Abstract
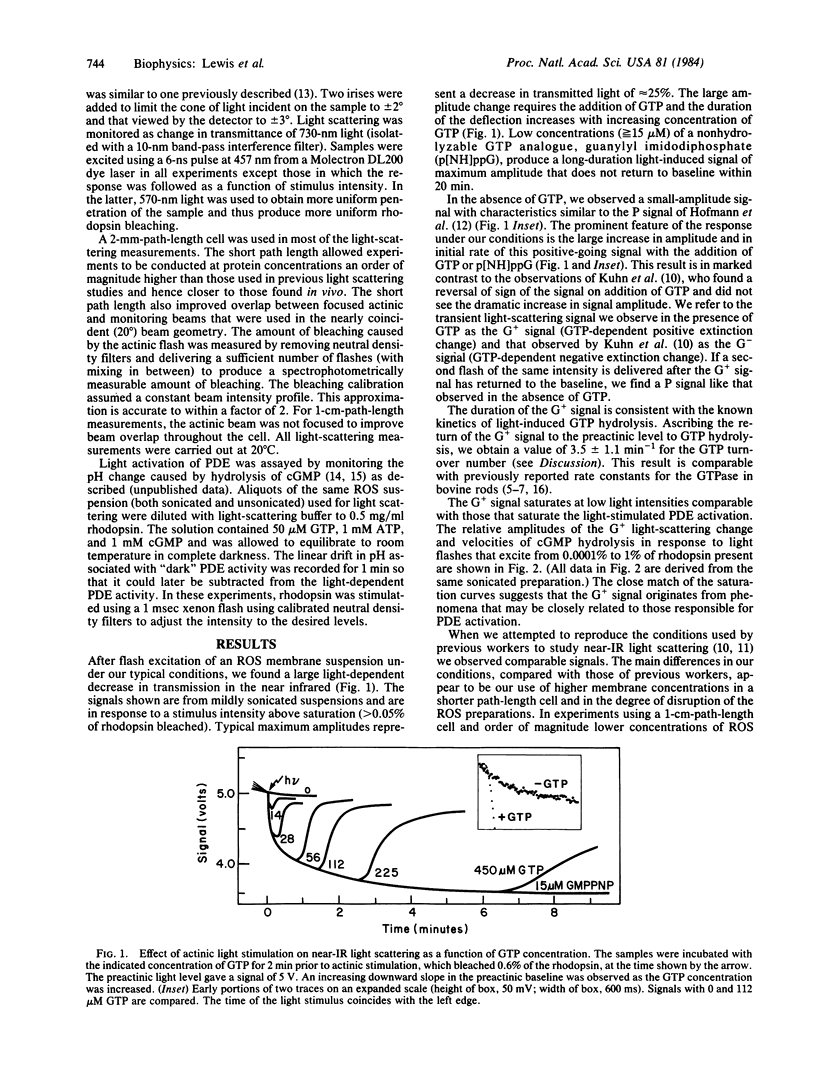
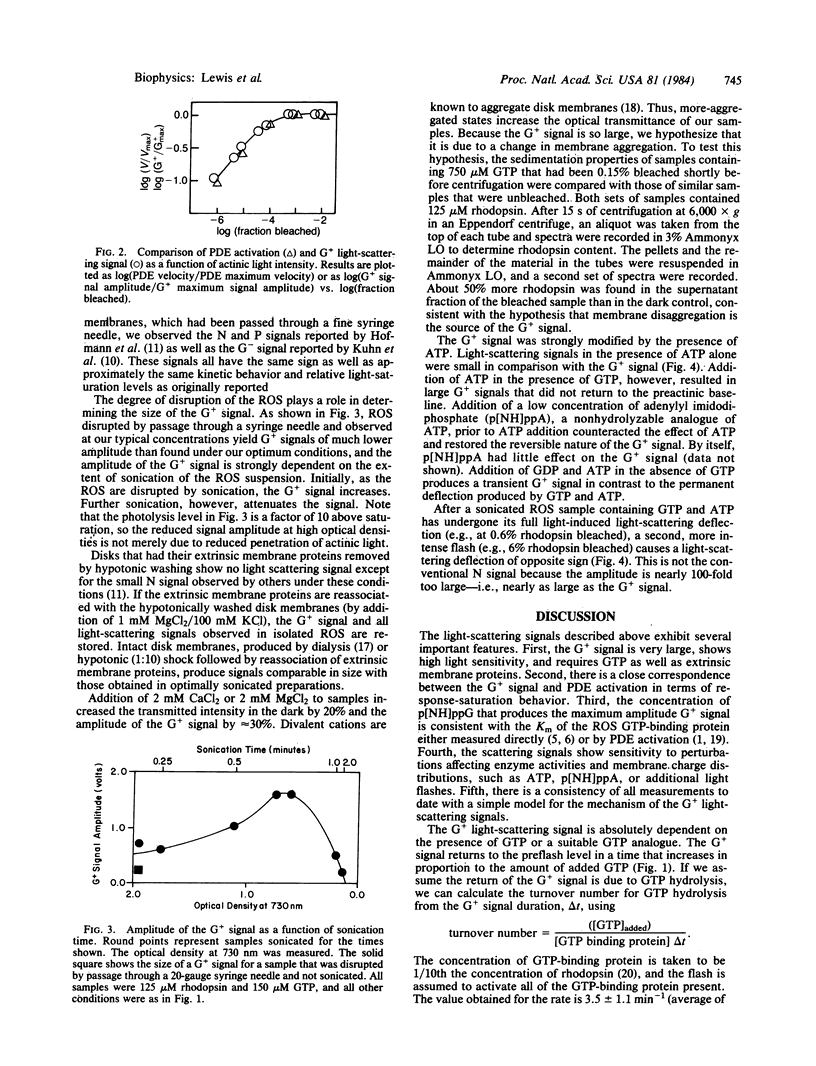
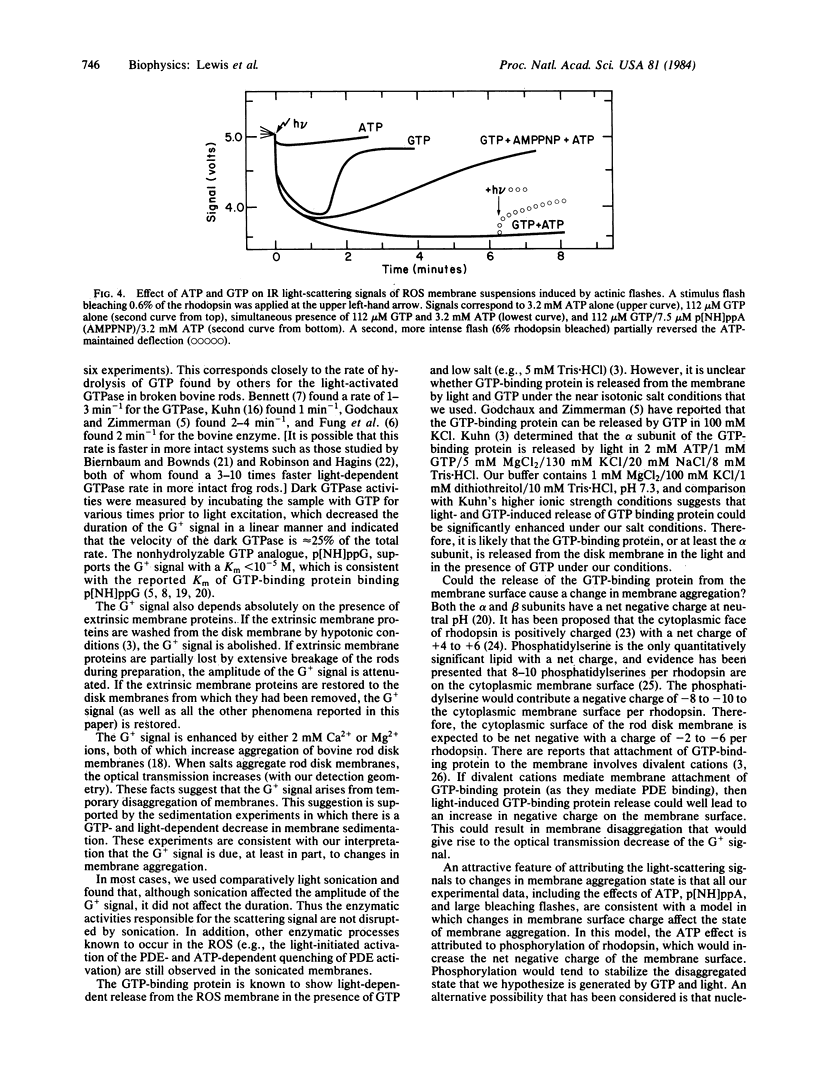
Light excitation of as little as 0.05% of the rhodopsin in a retinal rod membrane suspension reduces the near-IR optical transmission by 25%. This transmission decrease requires the presence of guanosine triphosphate, is opposite in sign and 25 times larger in amplitude than a GTP-dependent light-scattering signal previously reported in rod outer segment suspensions [Kuhn, H., Bennett, N., Michel-Vallez, M. & Chabre, M. (1981) Proc. Natl. Acad. Sci. USA, 78, 6873-6877], and is kinetically complex. The initial phase of the optical transmission decrease begins after about a 50-ms lag (at 0.05% bleach) and has a first-order time constant of 300-500 ms. The scattering signal returns to the preactinic baseline in a time dependent on the amount of GTP added. A nonhydrolyzable GTP analogue, guanylyl imidodiphosphate, produces a scattering signal that does not return to the preactinic baseline. Adenosine triphosphate strongly inhibits the return of the GTP-dependent transmission decrease to the preactinic baseline. This effect of ATP on the GTP signal apparently requires ATP hydrolysis because it is inhibited by the simultaneous presence of adenylyl imidodiphosphate, a nonhydrolyzable analogue of ATP. The light-scattering signal and the velocity of the activation of a rod outer segment phosphodiesterase saturate when >0.05% of the rhodopsin is bleached and both show nearly identical dependence on light stimulus. It is suggested that these nucleotide-dependent light-scattering signals arise from changes in the state of membrane aggregation that are controlled by enzymatic processes. This hypothesis is supported by the large amplitude of the signals, sedimentation experiments, and a strong membrane concentration dependence. The ATP effects can be rationalized within the above hypothesis as being due to ATP-dependent rhodopsin phosphorylation that adds negative charges to the membrane surface and tends to keep the membranes disaggregated. An additional signal, which increases light transmission, is produced by a second, much more intense flash. The latter signal is interpreted as the result of proton binding by bleached rhodopsin molecules that decreases the negative charge repulsion between the membranes and allows increased aggregation.
Keywords: vision, guanosine triphosphate-binding protein, phosphodiesterase, protein phosphorylation, rhodopsin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Rao J. K., Hargrave P. A. Structural prediction of membrane-bound proteins. Eur J Biochem. 1982 Nov 15;128(2-3):565–575. doi: 10.1111/j.1432-1033.1982.tb07002.x. [DOI] [PubMed] [Google Scholar]
- Baehr W., Morita E. A., Swanson R. J., Applebury M. L. Characterization of bovine rod outer segment G-protein. J Biol Chem. 1982 Jun 10;257(11):6452–6460. [PubMed] [Google Scholar]
- Bennett N. Light-induced interactions between rhodopsin and the GTP-binding protein. Relation with phosphodiesterase activation. Eur J Biochem. 1982 Mar;123(1):133–139. doi: 10.1111/j.1432-1033.1982.tb06509.x. [DOI] [PubMed] [Google Scholar]
- Biernbaum M. S., Bownds M. D. Influence of light and calcium on guanosine 5'-triphosphate in isolated frog rod outer segments. J Gen Physiol. 1979 Dec;74(6):649–669. doi: 10.1085/jgp.74.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Light-induced interfacial potentials in photoreceptor membranes. Biophys J. 1980 May;30(2):243–263. doi: 10.1016/S0006-3495(80)85092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M., Cavaggioni A. Light induced changes in ionic flux in the retinal rod. Nat New Biol. 1973 Jul 25;244(134):118–120. doi: 10.1038/newbio244118a0. [DOI] [PubMed] [Google Scholar]
- Emrich H. M., Reich R. Uber Primärreaktionen beim Schvorgang. Thermodynamischer und kinetischer Einfluss des pH-Wertes auf die Metarhodopsin-I-II-Umwandlung. Protonenverbrauch als Auswirkung einer Konformationsänderung. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):577–591. [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Zimmerman W. F. Membrane-dependent guanine nucleotide binding and GTPase activities of soluble protein from bovine rod cell outer segments. J Biol Chem. 1979 Aug 25;254(16):7874–7884. [PubMed] [Google Scholar]
- Harary H. H., Brown J. E., Pinto L. H. Rapid light-induced changes in near infrared transmission of rods in Bufo marinus. Science. 1978 Dec 8;202(4372):1083–1085. doi: 10.1126/science.102035. [DOI] [PubMed] [Google Scholar]
- Hermolin J., Karell M. A., Hamm H. E., Bownds M. D. Calcium and cyclic GMP regulation of light-sensitive protein phosphorylation in frog photoreceptor membranes. J Gen Physiol. 1982 Apr;79(4):633–655. doi: 10.1085/jgp.79.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K. P., Uhl R., Hoffmann W., Kreutz W. Measurements on fast light-induced light-scattering and -absorption changes in outer segments of vertebrate light sensitive rod cells. Biophys Struct Mech. 1976 Apr 15;2(1):61–77. doi: 10.1007/BF00535653. [DOI] [PubMed] [Google Scholar]
- Kühn H., Bennett N., Michel-Villaz M., Chabre M. Interactions between photoexcited rhodopsin and GTP-binding protein: kinetic and stoichiometric analyses from light-scattering changes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6873–6877. doi: 10.1073/pnas.78.11.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. W., Winterle J. S., Powers M. A., Kliger D. S., Dratz E. A. Kinetics of rhodopsin photolysis intermediates in retinal rod disk membranes--I. Temperature dependence of lumirhodopsin and metarhodopsin I kinetics. Photochem Photobiol. 1981 Sep;34(3):375–384. [PubMed] [Google Scholar]
- Liebman P. A., Evanczuk A. T. Real time assay of rod disk membrane cGMP phosphodiesterase and its controller enzymes. Methods Enzymol. 1982;81:532–542. doi: 10.1016/s0076-6879(82)81074-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr The control of phosphodiesterase in rod disk membranes: kinetics, possible mechanisms and significance for vision. Vision Res. 1979;19(4):375–380. doi: 10.1016/0042-6989(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Miljanich G. P., Nemes P. P., White D. L., Dratz E. A. The asymmetric transmembrane distribution of phosphatidylethanolamine, phosphatidylserine, and fatty acids of the bovine retinal rod outer segment disk membrane. J Membr Biol. 1981;60(3):249–255. doi: 10.1007/BF01992562. [DOI] [PubMed] [Google Scholar]
- Raubach R. A., Nemes P. P., Dratz E. A. Chemical labeling and freeze-fracture studies on the localization of rhodopsin in the rod outer segment disk membrane. Exp Eye Res. 1974 Jan;18(1):1–12. doi: 10.1016/0014-4835(74)90038-4. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Hagins W. A. GTP hydrolysis in intact rod outer segments and the transmitter cycle in visual excitation. Nature. 1979 Aug 2;280(5721):398–400. doi: 10.1038/280398a0. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Liebman P. A. Mechanism of ATP quench of phosphodiesterase activation in rod disc membranes. J Biol Chem. 1983 Jan 25;258(2):1205–1209. [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]



