+ T cell immunity. TNFRSF4 agonists were potent costimulators of ova/alum induced CD4+ conventional T cell proliferation but only weakly costimulated Treg proliferation and IgG2a production, while TNFRSF25 agonists were strong costimulators of Treg proliferation, production of IgG1, IgG2a and IgG2b and weak costimulators of CD4+ Tconv proliferation. Interestingly, antigen-specific cellular and humoral responses were uncoupled upon secondary immunization, which was dramatically effected by the presence of TNFRSF4 or TNFRSF25 costimulation. These studies highlight the overlapping but non-redundant activities of TNFRSF4 and TNFRSF25 in T cell immunity, which may guide the application of receptor agonistic agents as vaccine adjuvants for infectious disease and tumor immunity.
Introduction
T cell mediated immune responses are initiated by presentation of cognate antigen (signal 1) in the context of appropriate costimulatory molecules (signal 2), typically of the B7-family. Additional signals delivered via soluble cytokines or tumor necrosis factor superfamily (TNFSF) ligands may also influence the duration, magnitude and quality of T cell mediated immune responses, either in addition or instead of traditional B7 family members. The diversity of TNFSF members suggests that this family evolved to fine-tune adaptive immune responses by modulating specific phases of immunity for distinct cell types. Due to the activity of TNFSF members as antigen-dependent T cell costimulators, therapeutic stimulation of several receptors, including TNFRSF4 (CD134, OX40) and TNFRSF25, is a potential method to augment the activity of vaccines.
TNFRSF4 and TNFRSF25 are neighbors on chromosome 4 in mice and are proposed to signal upon ligation to their homotrimerized ligands, TNFSF4 (OX40L) and TNFSF15 (TL1A), respectively (1, 2). Each has a highly similar pattern of expression that is specific to lymphocytes, and in particular on activated CD4+ and CD8+ T cells (3). Signaling by TNFRSF4 or TNFRSF25 contributes to activation of CD4+ and CD8+ effector T cells in various murine autoimmunity and tumor models and selective blockade or stimulation of these receptors are both under investigation for the inhibition of autoimmunity or stimulation of anti-tumor immunity, respectively (3-5). In addition, both receptors are also constitutively expressed by CD4+FoxP3+ T regulatory cells (Treg) and can influence Treg activity and function (6, 7). Thus, TNFRSF4 and TNFRSF25 are reported to have highly similar activities in T cell activation despite their unique cytoplasmic domain structures and signaling pathways. Because coordinated signaling through multiple TNF receptors is believed to determine the specificity, magnitude and duration of T cell immunity, it is important that the apparently similar characteristics of TNFRSF4 and TNFRSF25 be comparatively evaluated in a systematic fashion.
In the current study we have performed a systematic comparison of TNFRSF4 and TNFRSF25 agonistic antibodies as costimulators of vaccine-induced T cell mediated immune responses. As vaccine we have utilized both traditional protein/adjuvant (ovalbumin/alum) based immunization, which leads to antigen presentation on both MHC I and MHC II, and also a cell-secreted heat shock protein, gp96-Ig, approach which specifically leads to antigen cross-presentation on MHC I(8). These studies demonstrate that TNFRSF4 and TNFRSF25 have overlapping and additive activity as costimulators of CD8+ T cell proliferation but diverge in stimulating proliferation of Treg and CD4+FoxP3-T conventional (Tconv) upon both primary and secondary immunization. Interestingly, there was also divergence in the antigen-specific cellular and humoral immune response to secondary immunization, which was dramatically influenced by TNFRSF4 and TNFRSF25 stimulation. These studies support the concept that therapeutic targeting of TNFSF members, including TNFRSF4 and TNFRSF25, can be utilized to enhance the activity of vaccine-primed immunity by targeting specific subsets of T cell mediated immunity.
Materials and Methods
Mice and Cell Lines
Wild type C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Foxp3+RFP+ reporter mice on a B6 background (generously provided by Dr. Richard Flavell (9)) and OT-II and OT-I mice were bred in our animal facility. Mice were used at 6-12 weeks of age and were maintained in pathogen-free conditions at the UM animal facilities. All animal use procedures were approved by the University of Miami Animal Care and Use Committee. 3T3-ova-gp96-Ig vaccine cells were maintained and utilized as previously described(10, 11).
Reagents, antibodies and flow cytometry
Commercial antibodies for use in flow cytometry, ELISA and in vivo studies were purchased from BD Pharmingen, eBioscience or BioLegend. The Armenian Hamster IgG Isotype control was bought from eBioscience. Armenian hamster hybridomas producing antibodies to mouse TNFRSF25 (4C12, agonistic) were generated as described previously(12). Briefly, 4C12 and OX86 were produced in hollow fiber bioreactors (Fibercell Systems, Frederick, MD) and purified from serum-free supernatants on a protein G column (GE Healthcare, UK). For flow cytometry analysis, single cell suspensions were prepared from spleen and lymph nodes. 106 cells were pre-blocked with anti-mouse CD16/CD32 and stained with different antibody combinations. Intracellular staining was performed according to standard procedures. Flow cytometric analysis was performed on a Becton Dickinson Fortessa instrument and DIVA or FlowJo software. Suspensions of ovalbumin (crystallized chicken egg ovalbumin Grade V, Sigma) and aluminum sulphate (Sigma) were prepared as previously described(12) and administered (66 μg/mouse unless otherwise indicated) by intraperitoneal injection. Serum immunoglobulin enzyme linked immunosorbent assays (ELISA) were performed using 96-well plates coated with 100 μg/ml ovalbumin in pH 7.7 bicarbonate buffer (Sigma-Aldrich, USA) and biotin-conjugated anti-mouse IgM, IgG1, IgG2a or IgG2b isotype antibodies for detection.
Adoptive transfer model
OT-I cells were isolated from total splenocytes of OT-I TCR transgenic mice using a CD8+ T cell isolation kit according to manufacturer instructions (Miltenyi Biotec). OT-II cells were isolated from total splenocytes of OT-II TCR transgenic mice using a CD4+ T cell isolation kit according to manufacturer instructions (Miltenyi Biotec). Cell enrichment purity was determined by flow cytometry (≥90% purity). OT-I (5×105) and OT-II (106) cells were then mixed and injected in a total volume of 100 μl HBSS per mouse by intravenous injection.
Statistical Analysis
All graphing and statistical analysis were performed using the ABI Prism program. Paired analysis was performed using the students t-test. Analysis of conditions with more than two conditions was performed using one-way ANOVA with Tukey’s post-hoc test. Significance is indicated as * (p<0.05), ** (p<0.01) and *** (p<0.001).
Results
T cell activation by adjuvanted versus cross-presented antigen
Aluminum salts (alum) are well known adjuvants that have been used in vaccine preparations for decades with, until very recently, limited understanding of how they contributed to enhanced immune activation. The mechanism of alum as an adjuvant remains unclear, but most reports agree that alum functions as an innate trigger for dendritic cells and macrophages to produce inflammatory and Th2 type cytokines, contributing to activation of both CD4 and CD8 mediated T cell immunity (13-19). Glycoprotein-96 (gp96, grp94) is an ER chaperone and naturally occurring danger associated molecular pattern (DAMP) released from damaged cells, which functions as a natural adjuvant for dendritic cells and macrophages and selectively delivers antigens for MHC I cross-presentation and cross-priming of CD8 mediated T cell immunity (20). Both alum and gp96 can stimulate antigen-specific T cell mediated immunity by either precipitation or co-expression, respectively, with a selected antigen. Like for alum, the specific mechanism of innate activation and antigen-delivery remains unclear for gp96.
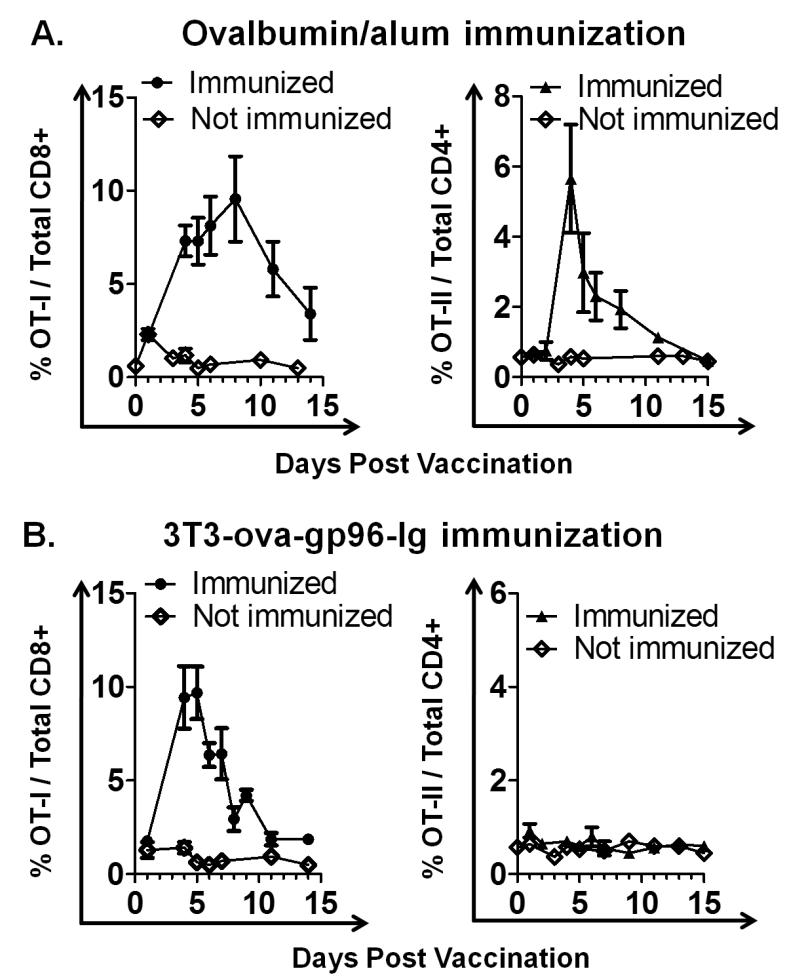
Antigen-specific activation of CD4+ and CD8+ T cell immunity using ovalbumin complexed to alum (ova/alum) was systematically compared to vaccination using a secretable form of gp96 (gp96-Ig) expressed in cis with ovalbumin by 3T3 cells (3T3-ova-gp96-Ig) (11). Vaccination with 3T3-ova-gp96-Ig leads to specific activation of TCR transgenic (Vα2+Vβ5+) ova-specific CD8+ T cells (OT-I) in vivo (10, 21, 22). C57BL/6 mice were adoptively transferred with a mixed population of TCR transgenic (Vα2+Vβ5+), ova-specific CD4+ cells (OT-II, 106 cells) and OT-I (5×105) cells. After 48 hrs these mice were vaccinated intraperitoneally (i.p.) with either 3T3-ova-gp96-Ig cells secreting 50 ng ova and 200 ng gp96-Ig (106 cells/24 hrs) or with 66μg ova/alum (Figure 1). This dose of ova/alum was selected (Figure 1A) because it leads to equivalent activation and expansion of OT-I cells as 3T3-ova-gp96-Ig (supplemental figure 1 and Figure 1B). These data illustrate that vaccination with ova/alum leads to the proliferation of both OT-I and OT-II cells, with responses peaking at 8 days post vaccination for OT-I cells and 4 days for OT-II cells (Figure 1A). In contrast, vaccination with 3T3-ova-gp96-Ig cells leads to the selective activation of OT-I cells, which peak at 4 days post vaccination without any detectable increase in the frequency of OT-II cells (Figure 1B). These data reinforce the concept that innate immune activation by gp96 leads to the selective activation of CD8+ T cells by MHC I antigen cross-presentation, which is distinct from activation of both OT-I and OT-II cells by ova/alum.
Figure 1. Comparative stimulation of OT-I and OT-II by adjuvanted versus cross-presented ovalbumin.
C57BL/6 mice expressing a FoxP3-RFP transgene (FIR mice) were adoptively transferred with a mixture of OT-I and OT-II cells two days prior to immunization with either ova/alum or 3T3-ova-gp96-Ig. Immunization with ova/alum stimulation proliferation of both OT-I (A, left panel) and OT-II (A, right panel) cells as detected in the peripheral blood, peaking at 8 and 4 days post immunization, respectively. Immunization with 3T3 cells expressing ovalbumin and gp96-Ig (3T3-ova-gp96-Ig) stimulated proliferation of OT-I cells (B, left panel), but did not stimulate proliferation of OT-II cells (B, right panel). OT-I proliferation by 3T3-ova-gp96-Ig immunization peaked at 4 days post immunization. Data indicate mean ± SEM for ≥ 5 mice in each of ≥ 2 independent experiments.
Costimulation of vaccine-primed OT-I by TNFRSF4 and TNFRSF25
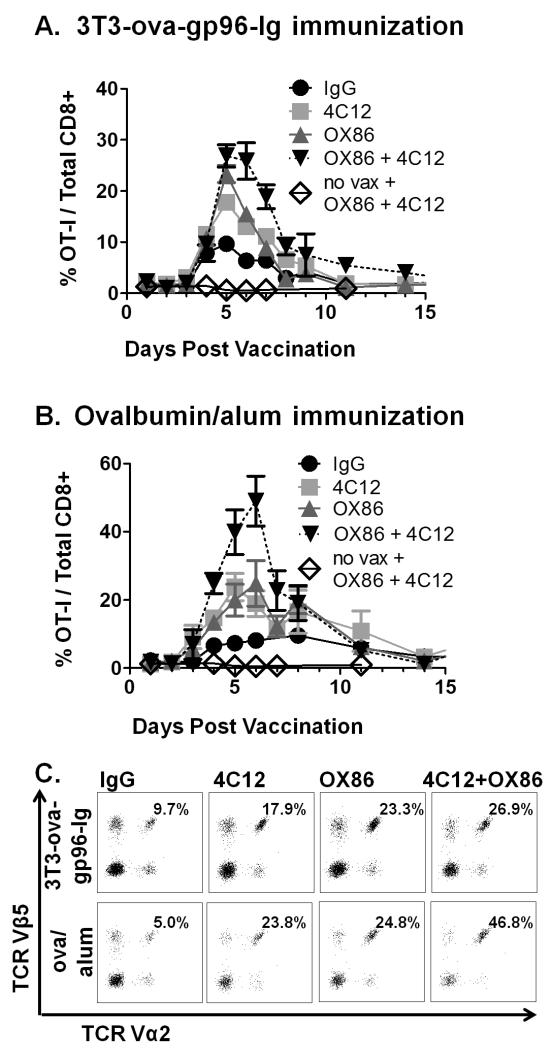
Both TNFRSF4 and TNFRSF25 have been independently described as T cell costimulators in the context of vaccination, but a systematic comparison of these receptors has not been performed (23, 24). Vaccinations were performed as described in Figure 1 with co-administration of the receptor agonistic antibody, OX86 (100μg), for TNFRSF4 or 4C12 (20μg) for TNFRSF25 at the time of vaccination (day 0).
Administration of OX86 or 4C12 together with 3T3-ova-gp96-Ig vaccination increased the proliferation of OT-I cells from 9.68 ± 1.4% to 23.33 ± 4.1% and 17.78 ± 3.5%, respectively, of all CD8+ T cells in the peripheral blood. Further, combined administration of OX86 and 4C12 stimulated additional increases in OT-I proliferation which extended through day 6, with OT-I cells peaking at 26.87 ± 5.3% of CD8+ cells (p=0.015 vs 4C12 alone) in the peripheral blood. No significant differences were observed in the expression of CD44, CD62L or CCR7 between the treatment groups (data not shown).
The kinetics of expansion by OX86 and 4C12 costimulation for ova/alum primed OT-I cells differed dramatically from that observed with ova/alum vaccination alone (Figure 2B). Both OX86 and 4C12 administration functioned independently to enhance OT-I proliferation at all time points, peaking in the peripheral blood at 5-6 days post vaccination with OT-I cells representing 24.84 ± 6.67% (OX86) and 23.8 ± 4.0% (4C12) of CD8+ cells. In addition, combined administration of OX86 and 4C12 led to a strong enhancement of OT-I proliferation, peaking at 7 days post-vaccination with OT-I cells representing nearly half (46.79 ± 7.8%, p=0.03) of the total peripheral CD8+ T cell population. Representative flow cytometry plots for each group on day 5 post immunization are illustrated (Figure 2C). Similar to 3T3-ova-gp96-Ig, no differences were observed in expression of CD44, CD62L or CCR7 between groups (data not shown).
Figure 2. Comparative costimulation of OT-I proliferation by TNFRSF4 and TNFRSF25 agonistic antibodies.
Adoptive transfer of OT-I and OT-II cells was performed as described in figure 1, followed by immunization with either 3T3-ova-gp96-Ig (A) or ova/alum (B) together with either IgG control antibody (100 μg, black circles), TNFRSF25 agonistic antibodies (clone 4C12, 20 μg, red squares), TNFRSF4 agonistic antibodies (clone OX86, 100 μg, green triangles) or both 4C12 and OX86 antibodies combined (blue squares). OT-I proliferation in the peripheral blood was measured by flow cytometry on the indicated days, and representative plots of Vα2+Vβ5+ cells (pre-gated on CD3+CD8+ cells) are illustrated in (C) on day 5 for each condition. Data indicate mean ± SEM for ≥ 5 mice in each of ≥ 2 independent experiments.
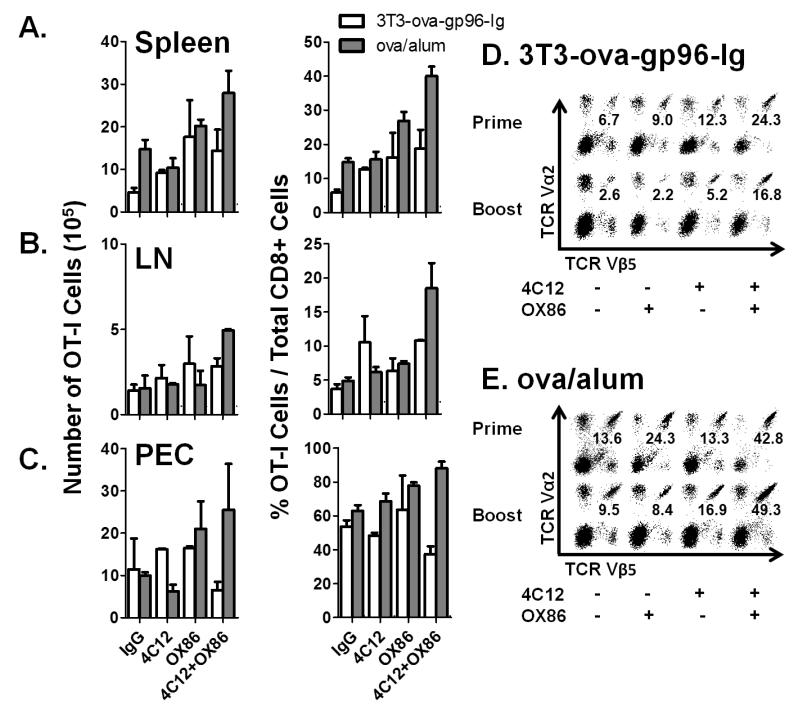
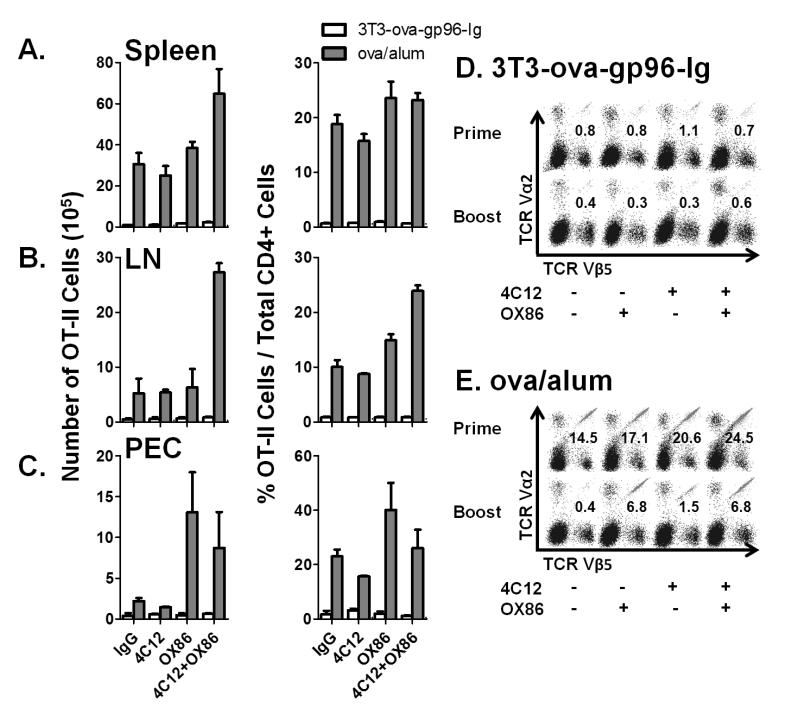
Analysis of spleen, lymph nodes and peritoneal cavity infiltrating cells indicate that the observed effects of TNFRSF4 and TNFRSF25 costimulation on peripheral blood OT-I cells are systemic (Figure 3). Both total numbers (left column) and frequencies (right column) of OT-I cells were determined in the spleen (Figure 3A), pooled mesenteric and inguinal lymph nodes (Figure 3B) and peritoneal cavity infiltrating cells (Figure 3C) 5 days after immunization with the indicated vaccine combination. This time point precedes the ‘peak’ of OT-I expansion following immunization with ova/alum but is at the time of peak expansion following immunization with 3T3-ova-gp96. Analysis of peritoneal cells indicates that in mice immunized with 3T3-ova-gp96, the observed enhancement of OT-I expansion by OX86 and 4C12 is already declining by day 5 at the site of immunization. In contrast, because the peak of OT-I expansion in the peripheral blood has not yet occurred for animals immunized with ova/alum, it is not surprising that the costimulatory effect of OX86 and 4C12 is magnified in the peritoneal cavity on day 5. Together, this tissue analysis provides a consistent picture of OT-I costimulation across several tissues, which appears to originate at the site of immunization (peritoneal cavity, as previously shown by Oizumi et al(10)) and subsequently distributes systemically with slightly delayed kinetics and can be observed in the lymph nodes, spleen and peripheral blood.
Figure 3. Tissue distribution and response of OT-I to secondary immunization.
The indicated groups were treated as shown in Figure 2 and the absolute numbers (left column) and frequencies (right column) of OT-I cells in the A) spleen, B) lymph nodes or C) peritoneal cavity determined on day 5 following primary immunization. Certain groups treated as indicated in (A-C) were monitored until day 52 following the primary immunization, at which time they were treated with a secondary immunization with D) 3T3-ova-gp96-Ig or E) ova/alum together with the indicated antibodies. Representative flow cytometry plots of splenocytes analyzed on day 57 and pre-gated on CD3+CD8+ cells are shown. The percentage of TCR Vα2+, Vβ5+ double-positive cells are overlayed on each plot. Data indicate mean ± SEM for 2 independent experiments.
To determine the influence of TNFRSF4 and TNFRSF25 costimulation at the time of priming on the magnitude of the memory response, the frequencies of OT-I cells were followed in the peripheral blood of primed animals from day 38 to day 52. These data demonstrate that the ‘set-point’ for OT-I cells following contraction from the primary immunization is equal between all groups immunized with 3T3-ova-gp96-Ig (~2% of total CD8+ cells, supplemental figure 2A). In contrast, mice immunized with ova/alum exhibited dramatic differences in the resting frequencies of OT-I cells depending on whether they were additionally costimulated with 4C12 (5.17±0.58% of total CD8+ cells), OX86 (8.16±1.5% of total CD8+ cells) or the combination of both 4C12 and OX86 (13.0±2.7% of total CD8+ cells) as compared to IgG controls (1.56±0.09% of total CD8+ cells, supplemental figure 2A).
On day 52, primed mice received a secondary immunization with the vaccine/costimulator combination administered to each mouse at the time of priming. These data indicate that for animals immunized with 3T3-ova-gp96-Ig, the OT-I proliferative response is weaker in the spleen on day 5 following the secondary immunization than observed on day 5 following the primary immunization (Figure 3D). The addition of either 4C12 antibody or 4C12 in addition to OX86 antibody significantly improved the magnitude of OT-I proliferation at the time of the recall response. Similarly, secondary immunization with ova/alum was observed to stimulate weaker OT-I proliferation unless combined with 4C12 antibody, and the effect of OX86 antibody was synergistic at the time of boosting with 4C12 antibody treatment (Figure 3E).
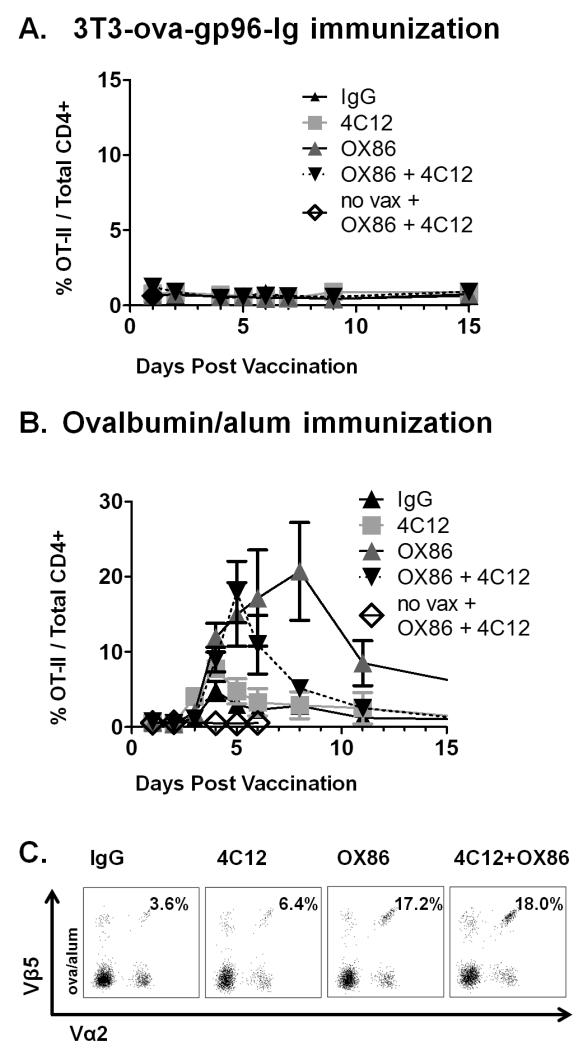
Costimulation of vaccine-primed OT-II by TNFRSF4 and TNFRSF25
Because these experiments were performed in animals adoptively transferred with both OT-I and OT-II cells, the activation of each subset was monitored simultaneously within individual mice. In all animals vaccinated with 3T3-ova-gp96-Ig, regardless of the addition of OX86 and/or 4C12 antibodies, there was absolutely no increase in the frequency of OT-II cells out of total CD4+ cells at any of the time points analyzed (Figure 4A). These findings are in accordance with previous reports demonstrating that the vaccine activity of gp96 functions as a powerful and exclusive antigen-delivery system to the MHC I-cross-presentation machinery of antigen presenting cells and can function in the absence of CD4+ cells (8, 10, 11, 25).
Figure 4. Comparative costimulation of OT-II proliferation by TNFRSF4 and TNFRSF25 agonistic antibodies.
Adoptive transfer of OT-I and OT-II cells was performed as described in figure 1, following by immunization with either 3T3-ova-gp96-Ig (A) or ova/alum (B) together with the indicated antibodies as described in figure 2. OT-II proliferation in the peripheral blood was measured by flow cytometry on the indicated days, and representative plots of Vα2+Vβ5+ cells (pre-gated on CD3+CD4+ cells) are illustrated in (C) on day 5 for ova/alum immunized mice. Data indicate mean ± SEM for ≥ 5 mice in each of ≥ 2 independent experiments.
Vaccination with ova/alum stimulated efficient proliferation of OT-II cells, which was further costimulated by administration OX40 agonistic antibodies (Figure 4B). Specifically, stimulation of TNFRSF4 by OX86 significantly increased the rate, amplitude and duration of OT-II cell proliferation in vivo. In contrast, administration of TNFRSF25 agonistic antibodies did not stimulate a significant increase in OT-II cell proliferation as compared to administration of IgG control antibodies. Addition of 4C12 antibodies reduced the costimulatory effect of OX86 antibodies to OT-II cell proliferation and is in stark contrast to the additive costimulatory effect of combined administration of OX86 and 4C12 to vaccine-primed OT-I cells. These data indicate that TNFRSF4 and TNFRSF25 differentially costimulate ova/alum primed OT-II effector T cells. Representative flow cytometry plots from day 5 post immunization are illustrated for each group (Figure 4C). Similar to OT-I cells, no differences were observed in the expression pattern of CD44, CD62L or CCR7 at 5 weeks post immunization for any of the groups (data not shown). Also similar to OT-I cells, the systemic distribution pattern of OT-II cells was observed to reflect observations made in the peripheral blood when analyzed in the spleen (Figure 5A), inguinal lymph nodes (Figure 5B) and peritoneal cavity (Figure 5C). As predicted by analysis of peripheral blood cells, OX86 appeared to provide the strongest costimulatory signal to OT-II cells systemically. Costimulation of OT-II cells in the peritoneal cavity was greatest by treatment with OX86 alone on day 5, but was already on the decline when combined with 4C12 (Figure 5C). Like OT-I cells, these observations suggest that OT-II proliferation originates at the site of vaccination (within the peritoneal cavity) and that peak proliferation at this tissue site precedes the peak of expansion observed in the peripheral blood, which does not peak until day 8 in OX86 treated animals but which has already peaked in the peripheral blood when used in combination with 4C12 (compare Figure 4B to Figure 5C).
Figure 5. Tissue distribution and response of OT-II to secondary immunization.
The indicated groups were treated as shown in Figure 4 and the absolute numbers (left column) and frequencies (right column) of OT-II cells in the A) spleen, B) lymph nodes or C) peritoneal cavity determined on day 5 following primary immunization. Certain groups treated as indicated in (A-C) were monitored until day 52 following the primary immunization, at which time they were treated with a secondary immunization with D) 3T3-ova-gp96-Ig or E) ova/alum together with the indicated antibodies. Representative flow cytometry plots of splenocytes analyzed on day 57 and pre-gated on CD3+CD4+ cells are shown. The percentage of TCR Vα2+, Vβ5+ double-positive cells are overlayed on each plot. Data indicate mean ± SEM for 2 independent experiments.
The frequency of OT-II cells was monitored in the peripheral blood following contraction from the primary immunization from day 38 to day 52. Interestingly, these data indicate that, similar to OT-I cells, there were no differences in the ‘set-point’ of memory OT-II cells for any mice immunized with 3T3-ova-gp96-Ig (supplemental figure 2B). In mice immunized with with ova/alum, there were also no differences in the set-point of peripheral blood OT-II cells except for mice that received OX86 at the time of immunization (3.36±0.69% of total CD4+ cells) as compared to IgG controls (0.43±0.09% of total CD4+ cells). In mice boosted with 3T3-ova-gp96-Ig, there was no primary proliferative response to immunization and there was also no proliferative response to secondary immunization (Figure 5D). Interestingly, in mice that received the secondary immunization with ova/alum, there was also no secondary proliferative response to immunization unless the immunization was combined with 4C12 antibody (1.5% of total CD4+ cells) or with OX86 antibody (6.8% of total CD4+ cells). In contrast to the synergistic effect of combined administration of 4C12 and OX86 antibodies on the proliferative response of OT-I cells (Figure 3D, 3E), no synergy was observed between 4C12 and OX86 on the proliferative response of OT-II cells upon secondary immunization (Figure 5E).
Antigen-specific serum immunoglobulin analysis
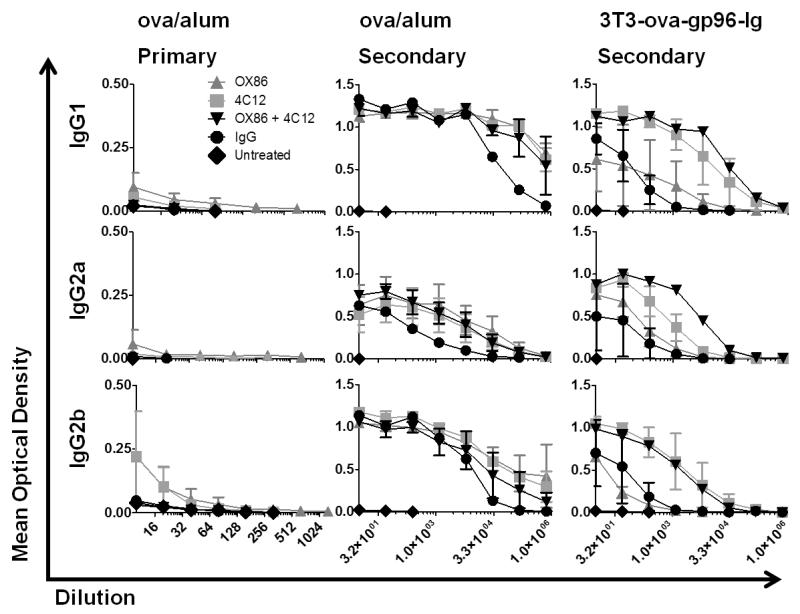
Serum was isolated from whole blood samples collected by cardiac puncture on day 5 following the primary (experimental day 5) or secondary (experimental day 57) immunization with the indicated treatment. The serum titer of ova-specific IgM, IgG1, IgG2a and IgG2b antibodies was determined using ELISA assays. Following primary immunization with 3T3-ova-gp96, ova-specific antibodies of all subtypes were below the limit of detection (data not shown). Low levels of IgG1, IgG2a and IgG2b were detectable in mice immunized with ova/alum, and levels of each of these immunoglobulin subtypes was increased by concurrent treatment with OX86 antibody (EC50 = 0.005, 0.035, 0.021, respectively; Figure 6, left column). TNFRSF25 stimulation with 4C12 antibody also increased the serum titer of ova-specific IgG2b following primary immunization with ova/alum (EC50 = 0.072). As predicted, the titers of ova-specific IgM antibody were significantly higher than any of the IgG sub-types following primary immunization with either ova/alum or 3T3-ova-gp96 (supplemental figure 3).
Figure 6. OVA-specific serum antibody analysis following primary and secondary immunization.
Serum samples were collected from mice on day 5 following primary immunization (experimental day 5) and secondary immunization (experimental day 57) as outlined in figures 3 and 5. The ova-specific antibody titers were determined for the indicated antibody isotypes using ELISA assays. Serially diluted titers of serum IgG1, IgG2a and IgG2b are shown for the indicated groups on day 5 following primary immunization with ova/alum (left column), secondary immunization with ova/alum (middle column) or secondary immunization with 3T3-ova-gp96-Ig (right column). Antibody titers following primary immunization with 3T3-ova-gp96-Ig were below the limit of detection and are not shown. Data indicate mean ± SEM for each dilution of serum collected from 2 mice per group per condition.
Secondary immunization with ova/alum or 3T3-ova-gp96 stimulated significantly higher serum titers of ova-specific IgM, IgG1, IgG2a and IgG2b than observed following primary immunization, as expected. In ova/alum immunized mice, the costimulatory effect of TNFRSF4 and TNFRSF25 stimulation was statistically equivalent for all IgG subtypes examined (IgG1 EC50=361674; IgG2a EC50=7308; IgG2b EC50=11167), and significantly increased the serum titer of IgG1 as compared to IgG antibody treated controls (EC50 = 361674 vs 35933, p=.0039; Figure 6, middle column). In 3T3-ova-gp96-Ig immunized mice, costimulation of TNFRSF25 led to a significant increase in the titer of IgG1 (EC50 = 7665 vs 100.5; p=.001) and a moderate increase in IgG2a (EC50=849.8) and IgG2b isotypes when compared to IgG antibody treated controls (EC50=1806; Figure 6, right column). Addition of OX86 did not further increase IgG1 or IgG2b concentrations following 3T3-ova-gp96-Ig immunization with 4C12 antibody, however it did lead to an additive increase in the concentration of IgG2a antibody (EC50 = 5856 vs 849.8, p=.003).
Differential Costimulation of Treg by TNFRSF4 and TNFRSF25
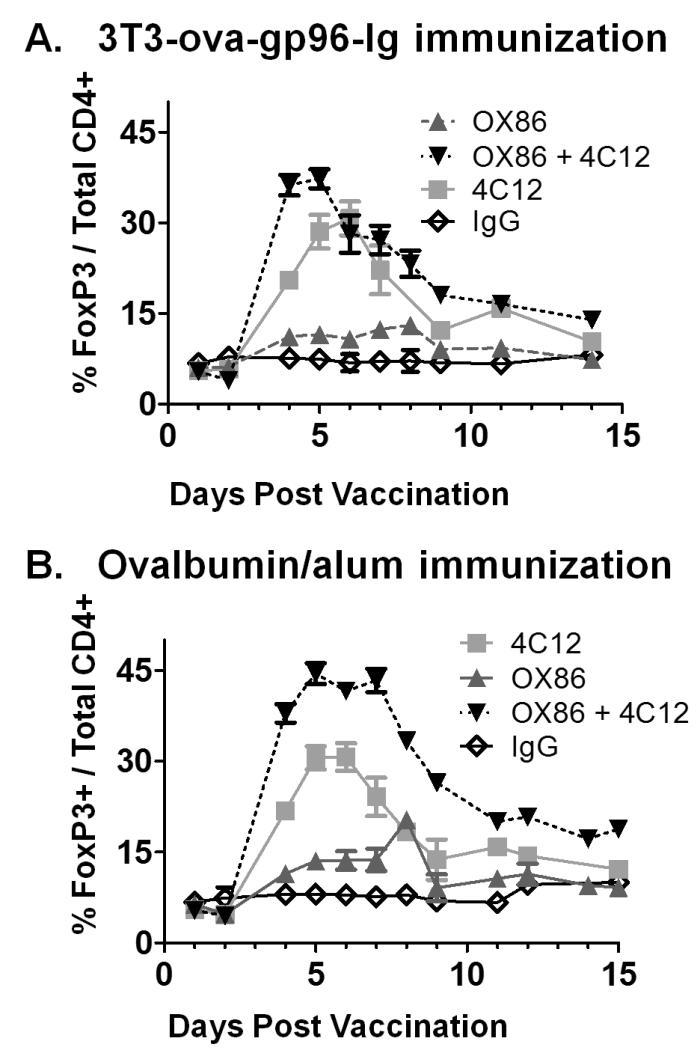
Because both TNFRSF4 and TNFRSF25 have been described to costimulate FoxP3+ Treg cells, the response of this CD4+ T cell subset was also analyzed. As previously reported, administration of 4C12 antibodies stimulated the proliferation of CD4+ Tregs to 3-4 fold above their resting frequency (Figure 7A,B)(7). The kinetics of expansion were slightly delayed in both vaccination groups as compared to naïve mice, peaking on day 6 in the 3T3-ova-gp96 immunized group at 30.72 ± 6.8% of all CD4+ cells (Figure 7A) and on day 5 in the ova/alum immunized group at 30.55 ± 5.0% of all CD4+ cells (Figure 7B). This proliferation occurred concurrently with the proliferation of OT-I cells in the context of 3T3-ova-gp96-Ig vaccination (Figure 2A and 7A) and together with proliferation of both OT-I and OT-II cells in ova/alum vaccinated mice (Figure 2B, 4B and 7B). Administration of OX86 antibodies led to minor proliferation of Treg cells (3T3-ova-gp96 immunization = 11.52 ± 1.1% of total CD4+ cells, ova/alum immunization = 14.6 ± 3.0% of total CD4+ cells), which occurred regardless of the vaccination method and is similar to the response to OX86 without immunization(7). When OX86 and 4C12 were combined, increased proliferation to roughly additive levels was observed for Treg cells: 37.35 ± 4.7% of all CD4+ cells and peaking on day 4 in the 3T3-ova-gp96 immunized group (Figure 7A) and to 44.47 ± 4.4% of all CD4+ cells and peaking on day 5 in the ova/alum immunized group (Figure 7B). In the setting of ova/alum vaccination, the combined influence of TNFRSF4 and TNFRSF25 stimulation led to Treg levels which peaked at nearly half of all CD4+ T cells (Figure 7B), at a time when roughly 20% of the CD4+ T cells were also OT-II cells (compare Figure 7B to Figure 4B). Proliferation of Treg cells to these levels concurrent with costimulation of antigen-specific OT-I and OT-II cells in the context of vaccination clearly indicates that absolute increases in Treg cells is not predictive of immune suppression.
Figure 7. Comparative costimulation of Treg cells by TNFRSF4 and TNFRSF25 agonistic antibodies.
In FIR mice adoptively transferred with OT-I and OT-II cells and immunized as described in figures 1-3, the expansion of CD4+FoxP3+ Treg cells (expressing the FoxP3-RFP transgene) were monitored by flow cytometry of peripheral blood cells. Data indicate mean ± SEM for ≥ 5 mice in each of ≥ 2 independent experiments.
The distinctions of ova/alum versus gp96-Ig chaperoned ovalbumin as OT-I and OT-II cell stimulators both alone and in conjunctions with TNFRSF4 and TNFRSF25 costimulation are summarized in table 1.
Table 1.
Summary of OT-I, OT-II and Treg responses to immunization with adjuvanted (alum) versus cross-presented (gp96-Ig) ovalbumin with and without costimulation by agonistic antibodies to TNFRSF4 (OX86) and TNFRSF25 (4C12).
| Vaccination Method | CD4+ Response |
CD8+ Response |
Treg Response |
|---|---|---|---|
| Ova/alum | + | + | − |
| Ova/alum + OX86 | ++ | ++ | + |
| Ova/alum + 4C12 | + | ++ | ++ |
| Ova/alum+OX86+4C12 | ++ | +++ | +++ |
| 3T3-ova-gp96-Ig | − | + | − |
| 3T3-ova-gp96-Ig + OX86 | − | ++ | + |
| 3T3-ova-gp96-Ig+ 4C12 | − | ++ | ++ |
| 3T3-ova-gp96-Ig + OX86 + 4C12 | − | +++ | +++ |
Discussion
Adjuvanted protein vaccines are a very common class of vaccines which typically lead to antigen presentation via the endosomal pathway to MHC II. We utilized ova/alum as a model for this class of vaccine and analyzed the proliferation of antigen-specific CD4+ and CD8+ T cells following vaccination. These studies demonstrated that ova/alum vaccination leads to the proliferation of both CD4+ and CD8+ T cells, and that the antigen threshold for CD4+ cell activation is lower than that for CD8+ T cells. This finding confirms that antigen presentation of ovalbumin derived peptides occurs on both MHC I and MHC II. In contrast, vaccination with allogeneic cells expressing ovalbumin and secreting gp96-Ig, which chaperones ovalbumin derived peptides, stimulated the exclusive proliferation of OT-I cells, indicating that antigen presentation was selective and exclusive to MHC I, likely via the antigen cross-presentation pathway. In addition, a ~600-fold larger dose of aluminum salt adjuvanted antigen was required to stimulate an equivalent OT-I response to gp96-Ig chaperoned antigen assuming that 100% of ovalbumin was chaperoned by gp96-Ig. If instead we assume that ovalbumin competes equally for gp96-Ig binding with the estimated 10,000 other proteins actively produced by a 3T3 cells and presented by MHC I, it is estimated that gp96-Ig chaperoned peptides in the sub-picogram range have equivalent CD8+ T cell stimulating activity to alum adjuvanted peptide in the microgram range. The two vaccine strategies are therefore distinct both in their efficiency of antigen presentation to MHC I (gp96-Ig being far superior) and to MHC II (ova/alum being far superior).
Next, we compared the relative activity of TNFRSF4 and TNFRSF25 as costimulators of vaccine-primed effector T cells based on their published similarities in activity. These studies demonstrate that TNFRSF4 and TNFRSF25 are similar and additive in their ability to costimulate CD8+ T cells regardless of the vaccine approach used following primary immunization. However, the two receptors differ significantly in their capacity to costimulate effector and regulatory subsets of CD4+ T cells. TNFRSF25 is, as reported, an excellent stimulator of Treg proliferation(7), but was not observed to alter the proliferation of OT-II cells primed by ova/alum following either primary or secondary immunization. In contrast, TNFRSF4 was a weak stimulator of Treg proliferation but, as reported, a powerful costimulator of OT-II proliferation(26). The activity of TNFRSF4 and TNFRSF25 was not complementary in the case of effector OT-II cells, however addition of TNFRSF4 agonists further increased the proliferation of CD8+ OT-I cells and CD4+ Treg cells stimulated by TNFRSF25 agonistic antibodies. The unique activities of TNFRSF4 and TNFRSF25 agonists in T cell function support the conclusion that the downstream signaling pathways from these receptors are also distinct.
One of the most striking observations from these studies is that the antigen-specific cellular responses differ so dramatically from the antigen-specific antibody responses at both the primary and secondary immunization. As is widely known and included in immunology textbooks, the antigen-specific antibody response is dominated by IgM-class antibodies following primary immunization and class-switches to predominantly IgG sub-types found at much higher titers in the serum following secondary immunization. Primary immunization with ova/alum programs antigen-specific antibody responses that do not appear to be further augmented by costimulation of TNFRSF4 or TNFRSF25. In contrast, despite the lack of OT-II proliferation at either the primary or secondary immunization with 3T3-ova-gp96-Ig, this strategy also led to high-titer antibody responses following secondary immunization that were preferentially class-switched to IgG1 and IgG2b, and to a lesser extent IgG2a, isotypes by addition of TNFRSF25 costimulation. In contrast to the increased antibody titer following secondary immunization, the proliferative OT-I and OT-II responses were diminished following secondary immunization unless combined with TNFRSF4 costimulation (increased OT-II proliferation) or TNFRSF25 costimulation (increased OT-I proliferation). These data indicate that the humoral and cellular antigen-specific immune responses are fundamentally uncoupled from one another at the time of secondary immunization. The side-by-side comparison of TNFRSF4 and TNFRSF25 agonists together with distinct vaccine approaches highlights the tremendous potential of combination vaccine strategies to selectively guide the activation and amplitude of specific T cell subsets and the dramatic influence these costimulators exert on memory T cell proliferation subsequent to secondary antigen challenge. The parallel observation that at a given point in time, the peripheral blood of an individual animal can simultaneously consist of 50% OT-I in the CD8+ subset as well as 50% Treg and 20% OT-II in the CD4+ T cell subset challenges the belief that Treg are immunosuppressive in an absolute sense, and implies that proliferative and effector responses for each of these cell types can be differentially modulated by both antigen and costimulatory receptors at the time of priming and boosting.
Supplementary Material
Acknowledgments
Financial support provided by NCI-5PO1CA109094-, NIAID-5RO1AI061807and NIAID 5R33 AI073234 to Eckhard R. Podack and a Bankhead-Coley Postdoctoral Fellowship to Taylor H. Schreiber.
References
- 1.Compaan DM, Hymowitz SG. The crystal structure of the costimulatory OX40-OX40L complex. Structure. 2006;14:1321–1330. doi: 10.1016/j.str.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 3.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber TH, Wolf D, Podack ER. The role of TNFRSF25:TNFSF15 in disease… and health? Adv Exp Med Biol. 691:289–298. doi: 10.1007/978-1-4419-6612-4_30. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg AD, Morris NP, Kovacsovics-Bankowski M, Urba WJ, Curti BD. Science gone translational: the OX40 agonist story. Immunol Rev. 244:218–231. doi: 10.1111/j.1600-065X.2011.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber TH, Wolf D, Tsai MS, Chirinos J, Deyev VV, Gonzalez L, Malek TR, Levy RB, Podack ER. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 120:3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 9.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oizumi S, Strbo N, Pahwa S, Deyev V, Podack ER. Molecular and cellular requirements for enhanced antigen cross-presentation to CD8 cytotoxic T lymphocytes. J Immunol. 2007;179:2310–2317. doi: 10.4049/jimmunol.179.4.2310. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki K, Nguyen T, Podack ER. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol. 1999;163:5178–5182. [PubMed] [Google Scholar]
- 12.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205:1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 14.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, Marrack P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 18.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 19.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber TH, Deyev VV, Rosenblatt JD, Podack ER. Tumor-induced suppression of CTL expansion and subjugation by gp96-Ig vaccination. Cancer Res. 2009;69:2026–2033. doi: 10.1158/0008-5472.CAN-08-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strbo N, Oizumi S, Sotosek-Tokmadzic V, Podack ER. Perforin is required for innate and adaptive immunity induced by heat shock protein gp96. Immunity. 2003;18:381–390. doi: 10.1016/s1074-7613(03)00056-6. [DOI] [PubMed] [Google Scholar]
- 23.Slebioda TJ, Rowley TF, Ferdinand JR, Willoughby JE, Buchan SL, Taraban VY, Al-Shamkhani A. Triggering of TNFRSF25 promotes CD8 T-cell responses and anti-tumor immunity. Eur J Immunol. 41:2606–2611. doi: 10.1002/eji.201141477. [DOI] [PubMed] [Google Scholar]
- 24.Jensen SM, Maston LD, Gough MJ, Ruby CE, Redmond WL, Crittenden M, Li Y, Puri S, Poehlein CH, Morris N, et al. Signaling through OX40 enhances antitumor immunity. Semin Oncol. 37:524–532. doi: 10.1053/j.seminoncol.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 26.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.