Abstract
Context
The unprecedented advances in cancer genetics and genomics are rapidly affecting clinical management and diagnostics in solid tumor oncology. Molecular diagnostics is now an integral part of routine clinical management in patients with lung, colon, and breast cancer. In sharp contrast, molecular biomarkers have been largely excluded from current management algorithms of urologic malignancies.
Objective
To discuss promising candidate biomarkers that may soon make their transition to the realm of clinical management of genitourologic malignancies. The need for new treatment alternatives that can improve upon the modest outcome so far in patients with several types of urologic cancer is evident. Well-validated prognostic molecular biomarkers that can help clinicians identify patients in need of early aggressive management are lacking. Identifying robust predictive biomarkers that will stratify response to emerging targeted therapeutics is another crucially needed development. A compiled review of salient studies addressing the topic could be helpful in focusing future efforts.
Data Sources
A PubMed (US National Library of Medicine) search for published studies with the following search terms was conducted: molecular, prognostic, targeted therapy, genomics, theranostics and urinary bladder cancer, prostate adenocarcinoma, and renal cell carcinoma. Articles with large cohorts and multivariate analyses were given preference.
Conclusions
Our recent understanding of the complex molecular alterations involved in the development and progression of urologic malignancies is yielding novel diagnostic and prognostic molecular tools and opening the doors for experimental targeted therapies for these prevalent, frequently lethal solid tumors.
The completion of the cancer genome project has paved the way to great advances in cancer genetics and genomics that are beginning to affect clinical management and diagnostics in solid tumor oncology. Molecular diagnostics is now an integral part of routine clinical management in patients with lung, colon, and breast cancer. In sharp contrast, molecular biomarkers have been largely excluded from current management algorithms of urologic malignancies. The need for new treatment alternatives and for prospectively validated prognostic molecular biomarkers cannot be overestimated. Furthermore, identifying robust predictive biomarkers that will stratify response to emerging targeted therapeutics is needed. The following discussion focuses on candidate biomarkers that will soon make their transition to daily clinical management of patients with urologic tumors.
UROTHELIAL CARCINOMA OF URINARY BLADDER
Pathogenetic Pathways in Urothelial Carcinoma: Parallels of 2 Distinct Biologic Phenotypes
Accumulating molecular genetic evidence supports 2 distinct broad pathogenetic pathways for bladder cancer (BC) development that seem to parallel the contrasting biologic and clinical phenotypes of superficial (non–muscle invasive) and muscle invasive urothelial carcinoma. While most invasive urothelial carcinomas are thought to originate through progression from dysplasia to flat carcinoma in situ (CIS) and high-grade noninvasive lesions, superficial urothelial lesions are thought to originate from benign urothelium through a process of urothelial hyperplasia. Progression from superficial to muscle invasive disease accounts for only a small percentage (10%–15%) of the entire pool of noninvasive lesions. Genetic instability is the key to the accumulation of genetic alterations required for progression to muscle invasive bladder cancer (MI-BC).1–4
Clinically, a significant proportion of superficial tumors (pTa and pT1) are deemed to recur after transurethral resection of the bladder (TURB) with only a minority of cases enduring progression to high-grade carcinoma that will ultimately progress to MI-BC.
Three primary genetic alterations have consistently been associated with the pathogenesis pathway of superficial non–muscle invasive bladder cancer (NMI-BC). These include tyrosine kinase receptor FGFR-3, HRAS,5 and PIK3CA.3,6,7 Alterations in the RAS-MAPK and PI3K-Akt pathways are in large part responsible for promoting cell growth in urothelial neoplasia. Activating mutations in RAS lead to activation of mitogen-activated protein kinase (MAPK) and PI3K pathways. Not surprisingly, activating mutations in upstream tyrosine kinase receptor (FGFR3) seem to be mutually exclusive with RAS mutations, given that both signal through a common downstream pathway in urothelial oncogenesis. PIK3CA and FGFR3 mutations generally co-occur, suggesting a potential synergistic additive oncogenic effect for PIK3CA mutations.
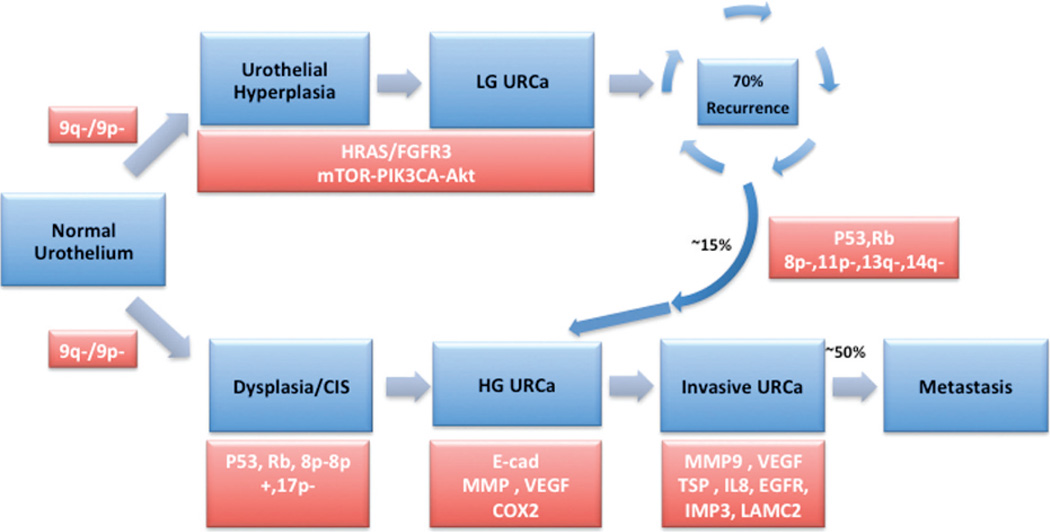
The pathogenic pathway for MI-BC primarily involves alterations in tumor suppressor genes involved in cell cycle control, including p53, p16, and Rb.1,4,8 As illustrated in Figure 1, progression of the subset of superficial BC into higher-grade muscle invasive disease is similarly based on alterations in p53 and Rb tumor suppressor genes (see Figures 1 and 2).
Figure 1.
Divergent molecular pathways of oncogenesis in superficial and muscle invasive urothelial carcinoma of urinary bladder. Genetic alterations are depicted in key stages of disease progression. Abbreviations: CIS, carcinoma in situ; EGFR, epidermal growth factor receptor; HG URCa, high-grade urothelial carcinoma; LG URCa, low-grade urothelial carcinoma; mTOR, mammalian target of rapamycin; URCa, urothelial carcinoma; VEGF, vascular endothelial growth factor.
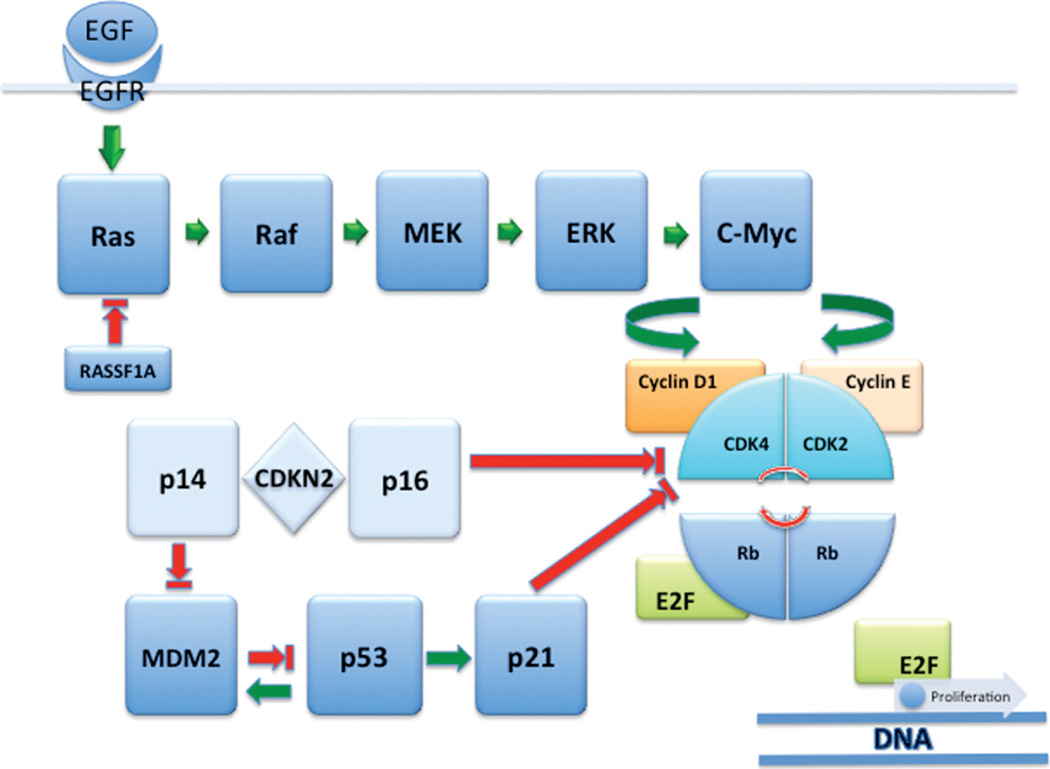
Figure 2.
Receptor tyrosine kinase (EGFR/Ras/Mek/ERK) and cell cycle regulator (p14, p16, p53, p21, cyclin D1, cyclin E, and Rb) pathways in urothelial carcinoma. Abbreviations: EGF, epidermal growth factor; EGFR, epidermal growth factor receptor.
Prognostic Biomarkers in Superficial Non–Muscle Invasive and Muscle Invasive Urothelial Carcinoma
Established clinicopathologic prognostic parameters for NMI-BC include pTNM stage, World Health Organization (WHO)/International Society of Urological Pathology grade, tumor size, tumor multifocality, presence of CIS, and frequency and rate of prior recurrences.9 Prognostic parameters that can accurately predict progression in patients with superficial tumors are actively sought to further facilitate identification of those in need of vigilant surveillance and an aggressive treatment plan. The latter is especially pertinent in a disease for which the financial burden and quality of life for patients under surveillance is significant. Per patient, bladder cancer is the most expensive single solid tumor in the United States, with a staggering $3 billion dollars estimated annual cost to our health care system.10 Furthermore, given the current poor outcome of muscle invasive disease (60% or less overall survival rate), markers that can improve prognostication in this group of patients are equally needed.11–13
As our understanding of molecular pathways involved in urothelial oncogenesis increasingly comes into focus, the translational field of molecular prognostication, theranostics, and targeted therapy in BC has sharply gained momentum.14–32 Evidently, a rigorous validation process ought to precede the incorporation of such molecular biomarkers in clinical management. Initial retrospective discovery studies need to be confirmed and validated in large independent cohorts. The subsequent crucial step is validating the robustness of the proposed biomarker in a well-controlled, multi-institutional, randomized prospective study. Such a prospective study should support an additive role for the inclusion of the new biomarkers over existing management algorithm(s).33,34 It is the lack of the latter crucial steps in biomarker development that has hindered the streamlining of clinical utilization of several promising markers in patient management of BC.35,36
Chromosomal Numerical Alteration
Chromosome 9 alterations are the earliest genetic alterations in both of the above-described divergent pathways of BC development. They are responsible for providing the necessary milieu of genetic instability that in turn allows for the accumulation of subsequent genetic defects. Several additional structural/numerical somatic chromosomal alterations are also a common occurrence in BC. Among these, gains of chromosome arms 3q, 7p, and 17q and deletions in 9p21 (p16 locus) are of special interest given their potential diagnostic and prognostic value.37,38 A multitarget interphase fluorescence in situ hybridization (FISH)–based urine cytogenetic assay was developed39 on the basis of the above numerical chromosomal alterations and is now commercially available and commonly used in clinical management. Initially approved by the US Food and Drug Administration (FDA) for surveillance of recurrence in previously diagnosed patients with BC, the test subsequently gained approval for screening in high-risk (smoking exposure) patients with hematuria. The multicolor FISH assay appears to enhance the sensitivity of routine urine cytology analysis and can be used in combination with routine cytology as a reflex test in cases with atypical cytology. A sensitivity range of 69% to 87% and a specificity range of 89% to 96% have been reported with the multitarget interphase FISH assay.40 With the exception of 1 study,41 the multitarget FISH urine assay has been shown to be more sensitive than routine cytology. An additional advantage of urine-based FISH testing could be the anticipatory positive category of patients identified by such assay. This refers to patients for whom the FISH assay detects molecular alteration of BC in urine cells several months before cancer detection by cystoscopy or routine cytology. In the study by Yoder et al,42 two-thirds of the 27% of patients categorized as “anticipatory positive” developed BC that was detected by cystoscopy up to 29 months later. Such encouraging results point to the great potential of molecular testing in early detection and allocation of vigorous frequent follow-up cystoscopy in at-risk patients.43–46
Finally, several recent studies37,38,47–49 have pointed to the potential prognostic role for multitarget FISH analysis. Maffezzini et al48 were able to demonstrate that low-risk patients with FISH-positive results, defined as 9p21 loss/chromosome 3 abnormalities, had a higher rate of recurrence as compared to FISH-negative patients. The recurrence rate was even greater for patients with a high-risk positive FISH result (chromosome 7/chromosome 17 abnormality). Both Kawauchi et al,37 using bladder washings, and Kruger et al,38 using formalin-fixed, paraffin-embedded transurethral biopsy samples, independently found that loss of 9p21 predicts recurrence but not progression in superficial BC. Furthermore, both Savic et al47 and Whitson et al49 found that urine cytology and FISH in post-BCG (Bacillus Calmette-Guerin) bladder washings were predictive of failure of BCG therapy in patients with non–muscle invasive disease. Such a promising prognostic role for multitarget FISH awaits prospective randomized trial before clinical integration into a practice algorithm. Clear guidelines for interpretation and test performance parameters in terms of interobserver reproducibility are also needed.50
Receptor Tyrosine Kinases
Recent studies have pointed to the potential prognostic value of evaluating the expression of receptor tyrosine kinases such as FGFR3, epidermal growth factor receptor (EGFR), and other ERB family members (HER2/neu and ERBB3)4,21,51–61 in superficial and muscle invasive bladder cancer disease.
FGFR3 mutations are a common occurrence in NMI-BC and can theoretically be used alone or combined with RAS and PIK3CA oncogenes as markers of early recurrence during surveillance. Both Zuiverloon et al62 and Miyake et al63 independently developed sensitive polymerase chain reaction (PCR) assays for detecting FGFR3 mutations in voided urine. A positive urine sample finding by the assay developed by Zuiverloon’s group was associated with concomitant or future recurrence in 81% of NMI-BC cases. An even higher positive predictive value of 90% was achieved in patients with consecutive FGFR3-positive urine samples. Similarly, Miyake et al63 were able to detect FGFR3 mutations in 53% of their 45 patients and found their assay to be superior to cytology (78% versus 0%) in detecting post-TURB recurrence in NMI-BC harboring FGFR3 mutations in primary tumors.
Kompier et al7 were recently able to develop a multiplex PCR assay for mutational analysis detecting the most frequent mutation hot spots of HRAS, KRAS, NRAS, FGFR3, and PIK3CA in formalin-fixed, paraffin-embedded TURB samples. They demonstrated evidence of at least 1 mutation in up to 88% of low-grade NMI-BC samples. Hernandez et al64 revealed that FGFR3 mutations were more common among low malignant potential neoplasms (77%) and TaG1/TaG2 tumors (61%/58%) than among TaG3 tumors (34%) and T1G3 tumors (17%). On multivariable analysis, mutations were associated with increased risk of recurrence in NMI-BC.
Van Rhijn et al65 previously proposed a molecular-grade parameter (mG), based on a combination of FGFR3 gene mutation status and MIB-1 index, as an alternative to pathologic grade in NMI-BC. Recently, the same group51 elegantly validated their previously proposed mG parameter and compared it to the European Organization for Research and Treatment of Cancer (EORTC) NMI-BC risk calculator66 (weighted score of 6 variables including WHO 1973 grade, stage, presence of CIS, multiplicity, size, and prior recurrence rate). The mG was more reproducible than the pathologic grade (89% versus 41%–74%). FGFR3 mutations significantly correlated with favorable disease parameters, whereas increased MIB-1 was frequently seen with pT1, high grade, and high EORTC risk scores. EORTC risk score remained significant in multivariable analyses for recurrence and progression. Importantly, mG also maintained independent significance for progression and disease-specific survival (DSS), and the addition of mG to the multivariable model for progression increased the predictive accuracy from 74.9% to 81.7%.
Several studies have suggested a negative prognostic role forHER2/neu amplification/overexpression in MI-BC.33,67–69 Most recently, Bolenz et al59 found that HER2-positive patients with MI-BC were at twice increased risk for recurrence and cancer-specific mortality on multivariable analyses adjusted for pathologic stage, grade, lymphovascular invasion, lymph node metastasis, and adjuvant chemotherapy.
p53, Cell Cycle Regulators, and Proliferation Index Markers
Early studies by Sarkis et al70–72 revealed p53 alterations to be a strong independent predictor of disease progression in BC (superficial, muscle invasive, as well as CIS). p53 has also been shown to be predictive of increased sensitivity to chemotherapeutic agents that lead to DNA damage.73–75 Recent studies have further supported the prognostic role of p5376 in pT1–pT2 tumors of patients after cystectomy, showing an independent role for p53 alteration in predicting disease-free survival and DSS.
Among other G1-S phase cell cycle regulators, cyclins D3 and D1, p16, p21, and p27 have also been evaluated as prognosticators in NMI-BC.32,75,77–81 Lopez-Beltran et al77 confirmed their initial finding75 of the independent prognostic role of cyclin D3 and cyclin D1 overexpression in predicting progression in pTa and pT1 tumors. Their findings, however, are in contrast to subsequent findings by Shariat et al,32 emphasizing the need for further validation in multi-institutional large cohorts of patients.
A synergistic prognostic role for combining p53 evaluation with other cell cycle control elements such as pRb, cyclin E1, p21, and p27 is emerging in both NMI-BC and MI-BC.24,26,73,82,83 In a study by Shariat et al,26 patients who have NMI-BC, with TURB demonstrating synchronous immunohistochemical alterations in all 4 tested markers (p53, p21, pRb, and p27), were at significantly lower likelihood of sustaining disease-free survival than patients with only 3 markers. The negative predictive effect was decreased with decreasing number of altered markers (3 versus 2 versus 1). Similarly, the same group83 later found that combining p53, p27, and Ki-67 assessment in pT1 radical cystectomy specimens improved the prediction of disease-free survival and DSS.
A similar synergistic prognostic role for the assessment of immunoexpression of multiple molecular markers (p53, pRb, and p21) was demonstrated by Chatterjee et al24 in patients undergoing cystectomy for MI-BC. The superiority of multimarker approach compared to prior single-marker approach certainly merits further assessment.70–72,84 Such multimarker approach of prognostication could soon be integrated in the standard of care in BC management once additional multi-institutional prospective trials confirm the above-mentioned promising findings.
Tumor proliferation index measured immunohistochemically by either Ki-67 or MIB-1 has been consistently shown to be a prognosticator in bladder cancer.51,65,75,80,85–88 As mentioned above, tumor proliferation index (MIB-1) in NMI-BC plays a prognostic role as one of the elements of the mG parameter forwarded by Van Rihjn et al.65 The independent prognostic role of proliferation index measured by Ki-67 has also been shown. In the study by Quintero et al,85 Ki-67 index in NMI-BC TURB biopsy samples was predictive of progression free survival and DSS.
A similar role for proliferation index assessment as prognosticator is established in MI-BC. Building on initial findings of significance in an organ-confined subset of MI-BC by Margulis et al,86 a recent report of the bladder consortium multi-institutional trial (7 institutions; 713 patients) again confirmed the role of proliferation index, measured in cystectomy specimens.87 In the later study, Ki-67 improved prediction of both progression free survival and DSS when added to standard prediction models, supporting a role for proliferation index assessment in stratifying patients for perioperative systemic chemotherapy. This has certainly taken Ki-67 assessment a step closer to clinical applicability in MI-BC.
Gene Expression and Genomic Analysis
Several recent gene expression studies have highlighted sets of differentially expressed genes that may play a role in diagnosis and in predicting recurrence and progression in BC.1,15–17,29,31,89–97 In a landmark study by Sanchez-Carbayo et al,15 oligonucleotide arrays were used to analyze transcript profiles of 105 cases of NMI-BC and MI-BC. Hierarchical clustering and supervised algorithms were used to stratify bladder tumors by stage, nodal metastases, and overall survival. Predictive algorithms were 89% accurate for tumor staging using genes differentially expressed in superficial versus muscle invasive tumors. Accuracies of 82% (entire cohort) and 90% (MI-BC) were also obtained for predicting overall survival. A genetic profile consisting of 174 probes was able to identify patients with positive lymph nodes and poor survival.
Recently, Birkhahn et al29 attempted to identify genes predictive for recurrence and progression in Ta category by using a quantitative pathway-specific approach in a set of 24 key genes by real-time PCR in tumor biopsy specimens at initial presentation. They found CCND3 expression to be highly sensitive and specific for recurrence (97% and 63%, respectively). While HRAS, E2F1, BIRC5/survivin, and VEGFR2 were predictive for progression by univariate analysis, on multivariable analysis the combination of HRAS, VEGFR2, and VEGF expression status predicted progression with an impressive 81% sensitivity and 94% specificity.
In a recent study, Lindgren et al95 suggested that a combined molecular and histopathologic classification of BC may prove more powerful in predicting outcome and stratifying treatment. The authors combined gene expression analysis, whole-genome array comparative genomic hybridization analysis, and mutational analysis of FGFR3, PIK3CA, KRAS, HRAS, NRAS, TP53, CDKN2A, and TSC1 to identify 2 intrinsic molecular signatures (MS1 and MS2). Genomic instability was the most distinguishing genomic feature of MS2 signature, independent of TP53/MDM2 alterations. Their genetic signatures were validated in 2 independent data sets that successfully classified urothelial carcinomas into low-grade and high-grade tumors, as well as NMI-BC and MI-BC, with high precision and sensitivity. Furthermore, a gene expression signature that independently predicts metastasis and disease free survival was also defined. This clearly supports the role of molecular grading as a complement to standard pathologic grading.
Mengual et al31 performed gene expression analysis in 341 urine samples from patients with NMI-BC and MI-BC and 235 controls by TaqMan Arrays (Applied Biosystems, Carlsbad, CA). A 12+2 gene expression signature demonstrated a staggering 98% sensitivity and 99% specificity in discriminating between BC and control and 79% sensitivity and 92% specificity in predicting tumor aggressiveness (NMI-BC versus MI-BC). The signature was then validated in voided urine samples and maintained accuracy. In an integrated genetic/epigenetic approach, Serizawa et al94 prospectively performed mutational screening of a set of 6 genes (FGFR3, PIK3CA, TP53, HRAS, NRAS, and KRAS) and quantitatively assessed promoter methylation status of 11 additional genes (APC, ARF, DBC1, INK4A, RARB, RASSF1A, SFRP1, SFRP2, SFRP4, SFRP5, and WIF1) in NMI-BC tumor biopsy specimens and corresponding urine samples from 118 patients and 33 controls. A total of 95 oncogenic mutations and 189 hypermethylation events were detected. The total panel of markers provided a sensitivity of 93% and 70% in biopsy specimens and urine samples, respectively. FGFR3 mutations in combination with 3 methylation markers (APC, RASSF1A, and SFRP2) provided a sensitivity of 90% in tumors and 62% in urine, with 100% specificity.
With the impending cost and turnaround time advantages of next-generation sequencing technology, the power of genomic approach in providing a noninvasive diagnostic and predictive tool should be actively pursued in a prospective large cohort.
Epigenetic Alterations
Epigenetic analysis is also gaining momentum in BC as a noninvasive diagnostic tool for screening and surveillance. As a prognostic tool, epigenetic analysis has similarly shown promising potential for patients with BC.94,98–110
In an early study by Catto et al,105 hypermethylation analysis at 11 CpG promoter islands was performed by methylation-specific PCR (MSP) in 116 bladder and 164 upper urinary tract tumors. Promoter methylation was found in 86% of all tumors and the incidence was relatively higher in upper tract tumors than BC. Methylation was associated with advanced tumor stage and higher tumor progression and mortality rates. Most importantly, on multivariate analysis, methylation at the RASSF1A and DAPK gene promoters was associated with disease progression independent of tumor stage and grade.
The same group,110 using quantitative MSP at 17 candidate gene promoters, found 5 loci that were associated with progression (RASSF1A, E-cadherin, TNFSR25, EDNRB, and APC). Multivariate analysis revealed that the overall degree of methylation was more significantly associated with subsequent progression and death than tumor stage. An epigenetic predictive model developed with artificial intelligence techniques identified the likelihood and the timing of progression with 97% specificity and 75% sensitivity.
Among the studies evaluating the diagnostic role of promoter hypermethylation, the study by Lin et al99 used MSP assay for 4 genes (E-cadherin, p16, p14, and RASSF1A) in primary tumor DNA and urine sediment DNA obtained from 57 patients with bladder cancer; MSP detected hypermethylation in the urine of 80% of tested patients. Hypermethylation analysis of E-cadherin, p14, or RASSF1A in urine sediment DNA detected 85% of superficial and low-grade bladder cancers, 79% of high-grade bladder cancers, and 75% of invasive bladder cancers. The study highlighted the great potential of such tests in detecting NMI-BC. A similar diagnostic role was also found by Cabello et al101 using a novel technology, methylation-specific multiplex ligation-dependent probe amplification assay, to analyze 25 tumor suppressor genes that have been thought to play a role in BC oncogenesis. The tumor suppressor genes included PTEN, CD44, WT1, GSTP1, BRCA2, RB1, TP53, BRCA1, TP73, RARB, VHL, ESR1, PAX5A, CDKN2A, and PAX6. The authors found BRCA1, WT1, and RARB to be the most frequently methylated tumor suppressor genes, with receiver operating characteristic curve analyses revealing significant diagnostic accuracies in 2 additional validation sets.
Finally, assessment of promoter hypermethylation is giving additional insights on BC oncogenesis. Promoter hypermethylation of CpG islands and “shores” controlling microRNA expression is one such example.103
Ploidy and Morphometric Analysis
Several studies have pointed to the independent prognostic role of ploidy and S phase analysis in NMI-BC.88,111–117 Ploidy analysis can be performed by flow cytometry or automated image cytometry and is applicable to urine cytology specimens as well as biopsy supernatant112 and disaggregated TURB formalin-fixed, paraffin-embedded specimens.113
In one of the largest studies assessing DNA ploidy in NMI-BC (377 [test set]; 156 [validation set]), Ali-El-Dein et al111 found that stage, DNA ploidy, tumor multiplicity, history of recurrence, tumor configuration, and type of adjuvant therapy independently predict recurrence. Recurrence at 3 months, grade, and DNA ploidy were the only predictors of progression to muscle invasion. The constructed “Predictive Index” model successfully stratified patients in a second validation set into 3 risk groups. Likewise, Baak et al113 were able to show ploidy status and S phase, measured by image cytometry, to be strong independent predictors of recurrence and progression in patients with pTa and pT1 tumors.
Despite all the above-mentioned encouraging data, ploidy analysis still awaits prospective randomized trials to bring image cytometry or flow cytometry technique into current standard management algorithms for NMI-BC.
Emerging Biomarkers
Other biomarkers with encouraging but less robust data on their potential prognostic role in BC include tumor microenvironment markers, such as cell adhesion markers E cadherin and N cadherin,20,118 and angiogenesis modulators such as HIF-1a, HIF-2, vascular endothelial growth factor (VEGF), CAIX, and thrombospondin-1.18,19,119–123 In addition, our group and others have demonstrated a potential prognostic role for mammalian target of rapamycin (mTOR) pathway markers.20,118,124–126 Other markers such as Aurora-A have also been investigated in this setting.127,128 Finally, microRNA profile alterations will certainly be a new area of heavy investigation as a noninvasive diagnostic tool and as a prognostic tool in patients with BC.104,129–131
Targeted Therapy and Predictive Markers in Bladder Cancer
As illustrated in Figure 1, RTK-HRAS-MAPK, mTOR, as well as angiogenesis pathway of the tumor microenvironment, offer promising opportunities for new targeted treatments of bladder cancer.7,25,124,126,132–145 Among receptor tyrosine kinases, HER2/neu has been targeted in a multicenter phase II trial reported in 2007 by Hussain et al.146 Forty-four patients with advanced BC who had metastatic disease and evidence of tumor HER2 positivity by either immunohistochemistry (IHC), FISH, or elevated serum extracellular HER2/neu domain levels were treated with a combination of carboplatin, paclitaxel, and gemcitabine with the humanized monoclonal anti-HER2 antibody trastuzumab. Approximately 70% of treated patients demonstrated partial (59%) or complete (11%) response with a median overall survival of 14.1 months. A higher response rate was associated with patients with 3+ HER2/neu expression by IHC or HER2/neu gene amplification by FISH than with those with 2+ HER2/neu expression and FISH-negative tumors. Interestingly, in contrast to the strongly correlated HER2/neu gene amplification and 3+ IHC HER2/neu overexpression usually seen in breast cancer, most HER2/neu overexpression in bladder urothelial carcinoma is not associated with HER2/neu gene amplification.147
A second ongoing randomized phase II trial is evaluating the role of the anti–EGFR recombinant humanized murine monoclonal antibody cetuximab. Patients with metastatic, locally recurrent, or nonresectable disease are treated with standard gemcitabine and carboplatin (GC) chemotherapy with or without cetuximab. By blocking epidermal growth factor binding to the extracellular EGFR domain, cetuximab inhibits downstream signal transduction pathway, accounting for its antiproliferative activity in solid tumors. In BC, a potential added synergistic antiangiogenic effect could be also at play.148,149 A separate phase II Cancer and Leukemia Group B trial investigated the role of a small molecule inhibitor of EGFR (gefitinb) in patients with advanced bladder cancer. Gefitinib in combination with GC had no survival or time to progression advantage over GC alone.150,151
Based on the results of a phase II single-arm trial152 suggesting a therapeutic advantage for lapatininb (a tyrosine kinase inhibitor targeting both EGFR and HER2) in EGFR- or HER2-positive BC tumors, a phase II randomized trial is underway that is looking at the role of maintenance with lapatininb (versus placebo) for patients with objective response to first-line chemotherapy who test positive for either marker by IHC or FISH studies.
In an attempt to target BC dependence on angiogenesis, monoclonal antibodies and small molecule inhibitors of angiogenesis are under investigation in advanced disease. An initial phase II trial evaluating the role of bevacizumab—a recombinant humanized monoclonal anti-VEGF antibody—in combination with GC as a first-line therapy in metastatic BC revealed objective response in two-thirds of patients, with 6 of 43 patients showing complete response albeit with significant treatment-related toxicity.153 A CALBG (Cancer and Leukemia Group B) phase III randomized trial for GC, with and without bevacizumab, for metastatic urothelial carcinoma is now underway, as well as other phase II trials for bevacizumab in combination with other chemotherapeutic agents such as M-VAC (methotrexate, vinblastine, adriamycin, and cisplatin).154
The role of multitarget tyrosine kinase inhibitors in BC has also been investigated with mixed results. While sorafenib (inhibits Raf kinase, PDGFR-B, VEGFR-2, and VEGFR-3) phase II trials have failed to show significant objective response, sunitinib (inhibits VEGFR-2 and PDGFR-B) has shown a more promising effect in a recent phase II trial involving 77 patients at Memorial Sloan-Kettering Cancer Center (New York, New York) in which clinical benefits were observed in almost one-third of the patients. A subsequent randomized double-blind phase II trial is underway, investigating the efficacy of sunitinib in delaying progression as a maintenance agent in patients with initial response to standard chemotherapy.155 Finally, given the recent evidence suggesting the presence of mTOR pathway alterations in BC, a phase II trial evaluating the potential role of everolimus, an inhibitor of mTOR pathway, in advanced BC is underway.124–126
In summary, as our understanding of the complex molecular mechanisms involved in BC development has come into a sharper focus, our approaches to diagnosis and management of bladder cancer continue to evolve. In the not-so-distant future, the current paradigm of clinicopathologic-based prognostic approach to predicting progression in superficial BC90,156–158 is to be supplemented by a molecular-guided approach based on some of the markers listed in Table 1.* Several new targeted therapy agents are under investigation in randomized trials in combination with standard chemotherapy agents, either as first-line treatment or on a maintenance basis to prolong response in patients with advanced bladder cancer.
Table 1.
Established Clinicopathologic and Potential Molecular Prognostic Parameters in Superficial and Muscle Invasive Urothelial Carcinoma of Bladder
| Clinicopathologic Prognostic Parameters in Urothelial Carcinoma | |
|---|---|
| Superficial Urothelial Carcinoma | Muscle Invasive Urothelial Carcinoma |
| WHO/ISUP grade | pTNM |
| pT stage | LVI |
| Presence of associated CIS/dysplasia | Resistance to neoadjuvant chemotherapy |
| Disease duration | |
| Time to and frequency of recurrences | Divergent histology |
| Multifocality | Micropapillary |
| Tumor size (>3 cm) | Osteoclast rich |
| Failure of prior BCG therapy | Undifferentiated/giant cell |
| Presence of LVI | Plasmacytoid |
| Depth of lamina propria invasion | |
| Emerging Molecular Prognostic Markers | |
| Superficial Non–Muscle Invasive Urothelial Carcinoma (NMI-BC) | Muscle Invasive Urothelial Carcinoma (MI-BC) |
| Proliferation index (Ki-67, MIB-1, S phase) | p53 inactivation/accumulation |
| FGFR3 mutation/overexpression (protective) | Alterations of Rb expression |
| mG (FGFR#/MIB-1) | Loss of p21 expression |
| p53 inactivation/accumulation | Alteration of p16 expression |
| DNA ploidy status | Loss of E-cadherin |
| Multitarget FISH | RTK |
| HRAS | EGFR overexpression |
| ERBB3, ERBB4 overexpression (protective) | HER2/neu overexpression/amplification |
| Loss of E-cadherin | |
| Angiogenesis markers | |
| Cell cycle control | VEGF overexpression |
| Down-regulation of Rb expression | HIF1A overexpression |
| Down-regulation of p21 expression | TSP1 overexpression |
| Down-regulation of p27 expression | |
| Cyclin D3 overexpression | mTOR-Akt pathway |
| Cyclin D1 overexpression | mTOR |
| Phos-S6 expression (protective) | |
| Multimarker immunoexpression analysis | |
| p53, p27, Ki-67, Rb, p21 | Genomic and gene expression array panels |
| Angiogenesis markers | Epigenetic alterations |
| VEGF overexpression | RASSF1 promoter hypermethylation |
| HIF1A overexpression | E-cadherin promoter hypermethylation |
| TSP1 overexpression | EDNRB promoter hypermethylation |
| Genomic and gene expression array panels | |
| Epigenetic alterations | |
| RASSF1 promoter hypermethylation | |
| DAPK promoter hypermethylation | |
| APC promoter hypermethylation | |
| E-cadherin promoter hypermethylation | |
| EDNRB promoter hypermethylation | |
Abbreviations: BC, bladder cancer; BCG, Bacillus Calmette-Guerin; CIS, carcinoma in situ; EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; ISUP, International Society of Urological Pathology; LVI, lymphovascular invasion; mG, molecular grade parameter; mTOR, mammalian target of rapamycin; RTK, receptor tyrosine kinase; VEGF, vascular endothelial growth factor; WHO, World Health Organization.
PROSTATE ADENOCARCINOMA
The continuous debate on whether current serum prostate-specific antigen (PSA)–based screening strategies are potentially leading to “overtreatment” of a subset of patients with prostate cancer (PCa) has further fueled the interest in pursuing clinicopathologic and molecular parameters that may help identify patients with biologically “significant” prostate cancers.162,163 A parallel pursuit of clinicopathologic algorithms and criteria that can accurately predict “insignificant” PCa tumors is also gaining momentum. The latter are generally defined as tumors that lack the biologic potential to affect disease-specific mortality and morbidity within a given patient life expectancy. As alternative PCa management approaches, such as “proactive surveillance,” are increasingly offered, accurate identification of insignificant PCa becomes more pressing.
Meanwhile, prostate needle biopsy continues to be the gold standard for establishing the diagnosis of PCa in patients with elevated serum PSA levels and/or positive digital rectal examination results. Established clinicopathologic parameters including clinical stage, pathologic stage, histologic Gleason grade, and serum PSA levels are the sole guiding tools of prognostication and disease management in PCa.164–166
Given the existing need to improve upon the prognostic and predictive power of the above-established parameters, an extensive list of molecular biomarkers have been evaluated in the last decade for their potential role in enhancing our ability to predict disease progression, response to therapy, and survival.167–171 These research efforts have been greatly facilitated by the wealth of information garnered from gene expression array studies and by sophisticated bioinformatics tools evaluating the overwhelming data sets generated from genomic, transcriptomic, and proteomic studies. These genomic technologies continue to yield new markers that can in turn be evaluated for clinical utility in a high-throughput manner with IHC and FISH-labeled tissue microarrays and state-of-the art image analysis systems.172–174
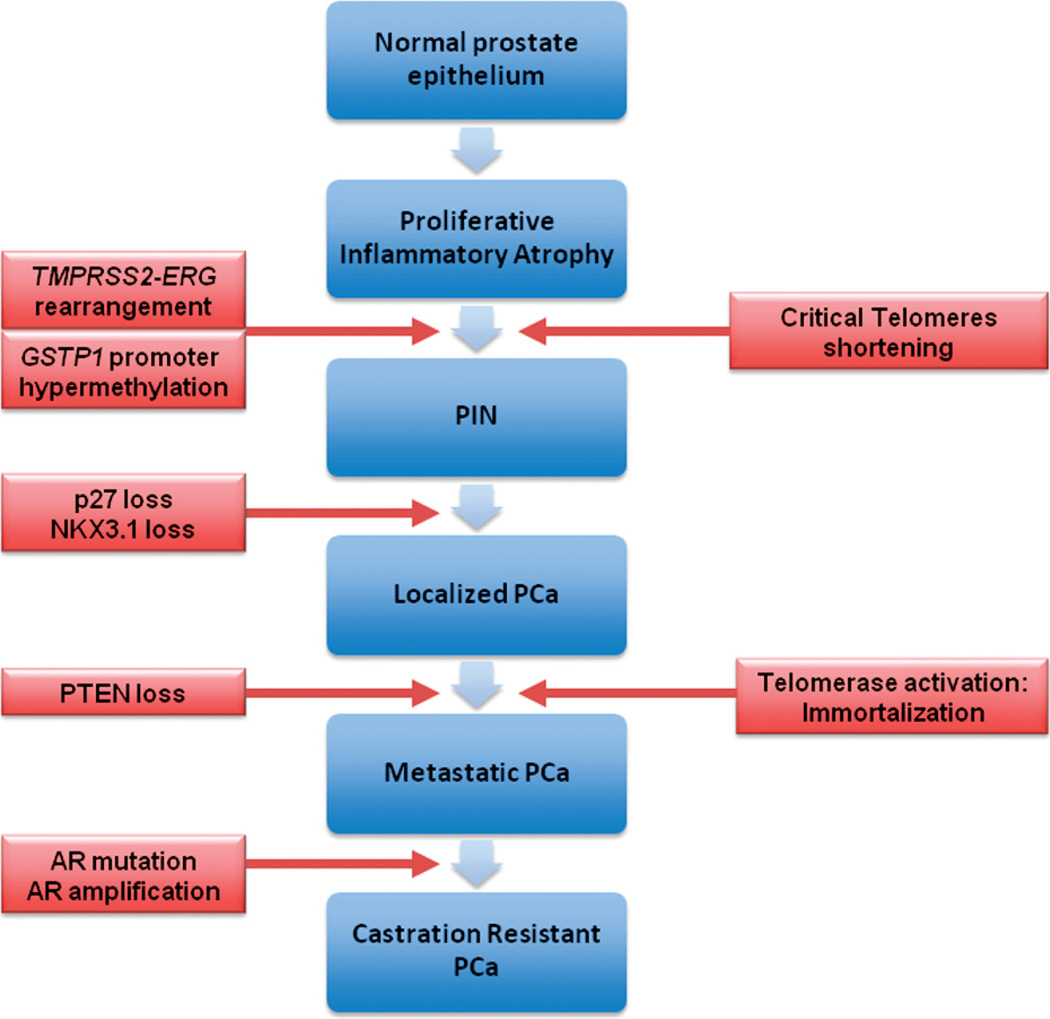
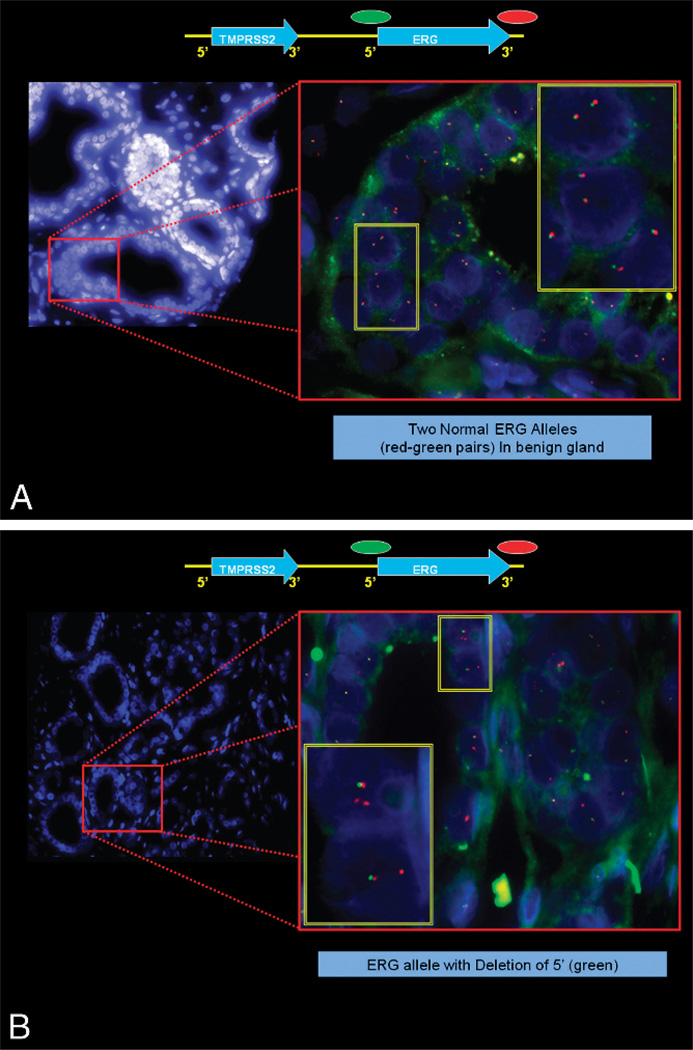
In the last decade, steps detailing the many genetic alterations involved in the progression of PCa have been unveiled (Figure 3). The discovery by Tomlins et al175,176 of a recurrent chromosomal rearrangement in more than one-half of their analyzed PCa cases is ranked as one of the most notable in solid tumor biology, given the shear prevalence of PCa. The recurrent chromosomal rearrangements lead to a fusion of the androgen-responsive promoter elements of the TMPRSS2 gene (21q22) to 1 of 3 members of the ETS transcription factor family members–ERG, ETV1, and ETV4–located at chromosome bands 21q22, 7p21, and 17q21, respectively. Although the prognostic role of assessing TMPRSS2-ETS rearrangements in PCa tissue samples has been called into question by recent, well-designed large cohort studies,177,178 the discovery will no doubt have great implications in terms of furthering our understanding of the steps involved in the development and pathogenesis of PCa and will provide a new marker for molecular diagnosis and potential target(s) of therapy in PCa.179–188 The potential diagnostic and prognostic role of detecting TMPRSS2-ERG in post–prostate massage urine samples requires further investigation.189–191 Figure 4 (A and B) depicts a commonly used FISH split apart–based approach for the evaluation of ERG gene fusion.
Figure 3.
Molecular alterations involved in oncogenesis and progression of prostate cancer. Abbreviations: PCa, prostate adenocarcinoma; PIN, prostatic intraepithelial neoplasia; PTEN, phosphatase and tensin homolog.
Figure 4.
Fluorescence in situ hybridization–based evaluation of ERG gene fusion in prostate carcinoma. A, A normal ERG allele will hybridize with the ERG 3′ and the ERG 5′ probes, leading to the formation of juxtaposed green-red signals or a yellow overlap signal. The 2 ERG rearrangements associated with TMPRSS2-ERG fusion lead to either a loss of 5′ (green) signal or to a split of the ERG 5′ (green) and ERG 3′ (red) signals. B, Example of prostate adenocarcinoma showing ERG fusion by deletion.
Recently, commercial anti-ERG monoclonal antibodies became available that make it possible to use IHC for evaluating ERG protein expression as a surrogate approach to detecting TMPRSS2-ERG fusion by FISH. We, and others, have demonstrated a strong correlation between ERG overexpression by IHC and ERG fusion status with rates greater than 86% for sensitivity and specificity (see Table 2 and Figure 5 [A through F]). Immunohistochemistry may offer an accurate, simpler, and less costly alternative for evaluation of ERG fusion status in PCa on needle biopsy and radical prostatectomy samples.192,193
Table 2.
Immunohistochemistry Expression of ERG Protein Strongly Correlates With TMRSS2-ERG Fusion Regardless of Mechanism of Fusiona
| FISH | No. Cases | No ERG Expression, No. (%) | ERG Expression, No. (%) | P |
|---|---|---|---|---|
| Esplit | <.001 | |||
| 108 | 17 (15.7) | 91 (84.3) | ||
| 319 | 218 (68.3) | 101 (31.7) | ||
| Edel | <.001 | |||
| 158 | 21 (13.3) | 137 (86.7) | ||
| 269 | 214 (79.6) | 55 (2.4) | ||
| 2Esplit | .008 | |||
| 6 | 0 (0) | 6 (100) | ||
| 421 | 235 (55.8) | 186 (44.2) | ||
| 2Edel | <.001 | |||
| 24 | 1 (4.2) | 23 (95.8) | ||
| 403 | 234 (58.1) | 169 (41.9) | ||
| Any fusion | <.001 | |||
| 195 | 28 (14.4) | 167 (85.6) | ||
| 232 | 207 (89.2) | 25 (10.8) |
Abbreviations: Edel, deletion; Esplit, insertion leading to FISH signal split; FISH, fluorescence in situ hybridization.
Reprinted from Chaux et al193 with permission from Lippincott Williams & Wilkins.
Figure 5.
Overexpression of ERG, as demonstrated by immunohistochemistry, is a simple surrogate method for evaluating TMPRSS2-ERG fusion in prostate adenocarcinoma. Positive expression of ERG in Gleason grades 6 and 8 cases that were also positive for TMPRSS2-ERG fusion by fluorescence in situ hybridization (FISH) are shown in A and B and in C and D, respectively. Lack of ERG expression in a Gleason grade 6 tumor that lacked TMPRSS2-ERG fusion by FISH is shown in E and F (ERG immunostain, original magnifications ×100 [A, C, and E] and ×200 [B, D, and F]).
The perceived need to identify “objective” markers to supplement, or conceivably supplant, the more “subjective” established histologic parameters has been a major driving force behind biomarker discovery efforts. It is crucial to recognize and account for the potential variability that can exist even with the new molecular parameters. Sources of variability include differences in molecular technique methodologies, tissue fixation and processing, interobserver and intraobserver variability (in IHC-based biomarkers), and differences in cutoff points.194 Furthermore, illustration of statistical significance for a particular biomarker does not alone assure its utility for a given patient. Therefore, a promising prognostic or therapeutic target biomarker should endure a rigorous “evidence-based” analysis and be validated in large, prospective clinical trials before transition into standard practice.195
Emerging Prognostic Factors
Currently pursued prognostic molecular biologic markers in PCa are categorized as College of American Pathologists Consensus Category III Prognostic Factors.196 Category III prognostic factors are those still needing additional studies to assure their prognostic utility before undergoing further clinical trials. In contrast, Category I factors are considered as proven to be useful in clinical practice and include preoperative PSA, TNM stage, Gleason grade, and surgical margins. Category II factors are factors that have been extensively studied but await statistically robust trials and include parameters such as tumor volume, histologic type, and DNA ploidy analysis.
The wide array of molecular–based PCa markers include proliferation index (Ki-67), microvessel density, nuclear morphometry, tumor suppression genes (eg, p53, p21, p27, NKX3.1, phosphatase and tensin homolog [PTEN], and retinoblastoma gene [Rb]), oncogenes (eg, Bcl2, c-myc, EZH2, and HER2), adhesion molecules (CD44, E-cadherin), PI3K/akt/mTOR pathway,197,198 apoptosis regulators (eg, survivin and transforming growth factor β 1), androgen receptor status, neuroendocrine differentiation markers, and prostate tissue lineage-specific marker expression (PSA, prostate-specific alkaline phosphatase, and prostate-specific membrane antigen).199–201
Proliferation Index
A single study202 has so far found proliferation index, as measured by Ki-67 and percentage of cells in S phase and G2M, to be superior to Gleason score in predicting biochemical recurrence after radical prostatectomy. Two additional studies203,204 have shown a similar role for Ki-67 index measurement as an independent prognosticator in prostatectomy specimens. Conflicting reports have been furthered by others.205–208
Angiogenesis
The mean number of microscopic blood vessels in tissue is higher in PCa and prostatic intraepithelial neoplasia than normal prostate tissue. In a study evaluating microvessel density (MVD) on needle biopsy, the authors209 found that MVD, when combined with Gleason score and preoperative PSA, provided improved ability to predict extraprostatic extension at radical prostatectomy. Although MVD was significant in the multivariate analysis, Gleason score and serum PSA levels were much more powerful predictors of extraprostatic disease. Three additional studies210–212 revealed a prognostic role for MVD in prostatectomy specimens. Others,213–215 however, failed to confirm such a role. Differences in vascular antibodies used and topography of vessel measurements could account for the variable results. It appears that MVD will have a marginal adjunctive role, if any, to established current parameters.
Tumor Suppressor Genes and Oncogenes
Among tumor suppressor genes, there is mounting evidence to support a role for p53 expression in predicting prognosis in PCa. Brewster et al found216 p53 expression and Gleason score in needle biopsy to be independent predictors of biochemical relapse after radical prostatectomy. Another study217 found p53 status on prostatectomy but not needle biopsies to be predictive, raising the issue of sampling. Many studies evaluating prostatectomy specimens found p53 to be of prognostic significance, independent of grade, stage, and margin status.207,215,218–223 The results of these studies suggest that p53 evaluation could become a clinically used parameter, at least in prostatectomy specimens, once standardization of cutoffs and immunostaining methodologies are achieved in large prospective studies. Most studies of another tumor suppressor gene, p27, a cell cycle inhibitor, have also supported a correlation with progression after prostatectomy.208,224
Several recent studies have demonstrated that the PTEN/PI3K/mTOR pathway plays an important role in cell growth, proliferation, and oncogenesis in prostate cancer.225–231 Phosphatase and tensin homolog is a negative regulator of this pathway. Loss of PTEN tumor suppressor gene activity and the ensuing mTOR pathway activation appear to be associated with poor prognosis in prostate cancer. The mTOR pathway is also a potential target for prostate cancer treatment, and several rapamycin analogs are currently being tested as potential therapeutic agents for PCa.197,230 We recently reported the results of a pilot study evaluating the pharmacodynamic efficacy of neoadjuvant rapamycin therapy in PCa.197 Using IHC analysis, we found a significant decrease in Phos-S6 protein, the main downstream effector of mTOR pathway, in patients receiving neoadjuvant therapy.197
While less robust evidence exists for the prognostic role of p21,232 a downstream mediator of p53, and transcription factors such as NKX3.1,172,233 preponderance of evidence supports a prognostic role for Bcl2203,216,218,220,222 and myc oncogenes234,235 as potential adjuncts to histologic prognostic parameters.
Despite great interest in HER2 and its potential use as a target of therapy, the data on its relation to prognosis in PCa are conflicting, with 1 study by Veltri et al236 showing HER2 to be an independent prognosticator and more recent studies using both IHC and FISH assessment showing lack of its utility in predicting progression.237
Genomic Data
In an elegant gene expression profiling study using cDNA microarrays containing 26 000 genes, Lapointe et al238 identified 3 subclasses of prostate tumors by distinct patterns of gene expression. High-grade and advanced-stage tumors, as well as tumors associated with recurrence, were disproportionately represented among 2 of the 3 subtypes, one of which also included mostly lymph node metastases. Furthermore, 2 surrogate genes were differentially expressed among tumor subgroups by IHC. These included MUC1, a gene highly expressed in the subgroups with “aggressive” clinicopathologic features, and AZGP1, a gene highly expressed in the favorable subgroup. The 2 surrogate markers were strong predictors of tumor recurrence, independent of tumor grade, stage, and preoperative PSA levels. Such study suggests that prostate tumors can be classified according to their gene expression patterns; these tumor subtypes may provide a basis for improved prognostication and treatment stratification.
In another study, Tomlins et al239 used laser-capture microdissection to isolate 101 cell populations to illustrate gene expression profiles of PCa progression from benign epithelium to metastatic disease. By analyzing expression signatures in the context of more than 14 000 “molecular concepts,” or sets of biologically connected genes, the authors generated an integrative model of progression. Molecular critical transitions in progression included protein biosynthesis, E26 transformation-specific (ETS) family transcriptional targets, androgen signaling, and cell proliferation. Known prognostic markers, such as grade, could be ascribed to noted attenuated androgen-signaling signature seen in high-grade cancer (Gleason pattern 4), similar to metastatic prostate cancer, which may reflect dedifferentiation and explain the clinical association of grade and prognosis. Taken together, these data show that analyzing gene expression signatures in the context of a compendium of molecular concepts is useful in understanding cancer biology.
Lapointe et al240 complemented their above-mentioned gene expression findings by looking for associated copy number alterations with array-based comparative genomic hybridization. They were able to identify recurrent copy number genetic aberrations240 corresponding to 3 prognostically distinct groups of PCa: (1) deletions at 5q21 and 6q15 deletion group, associated with favorable outcome; (2) a 8p21 (NKX3-1) and 21q22 (resulting in TMPRSS2-ERG fusion) deletion group, and (3) gains in 8q24 (MYC) and 16p13, and loss at 10q23 (PTEN) and 16q23 groups, correlating with metastatic disease and aggressive outcome.
Finally, in a recent genome-wide analysis of PCa, Taylor et al241 elegantly illustrated how detailed annotation of PCa genomes can affect our understanding of the disease and its treatment strategy. Assessing DNA copy number, messenger RNA expression, and focused exon resequencing in 218 prostate cancer tumors, the authors identified the role of nuclear receptor coactivator NCOA2 as a novel oncogene in 11% of PCa cases. TMPRSS2-ERG fusion was associated with novel prostate-specific deletion at chromosome band 3p14, which may implicate FOXP1, RYBP, and SHQ1 as potential cooperative tumor suppressors. Most intriguing was their ability to define clusters of lowrisk and high-risk disease beyond that achieved by Gleason score by using DNA copy number data. As shown in Figure 6 (A and B), six clusters of PCa tumors are identified by unsupervised hierarchical clustering with distinct risk for biochemical recurrence.
Figure 6.
Clinically distinct groups of prostate cancer are identified by genomic alterations. A, Unsupervised hierarchical clustering of copy number alterations identified 6 groups (clusters) of prostate cancers. B, Statistically significant differences in freedom from biochemical recurrence are found among the 6 groups. Adapted from Taylor et al241 with permission from Elsevier.
Genomic studies suggest that prostate cancers develop via a limited number of alternative preferred genetic pathways. The resultant molecular genetic subtypes provide a new framework for investigating PCa biology and explain, in part, the clinical heterogeneity of the disease.
Emerging Early Detection Markers and Targets of Therapy
Markers of PCa detection that can be applied to blood, urine, or prostatic secretion fluid (ejaculate or prostate massage fluids) are of great interest and have been the focus of active research. Markers that have been investigated in the urine or prostatic secretions include gene promoter hypermethylation profile assays242–245 and differential display code 3 (DD3), also known as PCA3 (Figure 7). DD3 is a gene that expresses a noncoding RNA and was initially identified by Bussemakers et al246 as one of the most specific markers of PCa. Quantitative real-time reverse transcriptase PCR assay detecting PCA3 can be applied to blood, urine, or prostatic fluid.247
Figure 7.
Structure of the PCA3/DD3 gene. The gene, which expresses a noncoding RNA, was mapped to chromosome 9q21–22 and consists of 4 exons. Alternative polyadenylation at 3 different positions in exon 4 (indicated as 4a, 4b, and 4c) gives rise to 3 different-sized transcripts. The most frequently found transcript contains exons 1, 3, 4a, and 4b.
Evaluation of PCA3 in postattentive prostate massage urine samples with transcription-mediated amplification technology has shown to be superior to serum PSA determination in predicting biopsy outcome, with sensitivity and specificity approximating 70% and 80%, respectively, and a negative predictive value of 90%248–251; it is currently under evaluation for FDA approval in the United States. Encouraging data from the REDUCE trial support a role for evaluation of PCA3 in postattentive prostate massage urine sample in predicting positive prostate needle biopsy findings in immediately subsequent, as well as future, biopsies after an initial negative biopsy result. PCA3 may also have a role in predicting the risk for higher Gleason score and larger tumor volume on radical retropubic prostatectomy. If confirmed, the latter could be of great value in treatment option algorithms and in delineation of candidates for active surveillance.252–255 Multiplex urine assays to include PCA3, TMPRSS-ERG, SPINK1, and GOLPH2 are also under evaluation, with recent data suggesting an improved performance of such assays compared to PCA3 alone.256
Finally, several markers are being investigated as potential targets of therapy for prostate cancer. The list includes tyrosine kinase receptors (eg, EGFR), angiogenesis targets (eg, VEGF),257 fatty acid synthase,258 PI3K/akt/mTOR,197,230,259 endothelin receptors,260,261 and prostate-specific membrane antigen,262–265 to name a few.
RENAL CELL CARCINOMA
Current established prognostic parameters in renal cell carcinoma (RCC) include pTNM stage, Fuhrman grade, histologic subtype, and clinical parameters such as the Eastern Cooperative Oncology Group (ECOG) performance status, hemoglobin level, and lactate dehydrogenase levels, among others.266–268 Continuous refinements of staging criteria and development of nomograms to integrate the factors listed above promise to yield better prognostic and management discriminators.269
A large number of biomarkers are under current intense investigation for their potential utility as prognosticators and/or therapy predictors in RCC.269–276 Table 3 lists some of these markers. Kim et al271 evaluated a set of immunohistochemical markers including Ki-67, CAIX, CAXII, p53, PTEN, gelsolin, EpCAM, and vimentin in combination with established parameters. Their study suggested that a new combined molecular and clinicopathologic prognostic model (CAIX, vimentin, p53, pTNM, ECOG performance status) is superior to prior models of clinicopathologic parameters alone, including the commonly used University of California Los Angeles integrated staging system model.
Table 3.
Current and Emerging Prognostic Parameters in Clear Cell Renal Cell Carcinoma (ccRCC)
| Current Prognostic Parameters in ccRCC |
|---|
| Patient factors |
| Age |
| Sex |
| Pathologic factors |
| pTNM |
| Histologic type |
| Fuhrman grade |
| LVI |
| Tumor necrosis |
| Clinical factors |
| ECOG performance status |
| Hgb level |
| Serum LDH |
| Emerging Potential Molecular Prognostic and Predictive Parameters in ccRCC |
| Hypoxia inducible |
| HIF-1 |
| CAIX |
| CAXII |
| CXCR4 |
| VEGF/VEGF-R |
| ILGF1 |
| Cell adhesion markers |
| EpCAM |
| E-cadherin |
| α-Catenin |
| Catenin-6 |
| Proliferation markers |
| Ki-67 |
| MCM2 |
| Cell cycle regulators |
| Cyclin |
| p27 |
| Apoptosis regulators |
| p53 |
| Bcl2 |
| Smac/DIABLO |
| mTOR pathway |
| PTEN |
| akt |
| Phos-S6k an |
Abbreviations: ccRCC, clear cell renal cell carcinoma; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; Hgb, hemoglobin; LVI, lymphovascular invasion; PTEN, phosphatase and tensin homolog; VEGF, vascular endothelial growth factor.
Pantuck et al277,278 recently highlighted a promising prognostic role for mTOR pathway members. Their study revealed an independent negative prognostic role for PTEN loss and phos-S6k overexpression. The same study showed that an increase in phos-Akt cytoplasmic expression and loss of phos-Akt nuclear expression were negative predictors of survival.
Bui et al279 demonstrated that both low expression of CAIX and high Ki-67 proliferation index were independent negative predictors of survival in clear cell renal cell carcinoma (ccRCC). Interestingly, CAIX overexpression predicted response to interleukin 2 immune therapy in metastatic RCC, a finding also documented in the study by Atkins et al280 and Stillebroer et al.281 A prognostic role for angiogenesis pathway has been revealed in RCC. In a study by Jacobsen et al,282 VEGF expression appeared to correlate with tumor size and pTNM stage in RCC. The authors found high VEGF expression to be a negative prognosticator for survival on univariate but not multivariate analysis. Separately, Kluger et al272 analyzed tissue microarrays containing 330 ccRCCs and papillary renal cell carcinoma (PRCC), using a novel method of automated quantitative analysis of VEGF and VEGF receptor expression by fluorescent IHC. Unsupervised hierarchical clustering classified tumors by coordinated expression of VEGF and VEGF receptors. The authors found that high expression of VEGF and VEGF receptors was associated with poor survival. Finally, a study by Lidgren et al283 revealed that high expression of hypoxia-inducible factor 1 α (HIF1A) was an independent negative prognosticator in ccRCC.
Among cell cycle control molecules, p27 (Kip1) and cyclin D1 appear to have a promising prognostic role in ccRCC. Migita et al284 found loss of p27 expression to be an independent predictor of poor DSS. Similar p27 findings were also documented by Hedberg et al.285,286
Genomic and Theranostic Applications
A tight correlation is maintained between morphologic phenotype and underlying genetic alterations in renal tumors. Lessons learned from the relatively rare familial renal cancer syndromes have helped unlock the complex molecular mechanisms involved in sporadic RCC tumorigenesis. As a result, many potential new targets of therapy are now under investigation in the heretofore unsuccessful endeavor of treating advanced RCC. The achieved understanding of the molecular basis of von Hippel-Lindau disease (VHL) best illustrates the great therapeutic and theranostic potentials of uncovering molecular mechanisms of oncogenesis.287 von Hippel-Lindau syndrome is a rare autosomal dominant familial cancer syndrome (retinal angiomas, hemangioblastomas, pheochromocytomas, ccRCC). Patients with VHL are born with a germline (constitutive) VHL gene mutation affecting all their cellular elements. Inactivation or silencing of the remaining wild-type allele in renal tissues facilitates the formation of ccRCC. The fact that similar defects in the VHL gene are found to be responsible for approximately 60% of sporadic ccRCCs288,289 has greatly widened the implications in understanding the molecular mechanisms of VHL inactivation.
HIF1A is a transcription factor that can be thought of as a cellular oxygen sensor. Under normoxic conditions, HIF1A interacts with normal VHL protein that, in turn, facilitates its elimination through ubiquitinization.290 Under hypoxic conditions, HIF1A escapes destruction by VHL and is allowed to exert its crucial role in triggering the transcription of angiogenesis factor (VEGF); cell growth factors (transforming growth factor α [TGFα] and transforming growth factor β [TGFβ]); and factors involved in glucose uptake and acid-base balance (GLUT-1 and CAIX, respectively). A defective VHL function in ccRCC leads to abnormal accumulation of HIF1A even under normal conditions, in turn resulting in the overexpression of the above-mentioned proteins that are normally inducible only during hypoxia. The overexpressed VEGF, platelet-derived growth factor β, and TGFβ act on neighboring vascular structures to promote tumor angiogenesis. The augmented tumor vasculature provides additional nutrients and oxygen to promote the growth of tumor cells. Furthermore, TGFα acts in an autocrine manner on tumor cells by signaling through EGFR, which promotes tumor cell proliferation and survival.
Several of the above-mentioned proteins are currently being investigated as targets of therapy for advanced ccRCC.291–298 A randomized phase II trial involving patients with metastatic ccRCC investigated the efficacy of bevacizumab, a humanized anti-VEGF antibody.299 Although the treatment resulted in only a few months extension of time to tumor progression, it provided a key “proof of principle” of the efficacy of antiangiogenic therapy. Inhibitors of VEGF receptor tyrosine kinase activity alone, and in combination with other tyrosine kinases, are also under study. The multitargeted kinase inhibitors sunitinib and sorafenib have shown great promise in phase II and phase III trials, with at least stabilization of disease in as many as 70% of patients with cytokine refractory disease.287,298
Another growth factor target is TGFα, which promotes ccRCC growth through its interaction with EGFR. A human monoclonal antibody against human EGFR (panitumumab [ABX-EGF]), as well as small molecule inhibitors of the EGFR tyrosine kinase activity (gefitinib and erlotinib), are 2 strategies being tested to target the TGFα/EGFR axis.292,294–296
Other options being pursued include the use of temsirolimus (CCI-779), a selective inhibitor of mTOR. Partial responses were noted in 7% of patients and minor responses in 26%. The median survival rate was 15 months. The notable activity of the drug for patients with poor prognostic features prompted a phase III trial.280,293 Finally, agents targeting HIF1A and CAIX, including anti-CAIX radiolabeled monoclonal antibody, are also under development.289,300
CONCLUSIONS
A wide array of molecular markers may be used in the near future as adjuncts to currently established prognostic parameters in urologic malignancies. For bladder cancer, molecular grade as a combination of proliferation index and FGFR3 mutation; multitarget FISH analysis for chromosomes 3,7,17, and chromosome band 9p21; and cell cycle and apoptosis control markers (p52, Rb, p21, p27, and p16) are among the leading markers that will soon enter routine use. In prostate adenocarcinoma, biologic markers that can refine early detection (eg, PCA3 and ERG) and serve as prognostic markers and potential targets of therapy (mTOR pathway) are expected to be among the first to enter the clinical arena. Finally, our detailed understanding of the complex molecular alterations involved in the development of renal cell carcinomas is yielding novel diagnostic and prognostic molecular tools and yielding many new targets of therapies including mTOR, VEGF, EGFR, HIF1A, and CAIX.
Footnotes
References
- 1.Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol. 2006;24:5552–5564. doi: 10.1200/JCO.2006.08.2073. [DOI] [PubMed] [Google Scholar]
- 2.Mitra AP, Cote RJ. Molecular screening for bladder cancer: progress and potential. Nat Rev Urol. 2010;7:11–20. doi: 10.1038/nrurol.2009.236. [DOI] [PubMed] [Google Scholar]
- 3.Mitra AP, Cote RJ. Molecular pathogenesis and diagnostics of bladder cancer. Annu Rev Pathol. 2009;4:251–285. doi: 10.1146/annurev.pathol.4.110807.092230. [DOI] [PubMed] [Google Scholar]
- 4.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 5.Oxford G, Theodorescu D. The role of ras superfamily proteins in bladder cancer progression. J Urol. 2003;170:1987–1993. doi: 10.1097/01.ju.0000088670.02905.78. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Knowles E, Hernandez S, Malats N, et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 2006;66:7401–7404. doi: 10.1158/0008-5472.CAN-06-1182. [DOI] [PubMed] [Google Scholar]
- 7.Kompier LC, Lurkin I, van der Aa MN, van Rhijn BW, van der Kwast TH, Zwarthoff EC. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubota Y, Miyamoto H, Noguchi S, et al. The loss of retinoblastoma gene in association with c-myc and transforming growth factor-beta 1 gene expression in human bladder cancer. J Urol. 1995;154:371–374. doi: 10.1097/00005392-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell MA. Advances in the management of superficial bladder cancer. Semin Oncol. 2007;34:85–97. doi: 10.1053/j.seminoncol.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon,France: IARC Press; 2004. World Health Organization Classification of Tumours; vol 7. [Google Scholar]
- 11.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 12.Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the bladder cancer research consortium. J Urol. 2006;176:2414–2422. doi: 10.1016/j.juro.2006.08.004. [discussion in J Urol. 2006; 176:2422]. [DOI] [PubMed] [Google Scholar]
- 13.Shariat SF, Chade DC, Karakiewicz PI, et al. Combination of multiple molecular markers can improve prognostication in patients with locally advanced and lymph node positive bladder cancer. J Urol. 2010;183:68–75. doi: 10.1016/j.juro.2009.08.115. [DOI] [PubMed] [Google Scholar]
- 14.Rabbani F, Koppie TM, Charytonowicz E, Drobnjak M, Bochner BH, Cordon-Cardo C. Prognostic significance of p27(Kip1) expression in bladder cancer. BJU Int. 2007;100:259–263. doi: 10.1111/j.1464-410X.2007.06927.x. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Carbayo M, Socci ND, Charytonowicz E, et al. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002;62:6973–6980. [PubMed] [Google Scholar]
- 17.Sanchez-Carbayo M, Cordon-Cardo C. Applications of array technology: identification of molecular targets in bladder cancer. Br J Cancer. 2003;89:2172–2177. doi: 10.1038/sj.bjc.6601406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ioachim E, Michael M, Salmas M, Michael MM, Stavropoulos NE, Malamou-Mitsi V. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha expression in bladder cancer and their associations with other angiogenesis-related proteins. Urol Int. 2006;77:255–263. doi: 10.1159/000094819. [DOI] [PubMed] [Google Scholar]
- 19.Ioachim E, Michael MC, Salmas M, et al. Thrombospondin-1 expression in urothelial carcinoma: prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. BMC Cancer. 2006;6:140. doi: 10.1186/1471-2407-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lascombe I, Clairotte A, Fauconnet S, et al. N-cadherin as a novel prognostic marker of progression in superficial urothelial tumors. Clin Cancer Res. 2006;12:2780–2787. doi: 10.1158/1078-0432.CCR-05-2387. [DOI] [PubMed] [Google Scholar]
- 21.Rotterud R, Nesland JM, Berner A, Fossa SD. Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int. 2005;95:1344–1350. doi: 10.1111/j.1464-410X.2005.05497.x. [DOI] [PubMed] [Google Scholar]
- 22.Highshaw RA, McConkey DJ, Dinney CP. Integrating basic science and clinical research in bladder cancer: update from the first bladder specialized program of research excellence (SPORE) Curr Opin Urol. 2004;14:295–300. doi: 10.1097/00042307-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Clairotte A, Lascombe I, Fauconnet S, et al. Expression of E-cadherin and alpha-, beta-, gamma-catenins in patients with bladder cancer: identification of gamma-catenin as a new prognostic marker of neoplastic progression in T1 superficial urothelial tumors. Am J Clin Pathol. 2006;125:119–126. [PubMed] [Google Scholar]
- 24.Chatterjee SJ, Datar R, Youssefzadeh D, et al. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22:1007–1013. doi: 10.1200/JCO.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 25.Beekman KW, Bradley D, Hussain M. New molecular targets and novel agents in the treatment of advanced urothelial cancer. Semin Oncol. 2007;34:154–164. doi: 10.1053/j.seminoncol.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J Urol. 2007;177:481–487. doi: 10.1016/j.juro.2006.09.038. [discussion in J Urol. 2007;177:487.] [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto H, Kubota Y, Fujinami K, et al. Infrequent somatic mutations of the p16 and p15 genes in human bladder cancer: p16 mutations occur only in low-grade and superficial bladder cancers. Oncol Res. 1995;7:327–330. [PubMed] [Google Scholar]
- 28.Miyamoto H, Kubota Y, Shuin T, et al. Analyses of p53 gene mutations in primary human bladder cancer. Oncol Res. 1993;5:245–249. [PubMed] [Google Scholar]
- 29.Birkhahn M, Mitra AP, Williams AJ, et al. Predicting recurrence and progression of noninvasive papillary bladder cancer at initial presentation based on quantitative gene expression profiles. Eur Urol. 2010;57:12–20. doi: 10.1016/j.eururo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng L, Davidson DD, Maclennan GT, et al. The origins of urothelial carcinoma. Expert Rev Anticancer Ther. 2010;10:865–880. doi: 10.1586/era.10.73. [DOI] [PubMed] [Google Scholar]
- 31.Mengual L, Burset M, Ribal MJ, et al. Gene expression signature in urine for diagnosing and assessing aggressiveness of bladder urothelial carcinoma. Clin Cancer Res. 2010;16:2624–2633. doi: 10.1158/1078-0432.CCR-09-3373. [DOI] [PubMed] [Google Scholar]
- 32.Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Association of cyclin D1 and E1 expression with disease progression and biomarkers in patients with nonmuscle-invasive urothelial cell carcinoma of the bladder. Urol Oncol. 2007;25:468–475. doi: 10.1016/j.urolonc.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Bolenz C, Lotan Y. Translational research in bladder cancer: from molecular pathogenesis to useful tissue biomarkers. Cancer Biol Ther. 2010;10:407–415. doi: 10.4161/cbt.10.5.13022. [DOI] [PubMed] [Google Scholar]
- 34.Bensalah K, Montorsi F, Shariat SF. Challenges of cancer biomarker profiling. Eur Urol. 2007;52:1601–1609. doi: 10.1016/j.eururo.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 35.Netto GJ. Molecular diagnostics in urologic malignancies: a work in progress. Arch Pathol Lab Med. 2011;135:610–621. doi: 10.5858/2010-0727-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 36.Netto GJ, Epstein JI. Theranostic and prognostic biomarkers: genomic applications in urological malignancies. Pathology. 2010;42:384–394. doi: 10.3109/00313021003779145. [DOI] [PubMed] [Google Scholar]
- 37.Kawauchi S, Sakai H, Ikemoto K, et al. 9p21 index as estimated by dual-color fluorescence in situ hybridization is useful to predict urothelial carcinoma recurrence in bladder washing cytology. Hum Pathol. 2009;40:1783–1789. doi: 10.1016/j.humpath.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Kruger S, Mess F, Bohle A, Feller AC. Numerical aberrations of chromosome 17 and the 9p21 locus are independent predictors of tumor recurrence in non-invasive transitional cell carcinoma of the urinary bladder. Int J Oncol. 2003;23:41–48. [PubMed] [Google Scholar]
- 39.Skacel M, Fahmy M, Brainard JA, et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol. 2003;169:2101–2105. doi: 10.1097/01.ju.0000066842.45464.cc. [DOI] [PubMed] [Google Scholar]
- 40.Sarosdy MF, Kahn PR, Ziffer MD, et al. Use of a multitarget fluorescence in situ hybridization assay to diagnose bladder cancer in patients with hematuria. J Urol. 2006;176:44–47. doi: 10.1016/S0022-5347(06)00576-3. [DOI] [PubMed] [Google Scholar]
- 41.Moonen PM, Merkx GF, Peelen P, Karthaus HF, Smeets DF, Witjes JA. UroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle-invasive bladder cancer. Eur Urol. 2007;51:1275–1280. doi: 10.1016/j.eururo.2006.10.044. [discussion in Eur Urol. 2007;51:1280]. [DOI] [PubMed] [Google Scholar]
- 42.Yoder BJ, Skacel M, Hedgepeth R, et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol. 2007;127:295–301. doi: 10.1309/ADJL7E810U1H42BJ. [DOI] [PubMed] [Google Scholar]
- 43.Fritsche HM, Burger M, Dietmaier W, et al. Multicolor FISH (UroVysion) facilitates follow-up of patients with high-grade urothelial carcinoma of the bladder. Am J Clin Pathol. 2010;134:597–603. doi: 10.1309/AJCPKKWBDSAOZ4RW. [DOI] [PubMed] [Google Scholar]
- 44.Karnwal A, Venegas R, Shuch B, Bassett J, Rajfer J, Reznichek R. The role of fluorescence in situ hybridization assay for surveillance of non-muscle invasive bladder cancer. Can J Urol. 2010;17:5077–5081. [PubMed] [Google Scholar]
- 45.Schlomer BJ, Ho R, Sagalowsky A, Ashfaq R, Lotan Y. Prospective validation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J Urol. 2010;183:62–67. doi: 10.1016/j.juro.2009.08.157. [DOI] [PubMed] [Google Scholar]
- 46.Ferra S, Denley R, Herr H, Dalbagni G, Jhanwar S, Lin O. Reflex UroVysion testing in suspicious urine cytology cases. Cancer. 2009;117:7–14. doi: 10.1002/cncy.20016. [DOI] [PubMed] [Google Scholar]
- 47.Savic S, Zlobec I, Thalmann GN, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical bacillus calmette-guerin therapy. Int J Cancer. 2009;124:2899–2904. doi: 10.1002/ijc.24258. [DOI] [PubMed] [Google Scholar]
- 48.Maffezzini M, Campodonico F, Capponi G, et al. Prognostic significance of fluorescent in situ hybridisation in the follow-up of non-muscle-invasive bladder cancer. Anticancer Res. 2010;30:4761–4765. [PubMed] [Google Scholar]
- 49.Whitson J, Berry A, Carroll P, Konety B. A multicolour fluorescence in situ hybridization test predicts recurrence in patients with high-risk superficial bladder tumours undergoing intravesical therapy. BJU Int. 2009;104:336–339. doi: 10.1111/j.1464-410X.2009.08375.x. [DOI] [PubMed] [Google Scholar]
- 50.Renshaw AA. UroVysion, urine cytology, and the College of American Pathologists: where should we go from here? Arch Pathol Lab Med. 2010;134:1106–1107. doi: 10.5858/2009-0731-RA.1. [DOI] [PubMed] [Google Scholar]
- 51.van Rhijn BW, Zuiverloon TC, Vis AN, et al. Molecular grade (FGFR3/MIB1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur Urol. 2010;58:433–441. doi: 10.1016/j.eururo.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 52.Mason RA, Morlock EV, Karagas MR, et al. EGFR pathway polymorphisms and bladder cancer susceptibility and prognosis. Carcinogenesis. 2009;30:1155–1160. doi: 10.1093/carcin/bgp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simonetti S, Russo R, Ciancia G, Altieri V, De Rosa G, Insabato L. Role of polysomy 17 in transitional cell carcinoma of the bladder: immunohistochemical study of HER2/neu expression and fish analysis of c-erbB-2 gene and chromosome 17. Int J Surg Pathol. 2009;17:198–205. doi: 10.1177/1066896909333415. [DOI] [PubMed] [Google Scholar]
- 54.Latif Z, Watters AD, Dunn I, Grigor K, Underwood MA, Bartlett JM. HER2/neu gene amplification and protein overexpression in G3 pT2 transitional cell carcinoma of the bladder: a role for anti-HER2 therapy? Eur J Cancer. 2004;40:56–63. doi: 10.1016/j.ejca.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 55.Gandour-Edwards R, Lara PN, Jr, Folkins AK, et al. Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer. 2002;95:1009–1015. doi: 10.1002/cncr.10808. [DOI] [PubMed] [Google Scholar]
- 56.Eissa S, Ali HS, Al Tonsi AH, Zaglol A, El Ahmady O. HER2/neu expression in bladder cancer: relationship to cell cycle kinetics. Clin Biochem. 2005;38:142–148. doi: 10.1016/j.clinbiochem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955–1959. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leibl S, Zigeuner R, Hutterer G, Chromecki T, Rehak P, Langner C. EGFR expression in urothelial carcinoma of the upper urinary tract is associated with disease progression and metaplastic morphology. APMIS. 2008;116:27–32. doi: 10.1111/j.1600-0463.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 59.Bolenz C, Shariat SF, Karakiewicz PI, et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int. 2010;106:1216–1222. doi: 10.1111/j.1464-410X.2009.09190.x. [DOI] [PubMed] [Google Scholar]
- 60.Al-Ahmadie HA, Iyer G, Janakiraman M, et al. Somatic mutation of fibroblast growth factor receptor-3 (FGFR3) defines a distinct morphological subtype of high-grade urothelial carcinoma. J Pathol. 2011;224:270–279. doi: 10.1002/path.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ling S, Chang X, Schultz L, et al. An EGFR-ERK-SOX9 signaling cascade links urothelial development and regeneration to cancer. Cancer Res. 2011;71:3812–3821. doi: 10.1158/0008-5472.CAN-10-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuiverloon TC, van der Aa MN, van der Kwast TH, et al. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low-grade non-muscle-invasive bladder cancer. Clin Cancer Res. 2010;16:3011–3018. doi: 10.1158/1078-0432.CCR-09-3013. [DOI] [PubMed] [Google Scholar]
- 63.Miyake M, Sugano K, Sugino H, et al. Fibroblast growth factor receptor 3 mutation in voided urine is a useful diagnostic marker and significant indicator of tumor recurrence in non-muscle invasive bladder cancer. Cancer Sci. 2010;101:250–258. doi: 10.1111/j.1349-7006.2009.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hernandez S, Lopez-Knowles E, Lloreta J, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006;24:3664–3671. doi: 10.1200/JCO.2005.05.1771. [DOI] [PubMed] [Google Scholar]
- 65.van Rhijn BW, Vis AN, van der Kwast TH, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol. 2003;21:1912–1921. doi: 10.1200/JCO.2003.05.073. [DOI] [PubMed] [Google Scholar]
- 66.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–465. doi: 10.1016/j.eururo.2005.12.031. [discussion in Eur Urol. 2006;49:475–477]. [DOI] [PubMed] [Google Scholar]
- 67.Chakravarti A, Winter K, Wu CL, et al. Expression of the epidermal growth factor receptor and her-2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle-invading bladder cancers treated by concurrent radiation and cisplatin-based chemotherapy: a report from the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2005;62:309–317. doi: 10.1016/j.ijrobp.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 68.Jimenez RE, Hussain M, Bianco FJ, Jr, et al. Her-2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res. 2001;7:2440–2447. [PubMed] [Google Scholar]
- 69.Ravery V, Grignon D, Angulo J, et al. Evaluation of epidermal growth factor receptor, transforming growth factor alpha, epidermal growth factor and c-erbB2 in the progression of invasive bladder cancer. Urol Res. 1997;25:9–17. doi: 10.1007/BF00941900. [DOI] [PubMed] [Google Scholar]
- 70.Sarkis AS, Dalbagni G, Cordon-Cardo C, et al. Nuclear overexpression of p53 protein in transitional cell bladder carcinoma: a marker for disease progression. J Natl Cancer Inst. 1993;85:53–59. doi: 10.1093/jnci/85.1.53. [DOI] [PubMed] [Google Scholar]
- 71.Sarkis AS, Dalbagni G, Cordon-Cardo C, et al. Association of P53 nuclear overexpression and tumor progression in carcinoma in situ of the bladder. J Urol. 1994;152:388–392. doi: 10.1016/s0022-5347(17)32745-3. [DOI] [PubMed] [Google Scholar]
- 72.Sarkis AS, Bajorin DF, Reuter VE, et al. Prognostic value of p53 nuclear overexpression in patients with invasive bladder cancer treated with neoadjuvant MVAC. J Clin Oncol. 1995;13:1384–1390. doi: 10.1200/JCO.1995.13.6.1384. [DOI] [PubMed] [Google Scholar]
- 73.Garcia del Muro X, Condom E, Vigues F, et al. p53 and p21 expression levels predict organ preservation and survival in invasive bladder carcinoma treated with a combined-modality approach. Cancer. 2004;100:1859–1867. doi: 10.1002/cncr.20200. [DOI] [PubMed] [Google Scholar]
- 74.Tzai TS, Tsai YS, Chow NH. The prevalence and clinicopathologic correlate of p16INK4a, retinoblastoma and p53 immunoreactivity in locally advanced urinary bladder cancer. Urol Oncol. 2004;22:112–118. doi: 10.1016/S1078-1439(03)00176-5. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, et al. Prognostic factors in stage T1 grade 3 bladder cancer survival: the role of G1-S modulators (p53, p21Waf1, p27kip1, cyclin D1, and cyclin D3) and proliferation index (ki67-MIB1) Eur Urol. 2004;45:606–612. doi: 10.1016/j.eururo.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 76.Shariat SF, Bolenz C, Karakiewicz PI, et al. P53 expression in patients with advanced urothelial cancer of the urinary bladder. BJU Int. 2010;105:489–495. doi: 10.1111/j.1464-410X.2009.08742.x. [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Beltran A, Requena MJ, Luque RJ, et al. Cyclin D3 expression in primary Ta/T1 bladder cancer. J Pathol. 2006;209:106–113. doi: 10.1002/path.1952. [DOI] [PubMed] [Google Scholar]
- 78.Fu TY, Tu MS, Tseng HH, Wang JS. Overexpression of p27kip1 in urinary bladder urothelial carcinoma. Int J Urol. 2007;14:1084–1087. doi: 10.1111/j.1442-2042.2007.01896.x. [DOI] [PubMed] [Google Scholar]
- 79.Yin M, Bastacky S, Parwani AV, McHale T, Dhir R. P16ink4 immunoreactivity is a reliable marker for urothelial carcinoma in situ. Hum Pathol. 2008;39:527–535. doi: 10.1016/j.humpath.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Kruger S, Mahnken A, Kausch I, Feller AC. P16 immunoreactivity is an independent predictor of tumor progression in minimally invasive urothelial bladder carcinoma. Eur Urol. 2005;47:463–467. doi: 10.1016/j.eururo.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 81.Sgambato A, Migaldi M, Faraglia B, et al. Loss of P27Kip1 expression correlates with tumor grade and with reduced disease-free survival in primary superficial bladder cancers. Cancer Res. 1999;59:3245–3250. [PubMed] [Google Scholar]
- 82.Shariat SF, Karakiewicz PI, Ashfaq R, et al. Multiple biomarkers improve prediction of bladder cancer recurrence and mortality in patients undergoing cystectomy. Cancer. 2008;112:315–325. doi: 10.1002/cncr.23162. [DOI] [PubMed] [Google Scholar]
- 83.Shariat SF, Bolenz C, Godoy G, et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol. 2009;182:78–84. doi: 10.1016/j.juro.2009.02.125. [discussion in J Urol. 2009;182:84]. [DOI] [PubMed] [Google Scholar]
- 84.Dalbagni G, Presti JC, Jr, Reuter VE, et al. Molecular genetic alterations of chromosome 17 and p53 nuclear overexpression in human bladder cancer. Diagn Mol Pathol. 1993;2:4–13. [PubMed] [Google Scholar]
- 85.Quintero A, Alvarez-Kindelan J, Luque RJ, et al. Ki-7 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. J Clin Pathol. 2006;59:83–88. doi: 10.1136/jcp.2004.022939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Margulis V, Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Ki-67 is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for organ-confined disease. Clin Cancer Res. 2006;12:7369–7373. doi: 10.1158/1078-0432.CCR-06-1472. [DOI] [PubMed] [Google Scholar]
- 87.Margulis V, Lotan Y, Karakiewicz PI, et al. Multi-institutional validation of the predictive value of ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst. 2009;101:114–119. doi: 10.1093/jnci/djn451. [DOI] [PubMed] [Google Scholar]
- 88.Ramos D, Ruiz A, Morell L, et al. Prognostic value of morphometry in low grade papillary urothelial bladder neoplasms. Anal Quant Cytol Histol. 2004;26:285–294. [PubMed] [Google Scholar]
- 89.Cheng L, Zhang S, Maclennan GT, Williamson SR, Lopez-Beltran A, Montironi R. Bladder cancer: translating molecular genetic insights into clinical practice. Hum Pathol. 2011;42(4):455–481. doi: 10.1016/j.humpath.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 90.Miyamoto H, Brimo F, Schultz L, et al. Low-grade papillary urothelial carcinoma of the urinary bladder: a clinicopathologic analysis of a post-World Health Organization/International Society of Urological Pathology classification cohort from a single academic center. Arch Pathol Lab Med. 2010;134:1160–1163. doi: 10.5858/2009-0403-OA.1. [DOI] [PubMed] [Google Scholar]
- 91.Mitra AP, Pagliarulo V, Yang D, et al. Generation of a concise gene panel for outcome prediction in urinary bladder cancer. J Clin Oncol. 2009;27:3929–3937. doi: 10.1200/JCO.2008.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rothman N, Garcia-Closas M, Chatterjee N, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. 2010;42:978–984. doi: 10.1038/ng.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanchez-Carbayo M, Cordon-Cardo C. Molecular alterations associated with bladder cancer progression. Semin Oncol. 2007;34:75–84. doi: 10.1053/j.seminoncol.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 94.Serizawa RR, Ralfkiaer U, Steven K, et al. Integrated genetic and epigenetic analysis of bladder cancer reveals an additive diagnostic value of FGFR3 mutations and hypermethylation events. Int J Cancer. 2011;129(1):78–87. doi: 10.1002/ijc.25651. [DOI] [PubMed] [Google Scholar]
- 95.Lindgren D, Frigyesi A, Gudjonsson S, et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res. 2010;70:3463–3472. doi: 10.1158/0008-5472.CAN-09-4213. [DOI] [PubMed] [Google Scholar]
- 96.Heidenblad M, Lindgren D, Jonson T, et al. Tiling resolution array CGH and high density expression profiling of urothelial carcinomas delineate genomic amplicons and candidate target genes specific for advanced tumors. BMC Med Genomics. 2008;1:3. doi: 10.1186/1755-8794-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lindgren D, Liedberg F, Andersson A, et al. Molecular characterization of early-stage bladder carcinomas by expression profiles, FGFR3 mutation status, and loss of 9q. Oncogene. 2006;25:2685–2696. doi: 10.1038/sj.onc.1209249. [DOI] [PubMed] [Google Scholar]
- 98.Nishiyama N, Arai E, Chihara Y, et al. Genome-wide DNA methylation profiles in urothelial carcinomas and urothelia at the precancerous stage. Cancer Sci. 2010;101:231–240. doi: 10.1111/j.1349-7006.2009.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin HH, Ke HL, Huang SP, Wu WJ, Chen YK, Chang LL. Increase sensitivity in detecting superficial, low grade bladder cancer by combination analysis of hypermethylation of E-cadherin, p16, p14, RASSF1A genes in urine. Urol Oncol. 2010;28:597–602. doi: 10.1016/j.urolonc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 100.Vinci S, Giannarini G, Selli C, et al. Quantitative methylation analysis of BCL2, hTERT, and DAPK promoters in urine sediment for the detection of non-muscle-invasive urothelial carcinoma of the bladder: a prospective, two-center validation study. Urol Oncol. 2011;29(2):150–156. doi: 10.1016/j.urolonc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 101.Cabello MJ, Grau L, Franco N, et al. Multiplexed methylation profiles of tumor suppressor genes in bladder cancer. J Mol Diagn. 2011;13:29–40. doi: 10.1016/j.jmoldx.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vallot C, Stransky N, Bernard-Pierrot I, et al. A novel epigenetic phenotype associated with the most aggressive pathway of bladder tumor progression. J Natl Cancer Inst. 2011;103:47–60. doi: 10.1093/jnci/djq470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dudziec E, Miah S, Choudhry H, et al. Hypermethylation of CpG islands and shores around specific MicroRNAs and mirtrons is associated with the phenotype and presence of bladder cancer. Clin Cancer Res. 2011;17(6):1287–1296. doi: 10.1158/1078-0432.CCR-10-2017. [DOI] [PubMed] [Google Scholar]
- 104.Wiklund ED, Bramsen JB, Hulf T, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 105.Catto JW, Azzouzi AR, Rehman I, et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol. 2005;23:2903–2910. doi: 10.1200/JCO.2005.03.163. [DOI] [PubMed] [Google Scholar]
- 106.Friedrich MG, Weisenberger DJ, Cheng JC, et al. Detection of methylated apoptosis-associated genes in urine sediments of bladder cancer patients. Clin Cancer Res. 2004;10:7457–7465. doi: 10.1158/1078-0432.CCR-04-0930. [DOI] [PubMed] [Google Scholar]
- 107.Chan MW, Chan LW, Tang NL, et al. Hypermethylation of multiple genes in tumor tissues and voided urine in urinary bladder cancer patients. Clin Cancer Res. 2002;8:464–470. [PubMed] [Google Scholar]
- 108.Yates DR, Rehman I, Meuth M, Cross SS, Hamdy FC, Catto JW. Methylational urinalysis: a prospective study of bladder cancer patients and age stratified benign controls. Oncogene. 2006;25:1984–1988. doi: 10.1038/sj.onc.1209209. [DOI] [PubMed] [Google Scholar]
- 109.Hoque MO, Begum S, Topaloglu O, et al. Quantitation of promoter methylation of multiple genes in urine DNA and bladder cancer detection. J Natl Cancer Inst. 2006;98:996–1004. doi: 10.1093/jnci/djj265. [DOI] [PubMed] [Google Scholar]
- 110.Yates DR, Rehman I, Abbod MF, et al. Promoter hypermethylation identifies progression risk in bladder cancer. Clin Cancer Res. 2007;13:2046–2053. doi: 10.1158/1078-0432.CCR-06-2476. [DOI] [PubMed] [Google Scholar]
- 111.Ali-El-Dein B, Sarhan O, Hinev A, Ibrahiem eI, Nabeeh A, Ghoneim MA. Superficial bladder tumours: analysis of prognostic factors and construction of a predictive index. BJU Int. 2003;92:393–399. doi: 10.1046/j.1464-410x.2003.04360.x. [DOI] [PubMed] [Google Scholar]
- 112.Loughman NT, Lin BP, Dent OF, Newland RC. DNA ploidy of bladder cancer using bladder biopsy supernate specimens. Anal Quant Cytol Histol. 2003;25:146–158. [PubMed] [Google Scholar]
- 113.Baak JP, Bol MG, van Diermen B, et al. DNA cytometric features in biopsies of TaT1 urothelial cell cancer predict recurrence and stage progression more accurately than stage, grade, or treatment modality. Urology. 2003;61:1266–1272. doi: 10.1016/s0090-4295(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 114.Bol MG, Baak JP, Diermen B, Janssen EA, Buhr-Wildhagen SB, Kjellevold KH. Correlation of grade of urothelial cell carcinomas and DNA histogram features assessed by flow cytometry and automated image cytometry. Anal Cell Pathol. 2003;25:147–153. doi: 10.1155/2003/410239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bellaoui H, Chefchaouni MC, Lazrak N, Khalfaoui LC, Yassine F, Elhamany Z. Flow cytometric DNA analysis and cytology in diagnosis and prognosis of bladder tumors: preliminary results of a comparative study of bladder lavage [in French] Ann Urol (Paris) 2002;36:45–52. doi: 10.1016/s0003-4401(01)00071-7. [DOI] [PubMed] [Google Scholar]
- 116.Caraway NP, Khanna A, Payne L, Kamat AM, Katz RL. Combination of cytologic evaluation and quantitative digital cytometry is reliable in detecting recurrent disease in patients with urinary diversions. Cancer. 2007;111:323–329. doi: 10.1002/cncr.22950. [DOI] [PubMed] [Google Scholar]
- 117.Falkman K, Tribukait B, Nyman CR, Larsson P, Norming U. S-phase fraction in superficial urothelial carcinoma of the bladder–a prospective, long-term, follow-up study. Scand J Urol Nephrol. 2004;38:278–284. doi: 10.1080/00365590410031760. [DOI] [PubMed] [Google Scholar]
- 118.Lin J, Dinney CP, Grossman HB, et al. E-cadherin promoter polymorphism (C-160A) and risk of recurrence in patients with superficial bladder cancer. Clin Genet. 2006;70:240–245. doi: 10.1111/j.1399-0004.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 119.Palit V, Phillips RM, Puri R, Shah T, Bibby MC. Expression of HIF-1alpha and glut-1 in human bladder cancer. Oncol Rep. 2005;14:909–913. doi: 10.3892/or.14.4.909. [DOI] [PubMed] [Google Scholar]
- 120.Chai CY, Chen WT, Hung WC, et al. Hypoxia-inducible factor-1alpha expression correlates with focal macrophage infiltration, angiogenesis and unfavourable prognosis in urothelial carcinoma. J Clin Pathol. 2008;61:658–664. doi: 10.1136/jcp.2007.050666. [DOI] [PubMed] [Google Scholar]
- 121.Crew JP, O’Brien T, Bicknell R, Fuggle S, Cranston D, Harris AL. Urinary vascular endothelial growth factor and its correlation with bladder cancer recurrence rates. J Urol. 1999;161:799–804. [PubMed] [Google Scholar]
- 122.Crew JP, O’Brien T, Bradburn M, et al. Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. Cancer Res. 1997;57:5281–5285. [PubMed] [Google Scholar]
- 123.Turner KJ, Crew JP, Wykoff CC, et al. The hypoxia-inducible genes VEGF and CA9 are differentially regulated in superficial vs invasive bladder cancer. Br J Cancer. 2002;86:1276–1282. doi: 10.1038/sj.bjc.6600215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tickoo SK, Milowsky MI, Dhar N, et al. Hypoxia-inducible factor and mammalian target of rapamycin pathway markers in urothelial carcinoma of the bladder: possible therapeutic implications. BJU Int. 2011;107:844–849. doi: 10.1111/j.1464-410X.2010.09517.x. [DOI] [PubMed] [Google Scholar]
- 125.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–6017. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 126.Schultz L, Albadine R, Hicks J, et al. Expression status and prognostic significance of mammalian target of rapamycin pathway members in urothelial carcinoma of urinary bladder after cystectomy. Cancer. 2010;116:5517–5526. doi: 10.1002/cncr.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Comperat E, Camparo P, Haus R, et al. Aurora-A/STK-15 is a predictive factor for recurrent behaviour in non-invasive bladder carcinoma: a study of 128 cases of non-invasive neoplasms. Virchows Arch. 2007;450:419–424. doi: 10.1007/s00428-007-0383-x. [DOI] [PubMed] [Google Scholar]
- 128.Mhawech-Fauceglia P, Fischer G, Beck A, Cheney RT, Herrmann FR. Raf1, aurora-A/STK15 and E-cadherin biomarkers expression in patients with pTa/pT1 urothelial bladder carcinoma; a retrospective TMA study of 246 patients with long-term follow-up. Eur J Surg Oncol. 2006;32:439–444. doi: 10.1016/j.ejso.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 129.Veerla S, Panagopoulos I, Jin Y, Lindgren D, Hoglund M. Promoter analysis of epigenetically controlled genes in bladder cancer. Genes Chromosomes Cancer. 2008;47:368–378. doi: 10.1002/gcc.20542. [DOI] [PubMed] [Google Scholar]
- 130.Wszolek MF, Rieger-Christ KM, Kenney PA, et al. A MicroRNA expression profile defining the invasive bladder tumor phenotype. Urol Oncol. 2011;29(6):794.e1–801.e1. doi: 10.1016/j.urolonc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 131.Catto JW, Miah S, Owen HC, et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res. 2009;69:8472–8481. doi: 10.1158/0008-5472.CAN-09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iyer G, Milowsky MI, Bajorin DF. Novel strategies for treating relapsed/refractory urothelial carcinoma. Expert Rev Anticancer Ther. 2010;10:1917–1932. doi: 10.1586/era.10.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wallerand H, Reiter RR, Ravaud A. Molecular targeting in the treatment of either advanced or metastatic bladder cancer or both according to the signalling pathways. Curr Opin Urol. 2008;18:524–532. doi: 10.1097/MOU.0b013e3283097889. [DOI] [PubMed] [Google Scholar]
- 134.Amit D, Tamir S, Birman T, Gofrit ON, Hochberg A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of IGF2-P3 and IGF2-P4 regulatory sequences. Int J Clin Exp Med. 2011;4:91–102. [PMC free article] [PubMed] [Google Scholar]
- 135.Gerullis H, Ecke TH, Janusch B, et al. Long-term response in advanced bladder cancer involving the use of temsirolimus and vinflunine after platin resistance. Anticancer Drugs. 2011;22(9):940–943. doi: 10.1097/CAD.0b013e328347a86a. [DOI] [PubMed] [Google Scholar]
- 136.Yafi FA, North S, Kassouf W. First- and second-line therapy for metastatic urothelial carcinoma of the bladder. Curr Oncol. 2011;18:e25–e34. doi: 10.3747/co.v18i1.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang X, Godbey WT. Preclinical evaluation of a gene therapy treatment for transitional cell carcinoma. Cancer Gene Ther. 2011;18:34–41. doi: 10.1038/cgt.2010.50. [DOI] [PubMed] [Google Scholar]
- 138.Smaldone MC, Davies BJ. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr Opin Mol Ther. 2010;12:607–616. [PubMed] [Google Scholar]
- 139.Kramer MW, Krege S, Peters I, Merseburger AS, Kuczyk MA. Targeted therapy of urological tumours. experimental field or established therapeutic approach? [in German] Urologe A. 2010;49:1260–1265. doi: 10.1007/s00120-010-2397-2. [DOI] [PubMed] [Google Scholar]
- 140.Ching CB, Hansel DE. Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Lab Invest. 2010;90:1406–1414. doi: 10.1038/labinvest.2010.133. [DOI] [PubMed] [Google Scholar]
- 141.Black PC, Agarwal PK, Dinney CP. Targeted therapies in bladder cancer—an update. Urol Oncol. 2007;25:433–438. doi: 10.1016/j.urolonc.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 142.Black PC, Dinney CP. Bladder cancer angiogenesis and metastasis—translation from murine model to clinical trial. Cancer Metastasis Rev. 2007;26:623–634. doi: 10.1007/s10555-007-9084-9. [DOI] [PubMed] [Google Scholar]
- 143.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 144.Bellmunt J, Hussain M, Dinney CP. Novel approaches with targeted therapies in bladder cancer. therapy of bladder cancer by blockade of the epidermal growth factor receptor family. Crit Rev Oncol Hematol. 2003;46(suppl):S85–S104. doi: 10.1016/s1040-8428(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 145.Wallerand H, Robert G, Bernhard JC, Ravaud A, Ferriere JM. Targeted therapy for locally advanced and/or metastatic bladder cancer. Prog Urol. 2008;18:407–417. doi: 10.1016/j.purol.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 146.Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II national cancer institute trial. J Clin Oncol. 2007;25:2218–2224. doi: 10.1200/JCO.2006.08.0994. [DOI] [PubMed] [Google Scholar]
- 147.Hansel DE, Swain E, Dreicer R, Tubbs RR. HER2 overexpression and amplification in urothelial carcinoma of the bladder is associated with MYC coamplification in a subset of cases. Am J Clin Pathol. 2008;130:274–281. doi: 10.1309/41VLTFX3YPP1HF6F. [DOI] [PubMed] [Google Scholar]
- 148.Inoue K, Slaton JW, Perrotte P, et al. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin Cancer Res. 2000;6:4874–4884. [PubMed] [Google Scholar]
- 149.Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- 150.Philips GK, Halabi S, Sanford BL, Bajorin D, Small EJ Cancer and Leukaemia Group B. A phase II trial of cisplatin, fixed dose-rate gemcitabine and gefitinib for advanced urothelial tract carcinoma: results of the cancer and leukaemia group B 90102. BJU Int. 2008;101:20–25. doi: 10.1111/j.1464-410X.2007.07226.x. [DOI] [PubMed] [Google Scholar]
- 151.Philips GK, Halabi S, Sanford BL, Bajorin D, Small EJ Cancer and Leukemia Group B. A phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of cancer and leukemia group B (CALGB) 90102. Ann Oncol. 2009;20:1074–1079. doi: 10.1093/annonc/mdn749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wulfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115:2881–2890. doi: 10.1002/cncr.24337. [DOI] [PubMed] [Google Scholar]
- 153.Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol. 2011;29:1525–1530. doi: 10.1200/JCO.2010.31.6067. [DOI] [PubMed] [Google Scholar]
- 154.Elfiky AA, Rosenberg JE. Targeting angiogenesis in bladder cancer. Curr Oncol Rep. 2009;11:244–249. doi: 10.1007/s11912-009-0034-2. [DOI] [PubMed] [Google Scholar]
- 155.Bradley DA, Dunn R, Nanus D, et al. Randomized, double-blind, placebo-controlled phase II trial of maintenance sunitinib versus placebo after chemotherapy for patients with advanced urothelial carcinoma: scientific rationale and study design. Clin Genitourin Cancer. 2007;5:460–463. doi: 10.3816/CGC.2007.n.037. [DOI] [PubMed] [Google Scholar]
- 156.Miyamoto H, Miller JS, Fajardo DA, Lee TK, Netto GJ, Epstein JI. Non-invasive papillary urothelial neoplasms: the 2004 WHO/ISUP classification system. Pathol Int. 2010;60:1–8. doi: 10.1111/j.1440-1827.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 157.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodriguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000;163:73–78. doi: 10.1016/s0022-5347(05)67975-x. [DOI] [PubMed] [Google Scholar]
- 158.Soloway MS, Sofer M, Vaidya A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J Urol. 2002;167:1573–1583. [PubMed] [Google Scholar]
- 159.Malekzadeh K, Sobti RC, Nikbakht M, et al. Methylation patterns of Rb1 and casp-8 promoters and their impact on their expression in bladder cancer. Cancer Invest. 2009;27:70–80. doi: 10.1080/07357900802172085. [DOI] [PubMed] [Google Scholar]
- 160.van der Kwast TH, Bapat B. Predicting favourable prognosis of urothelial carcinoma: gene expression and genome profiling. Curr Opin Urol. 2009;19:516–521. doi: 10.1097/MOU.0b013e32832eb45f. [DOI] [PubMed] [Google Scholar]
- 161.Wilhelm-Benartzi CS, Koestler DC, Houseman EA, et al. DNA methylation profiles delineate etiologic heterogeneity and clinically important subgroups of bladder cancer. Carcinogenesis. 2010;31:1972–1976. doi: 10.1093/carcin/bgq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized european study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 164.Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715–717. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stephenson AJ, Scardino PT, Eastham JA, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005–7012. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and gleason score to predict pathological stage of localized prostate cancer: a multi-institutional update. JAMA. 1997;277:1445–1451. [PubMed] [Google Scholar]
- 167.Amin M, Boccon-Gibod L, Egevad L, et al. Prognostic and predictive factors and reporting of prostate carcinoma in prostate needle biopsy specimens. Scand J Urol Nephrol Suppl. 2005;(216):20–33. doi: 10.1080/03008880510030923. [DOI] [PubMed] [Google Scholar]
- 168.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–964. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 169.De Marzo AM, DeWeese TL, Platz EA, et al. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91:459–477. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 170.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 171.Srigley JR, Amin M, Boccon-Gibod L, et al. Prognostic and predictive factors in prostate cancer: historical perspectives and recent international consensus initiatives. Scand J Urol Nephrol Suppl. 2005;(216):8–19. doi: 10.1080/03008880510030914. [DOI] [PubMed] [Google Scholar]
- 172.Bethel CR, Faith D, Li X, et al. Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: association with gleason score and chromosome 8p deletion. Cancer Res. 2006;66:10683–10690. doi: 10.1158/0008-5472.CAN-06-0963. [DOI] [PubMed] [Google Scholar]
- 173.Prowatke I, Devens F, Benner A, et al. Expression analysis of imbalanced genes in prostate carcinoma using tissue microarrays. Br J Cancer. 2007;96:82–88. doi: 10.1038/sj.bjc.6603490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khan MA, Partin AW. Tissue microarrays in prostate cancer research. Rev Urol. 2004;6:44–46. [PMC free article] [PubMed] [Google Scholar]
- 175.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 176.Tomlins SA, Mehra R, Rhodes DR, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66:3396–3400. doi: 10.1158/0008-5472.CAN-06-0168. [DOI] [PubMed] [Google Scholar]
- 177.Toubaji A, Albadine R, Meeker AK, et al. Increased gene copy number of ERG on chromosome 21 but not TMPRSS2-ERG fusion predicts outcome in prostatic adenocarcinomas. Mod Pathol. 2011;24(11):1511–1520. doi: 10.1038/modpathol.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gopalan A, Leversha MA, Satagopan JM, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69:1400–1406. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Demichelis F, Fall K, Perner S, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–4599. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 180.Lotan TL, Toubaji A, Albadine R, et al. TMPRSS2-ERG gene fusions are infrequent in prostatic ductal adenocarcinomas. Mod Pathol. 2009;22(3):359–365. doi: 10.1038/modpathol.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yoshimoto M, Joshua AM, Cunha IW, et al. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol. 2008;21:1451–1460. doi: 10.1038/modpathol.2008.96. [DOI] [PubMed] [Google Scholar]
- 182.FitzGerald LM, Agalliu I, Johnson K, et al. Association of TMPRSS2-ERG gene fusion with clinical characteristics and outcomes: results from a population-based study of prostate cancer. BMC Cancer. 2008;8:230. doi: 10.1186/1471-2407-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mao X, Shaw G, James SY, et al. Detection of TMPRSS2:ERG fusion gene in circulating prostate cancer cells. Asian J Androl. 2008;10:467–473. doi: 10.1111/j.1745-7262.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 184.Perner S, Mosquera JM, Demichelis F, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–888. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 185.Saramaki OR, Harjula AE, Martikainen PM, Vessella RL, Tammela TL, Visakorpi T. TMPRSS2:ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res. 2008;14:3395–3400. doi: 10.1158/1078-0432.CCR-07-2051. [DOI] [PubMed] [Google Scholar]
- 186.Falzarano SM, Navas M, Simmerman K, et al. ERG rearrangement is present in a subset of transition zone prostatic tumors. Mod Pathol. 2010;23:1499–1506. doi: 10.1038/modpathol.2010.150. [DOI] [PubMed] [Google Scholar]
- 187.Netto GJ. TMPRSS2-ERG fusion as a marker of prostatic lineage in small-cell carcinoma [author reply in Histopathology. 2010;57:633–634] Histopathology. 2010;57:633. doi: 10.1111/j.1365-2559.2010.03659.x. [DOI] [PubMed] [Google Scholar]
- 188.Albadine R, Latour M, Toubaji A, et al. TMPRSS2-ERG gene fusion status in minute (minimal) prostatic adenocarcinoma. Mod Pathol. 2009;22:1415–1422. doi: 10.1038/modpathol.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rostad K, Hellwinkel OJ, Haukaas SA, et al. TMPRSS2:ERG fusion transcripts in urine from prostate cancer patients correlate with a less favorable prognosis. APMIS. 2009;117:575–582. doi: 10.1111/j.1600-0463.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- 190.Rice KR, Chen Y, Ali A, et al. Evaluation of the ETS-related gene mRNA in urine for the detection of prostate cancer. Clin Cancer Res. 2010;16:1572–1576. doi: 10.1158/1078-0432.CCR-09-2191. [DOI] [PubMed] [Google Scholar]
- 191.Nguyen PN, Violette P, Chan S, et al. A panel of TMPRSS2:ERG fusion transcript markers for urine-based prostate cancer detection with high specificity and sensitivity. Eur Urol. 2011;59:407–414. doi: 10.1016/j.eururo.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 192.Park K, Tomlins SA, Mudaliar KM, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–598. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chaux A, Albadine R, Toubaji A, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol. 2011;35:1014–1020. doi: 10.1097/PAS.0b013e31821e8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Epstein JI, Amin M, Boccon-Gibod L, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl. 2005;(216):34–63. doi: 10.1080/03008880510030932. [DOI] [PubMed] [Google Scholar]
- 195.Hammond ME, Fitzgibbons PL, Compton CC, et al. Cancer Committee and Conference Participants. College of American Pathologists Conference XXXV: solid tumor prognostic factors—which, how and so what: summary document and recommendations for implementation. Arch Pathol Lab Med. 2000;124:958–965. doi: 10.5858/2000-124-0958-COAPCX. [DOI] [PubMed] [Google Scholar]
- 196.Bostwick DG, Grignon DJ, Hammond ME, et al. Prognostic factors in prostate cancer: College of American Pathologists consensus statement 1999. Arch Pathol Lab Med. 2000;124:995–1000. doi: 10.5858/2000-124-0995-PFIPC. [DOI] [PubMed] [Google Scholar]
- 197.Armstrong AJ, Netto GJ, Rudek MA, et al. A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res. 2010;16:3057–3066. doi: 10.1158/1078-0432.CCR-10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kremer CL, Klein RR, Mendelson J, et al. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate. 2006;66:1203–1212. doi: 10.1002/pros.20410. [DOI] [PubMed] [Google Scholar]
- 199.Sanchez D, Rosell D, Honorato B, Lopez J, Arocena J, Sanz G. Androgen receptor mutations are associated with gleason score in localized prostate cancer. BJU Int. 2006;98:1320–1325. doi: 10.1111/j.1464-410X.2006.06438.x. [DOI] [PubMed] [Google Scholar]
- 200.Wikstrom P, Bergh A, Damber JE. Transforming growth factor-beta1 and prostate cancer. Scand J Urol Nephrol. 2000;34:85–94. doi: 10.1080/003655900750016689. [DOI] [PubMed] [Google Scholar]
- 201.Wikstrom P, Damber J, Bergh A. Role of transforming growth factor-beta1 in prostate cancer. Microsc Res Tech. 2001;52:411–419. doi: 10.1002/1097-0029(20010215)52:4<411::AID-JEMT1026>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 202.Diaz JI, Mora LB, Austin PF, et al. Predictability of PSA failure in prostate cancer by computerized cytometric assessment of tumoral cell proliferation. Urology. 1999;53:931–938. doi: 10.1016/s0090-4295(98)00625-6. [DOI] [PubMed] [Google Scholar]
- 203.Keshgegian AA, Johnston E, Cnaan A. Bcl-2 oncoprotein positivity and high MIB1 (ki-67) proliferative rate are independent predictive markers for recurrence in prostate carcinoma. Am J Clin Pathol. 1998;110:443–449. doi: 10.1093/ajcp/110.4.443. [DOI] [PubMed] [Google Scholar]
- 204.Bubendorf L, Tapia C, Gasser TC, et al. Ki67 labeling index in core needle biopsies independently predicts tumor-specific survival in prostate cancer. Hum Pathol. 1998;29:949–954. doi: 10.1016/s0046-8177(98)90199-x. [DOI] [PubMed] [Google Scholar]
- 205.Bettencourt MC, Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, Moul JW. Ki-67 expression is a prognostic marker of prostate cancer recurrence after radical prostatectomy. J Urol. 1996;156:1064–1068. [PubMed] [Google Scholar]
- 206.Cheng L, Pisansky TM, Sebo TJ, et al. Cell proliferation in prostate cancer patients with lymph node metastasis: a marker for progression. Clin Cancer Res. 1999;5:2820–2823. [PubMed] [Google Scholar]
- 207.Stapleton AM, Zbell P, Kattan MW, et al. Assessment of the biologic markers p53, ki-67, and apoptotic index as predictive indicators of prostate carcinoma recurrence after surgery. Cancer. 1998;82:168–175. doi: 10.1002/(sici)1097-0142(19980101)82:1<168::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 208.Vis AN, van Rhijn BW, Noordzij MA, Schroder FH, van der Kwast TH. Value of tissue markers p27(kip1), MIB1, and CD44s for the pre-operative prediction of tumour features in screen-detected prostate cancer. J Pathol. 2002;197:148–154. doi: 10.1002/path.1084. [DOI] [PubMed] [Google Scholar]
- 209.Bostwick DG, Wheeler TM, Blute M, et al. Optimized microvessel density analysis improves prediction of cancer stage from prostate needle biopsies. Urology. 1996;48:47–57. doi: 10.1016/s0090-4295(96)00149-5. [DOI] [PubMed] [Google Scholar]
- 210.Silberman MA, Partin AW, Veltri RW, Epstein JI. Tumor angiogenesis correlates with progression after radical prostatectomy but not with pathologic stage in gleason sum 5 to 7 adenocarcinoma of the prostate. Cancer. 1997;79:772–779. doi: 10.1002/(sici)1097-0142(19970215)79:4<772::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 211.Strohmeyer D, Rossing C, Strauss F, Bauerfeind A, Kaufmann O, Loening S. Tumor angiogenesis is associated with progression after radical prostatectomy in pT2/pT3 prostate cancer. Prostate. 2000;42:26–33. doi: 10.1002/(sici)1097-0045(20000101)42:1<26::aid-pros4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 212.Strohmeyer D, Strauss F, Rossing C, et al. Expression of bFGF, VEGF and c-met and their correlation with microvessel density and progression in prostate carcinoma. Anticancer Res. 2004;24:1797–1804. [PubMed] [Google Scholar]
- 213.Gettman MT, Bergstralh EJ, Blute M, Zincke H, Bostwick DG. Prediction of patient outcome in pathologic stage T2 adenocarcinoma of the prostate: lack of significance for microvessel density analysis. Urology. 1998;51:79–85. doi: 10.1016/s0090-4295(97)00464-0. [DOI] [PubMed] [Google Scholar]
- 214.Gettman MT, Pacelli A, Slezak J, et al. Role of microvessel density in predicting recurrence in pathologic stage T3 prostatic adenocarcinoma. Urology. 1999;54:479–485. doi: 10.1016/s0090-4295(99)00202-2. [DOI] [PubMed] [Google Scholar]
- 215.Krupski T, Petroni GR, Frierson HF, Jr, Theodorescu JU. Microvessel density, p53, retinoblastoma, and chromogranin A immunohistochemistry as predictors of disease-specific survival following radical prostatectomy for carcinoma of the prostate. Urology. 2000;55:743–749. doi: 10.1016/s0090-4295(99)00598-1. [DOI] [PubMed] [Google Scholar]
- 216.Brewster SF, Oxley JD, Trivella M, Abbott CD, Gillatt DA. Preoperative p53, bcl-2, CD44 and E-cadherin immunohistochemistry as predictors of biochemical relapse after radical prostatectomy. J Urol. 1999;161:1238–1243. [PubMed] [Google Scholar]
- 217.Stackhouse GB, Sesterhenn IA, Bauer JJ, et al. p53 and bcl-2 immunohistochemistry in pretreatment prostate needle biopsies to predict recurrence of prostate cancer after radical prostatectomy. J Urol. 1999;162:2040–2045. doi: 10.1016/S0022-5347(05)68095-0. [DOI] [PubMed] [Google Scholar]
- 218.Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, Srivastava S, Moul JW. Elevated levels of apoptosis regulator proteins p53 and bcl-2 are independent prognostic biomarkers in surgically treated clinically localized prostate cancer. J Urol. 1996;156:1511–1516. [PubMed] [Google Scholar]
- 219.Bauer JJ, Sesterhenn IA, Mostofi KF, McLeod DG, Srivastava S, Moul JW. p53 nuclear protein expression is an independent prognostic marker in clinically localized prostate cancer patients undergoing radical prostatectomy. Clin Cancer Res. 1995;1:1295–1300. [PubMed] [Google Scholar]
- 220.Moul JW, Bettencourt MC, Sesterhenn IA, et al. Protein expression of p53, bcl-2, and KI-67 (MIB1) as prognostic biomarkers in patients with surgically treated, clinically localized prostate cancer. Surgery. 1996;120:159–166. doi: 10.1016/s0039-6060(96)80283-2. [discussion in Surgery. 1996;120:166–167]. [DOI] [PubMed] [Google Scholar]
- 221.Osman I, Drobnjak M, Fazzari M, Ferrara J, Scher HI, Cordon-Cardo C. Inactivation of the p53 pathway in prostate cancer: Impact on tumor progression. Clin Cancer Res. 1999;5:2082–2088. [PubMed] [Google Scholar]
- 222.Theodorescu D, Broder SR, Boyd JC, Mills SE, Frierson HF., Jr p53, bcl-2 and retinoblastoma proteins as long-term prognostic markers in localized carcinoma of the prostate. J Urol. 1997;158:131–137. doi: 10.1097/00005392-199707000-00040. [DOI] [PubMed] [Google Scholar]
- 223.Kuczyk MA, Serth J, Bokemeyer C, et al. The prognostic value of p53 for long-term and recurrence-free survival following radical prostatectomy. Eur J Cancer. 1998;34:679–686. doi: 10.1016/s0959-8049(97)10112-5. [DOI] [PubMed] [Google Scholar]
- 224.Cheng L, Lloyd RV, Weaver AL, et al. The cell cycle inhibitors p21WAF1 and p27KIP1 are associated with survival in patients treated by salvage prostatectomy after radiation therapy. Clin Cancer Res. 2000;6:1896–1899. [PubMed] [Google Scholar]
- 225.Wu Y, Chhipa RR, Cheng J, Zhang H, Mohler JL, Ip C. Androgen receptor-mTOR crosstalk is regulated by testosterone availability: Implication for prostate cancer cell survival. Anticancer Res. 2010;30:3895–3901. [PMC free article] [PubMed] [Google Scholar]
- 226.Bismar TA, Yoshimoto M, Vollmer RT, et al. PTEN genomic deletion is an early event associated with ERG gene rearrangements in prostate cancer. BJU Int. 2011;107(3):477–485. doi: 10.1111/j.1464-410X.2010.09470.x. [DOI] [PubMed] [Google Scholar]
- 227.Bubendorf L. Words of wisdom, re: aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Eur Urol. 2009;56:882–883. doi: 10.1016/j.eururo.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 228.Han B, Mehra R, Lonigro RJ, et al. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol. 2009;22:1083–1093. doi: 10.1038/modpathol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.King JC, Xu J, Wongvipat J, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 231.Squire JA. TMPRSS2-ERG and PTEN loss in prostate cancer. Nat Genet. 2009;41:509–510. doi: 10.1038/ng0509-509. [DOI] [PubMed] [Google Scholar]
- 232.Lacombe L, Maillette A, Meyer F, Veilleux C, Moore L, Fradet Y. Expression of p21 predicts PSA failure in locally advanced prostate cancer treated by prostatectomy. Int J Cancer. 2001;95:135–139. doi: 10.1002/1097-0215(20010520)95:3<135::aid-ijc1023>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 233.Aslan G, Irer B, Tuna B, Yorukoglu K, Saatcioglu F, Celebi I. Analysis of NKX3.1 expression in prostate cancer tissues and correlation with clinicopathologic features. Pathol Res Pract. 2006;202:93–98. doi: 10.1016/j.prp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 234.Gurel B, Iwata T, Koh CM, et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod Pathol. 2008;21:1156–1167. doi: 10.1038/modpathol.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gurel B, Iwata T, Koh CM, Yegnasubramanian S, Nelson WG, De Marzo AM. Molecular alterations in prostate cancer as diagnostic, prognostic, and therapeutic targets. Adv Anat Pathol. 2008;15:319–331. doi: 10.1097/PAP.0b013e31818a5c19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Veltri RW, Partin AW, Epstein JE, et al. Quantitative nuclear morphometry, markovian texture descriptors, and DNA content captured on a CAS-200 image analysis system, combined with PCNA and HER-2/neu immunohistochemistry for prediction of prostate cancer progression. J Cell Biochem Suppl. 1994;19:249–258. [PubMed] [Google Scholar]
- 237.Ross JS, Sheehan CE, Hayner-Buchan AM, et al. Prognostic significance of HER-2/neu gene amplification status by fluorescence in situ hybridization of prostate carcinoma. Cancer. 1997;79:2162–2170. doi: 10.1002/(sici)1097-0142(19970601)79:11<2162::aid-cncr14>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 238.Lapointe J, Li C, Higgins JP, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 240.Lapointe J, Li C, Giacomini CP, et al. Genomic profiling reveals alternative genetic pathways of prostate tumorigenesis. Cancer Res. 2007;67:8504–8510. doi: 10.1158/0008-5472.CAN-07-0673. [DOI] [PubMed] [Google Scholar]
- 241.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bastian PJ, Ellinger J, Wellmann A, et al. Diagnostic and prognostic information in prostate cancer with the help of a small set of hypermethylated gene loci. Clin Cancer Res. 2005;11:4097–4106. doi: 10.1158/1078-0432.CCR-04-1832. [DOI] [PubMed] [Google Scholar]
- 243.Bastian PJ, Nakayama M, De Marzo AM, Nelson WG. GSTP1 CpG island hypermethylation as a molecular marker of prostate cancer [in German] Urologe A. 2004;43:573–579. doi: 10.1007/s00120-004-0540-7. [DOI] [PubMed] [Google Scholar]
- 244.Bastian PJ, Palapattu GS, Lin X, et al. Preoperative serum DNA GSTP1 CpG island hypermethylation and the risk of early prostate-specific antigen recurrence following radical prostatectomy. Clin Cancer Res. 2005;11:4037–4043. doi: 10.1158/1078-0432.CCR-04-2446. [DOI] [PubMed] [Google Scholar]
- 245.Bastian PJ, Yegnasubramanian S, Palapattu GS, et al. Molecular biomarker in prostate cancer: the role of CpG island hypermethylation. Eur Urol. 2004;46:698–708. doi: 10.1016/j.eururo.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 246.Bussemakers MJ, van Bokhoven A, Verhaegh GW, et al. DD3: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–5979. [PubMed] [Google Scholar]
- 247.de Kok JB, Verhaegh GW, Roelofs RW, et al. DD3(PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer Res. 2002;62:2695–2698. [PubMed] [Google Scholar]
- 248.Groskopf J, Aubin SM, Deras IL, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52:1089–1095. doi: 10.1373/clinchem.2005.063289. [DOI] [PubMed] [Google Scholar]
- 249.Deras IL, Aubin SM, Blase A, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol. 2008;179:1587–1592. doi: 10.1016/j.juro.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 250.Haese A, de la Taille A, van Poppel H, et al. Clinical utility of the PCA3 urine assay in european men scheduled for repeat biopsy. Eur Urol. 2008;54:1081–1088. doi: 10.1016/j.eururo.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 251.Sokoll LJ, Ellis W, Lange P, et al. A multicenter evaluation of the PCA3 molecular urine test: pre-analytical effects, analytical performance, and diagnostic accuracy. Clin Chim Acta. 2008;389:1–6. doi: 10.1016/j.cca.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 252.Aubin SM, Reid J, Sarno MJ, et al. PCA3 molecular urine test for predicting repeat prostate biopsy outcome in populations at risk: validation in the placebo arm of the dutasteride REDUCE trial. J Urol. 2010;184:1947–1952. doi: 10.1016/j.juro.2010.06.098. [DOI] [PubMed] [Google Scholar]
- 253.Nakanishi H, Groskopf J, Fritsche HA, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol. 2008;179:1804–1809. doi: 10.1016/j.juro.2008.01.013. [discussion in J Urol. 2008;179:1809–1810]. [DOI] [PubMed] [Google Scholar]
- 254.van Poppel H, Haese A, Graefen M, et al. The relationship between Prostate Cancer gene 3 (PCA3) and prostate cancer significance. BJU Int. doi: 10.1111/j.1464-410X.2011.10377.x. [published online ahead of print August 26, 2011]. [DOI] [PubMed] [Google Scholar]
- 255.Aubin SM, Reid J, Sarno MJ, et al. Prostate cancer gene 3 score predicts prostate biopsy outcome in men receiving dutasteride for prevention of prostate cancer: results from the REDUCE trial. Urology. 2011;78:380–385. doi: 10.1016/j.urology.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 256.Laxman B, Morris DS, Yu J, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Re. s. 2008;68:645–649. doi: 10.1158/0008-5472.CAN-07-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kantoff P. Recent progress in management of advanced prostate cancer. Oncology (Williston Park) 2005;19:631–636. [PubMed] [Google Scholar]
- 258.Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, Nelson JB. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102–110. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 259.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 260.Jimeno A, Carducci M. Atrasentan: a rationally designed targeted therapy for cancer. Drugs Today (Barc) 2006;42:299–312. doi: 10.1358/dot.2006.42.5.973590. [DOI] [PubMed] [Google Scholar]
- 261.Jimeno A, Carducci M. Atrasentan: a novel and rationally designed therapeutic alternative in the management of cancer. Expert Rev Anticancer Ther. 2005;5:419–427. doi: 10.1586/14737140.5.3.419. [DOI] [PubMed] [Google Scholar]
- 262.Aggarwal S, Singh P, Topaloglu O, Isaacs JT, Denmeade SR. A dimeric peptide that binds selectively to prostate-specific membrane antigen and inhibits its enzymatic activity. Cancer Res. 2006;66:9171–9177. doi: 10.1158/0008-5472.CAN-06-1520. [DOI] [PubMed] [Google Scholar]
- 263.Elsasser-Beile U, Wolf P, Gierschner D, Buhler P, Schultze-Seemann W, Wetterauer U. A new generation of monoclonal and recombinant antibodies against cell-adherent prostate specific membrane antigen for diagnostic and therapeutic targeting of prostate cancer. Prostate. 2006;66:1359–1370. doi: 10.1002/pros.20367. [DOI] [PubMed] [Google Scholar]
- 264.Ikegami S, Yamakami K, Ono T, et al. Targeting gene therapy for prostate cancer cells by liposomes complexed with anti-prostate-specific membrane antigen monoclonal antibody. Hum Gene Ther. 2006;17:997–1005. doi: 10.1089/hum.2006.17.997. [DOI] [PubMed] [Google Scholar]
- 265.Jayaprakash S, Wang X, Heston WD, Kozikowski AP. Design and synthesis of a PSMA inhibitor-doxorubicin conjugate for targeted prostate cancer therapy. ChemMedChem. 2006;1:299–302. doi: 10.1002/cmdc.200500044. [DOI] [PubMed] [Google Scholar]
- 266.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 267.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 268.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 269.Lane BR, Kattan MW. Prognostic models and algorithms in renal cell carcinoma. Urol Clin North Am. 2008;35:613–625. doi: 10.1016/j.ucl.2008.07.003. vii. [DOI] [PubMed] [Google Scholar]
- 270.Kim HL, Seligson D, Liu X, et al. Using tumor markers to predict the survival of patients with metastatic renal cell carcinoma. J Urol. 2005;173:1496–1501. doi: 10.1097/01.ju.0000154351.37249.f0. [DOI] [PubMed] [Google Scholar]
- 271.Kim HL, Seligson D, Liu X, et al. Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res. 2004;10:5464–5471. doi: 10.1158/1078-0432.CCR-04-0488. [DOI] [PubMed] [Google Scholar]
- 272.Kluger HM, Siddiqui SF, Angeletti C, et al. Classification of renal cell carcinoma based on expression of VEGF and VEGF receptors in both tumor cells and endothelial cells. Lab Invest. 2008;88:962–972. doi: 10.1038/labinvest.2008.65. [DOI] [PubMed] [Google Scholar]
- 273.Hager M, Haufe H, Kemmerling R, et al. Increased activated akt expression in renal cell carcinomas and prognosis. J Cell Mol Med. 2009;13(8B):2181–2188. doi: 10.1111/j.1582-4934.2008.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Eichelberg C, Junker K, Ljungberg B, Moch H. Diagnostic and prognostic molecular markers for renal cell carcinoma: a critical appraisal of the current state of research and clinical applicability. Eur Urol. 2009;55(4):851–863. doi: 10.1016/j.eururo.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 275.Djordjevic G, Mozetic V, Mozetic DV, et al. Prognostic significance of vascular endothelial growth factor expression in clear cell renal cell carcinoma. Pathol Res Pract. 2007;203:99–106. doi: 10.1016/j.prp.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 276.Bensalah K, Pantuck AJ, Crepel M, et al. Prognostic variables to predict cancer-related death in incidental renal tumours. BJU Int. 2008;102:1376–1380. doi: 10.1111/j.1464-410X.2008.07847.x. [DOI] [PubMed] [Google Scholar]
- 277.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 278.Pantuck AJ, Thomas G, Belldegrun AS, Figlin RA. Mammalian target of rapamycin inhibitors in renal cell carcinoma: current status and future applications. Semin Oncol. 2006;33:607–613. doi: 10.1053/j.seminoncol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 279.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 280.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 281.Stillebroer AB, Mulders PF, Boerman OC, Oyen WJ, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol. 2010;58:75–83. doi: 10.1016/j.eururo.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 282.Jacobsen J, Grankvist K, Rasmuson T, Bergh A, Landberg G, Ljungberg B. Expression of vascular endothelial growth factor protein in human renal cell carcinoma. BJU Int. 2004;93:297–302. doi: 10.1111/j.1464-410x.2004.04605.x. [DOI] [PubMed] [Google Scholar]
- 283.Lidgren A, Hedberg Y, Grankvist K, Rasmuson T, Vasko J, Ljungberg B. The expression of hypoxia-inducible factor 1alpha is a favorable independent prognostic factor in renal cell carcinoma. Clin Cancer Res. 2005;11:1129–1135. [PubMed] [Google Scholar]
- 284.Migita T, Oda Y, Naito S, Tsuneyoshi M. Low expression of p27(Kip1) is associated with tumor size and poor prognosis in patients with renal cell carcinoma. Cancer. 2002;94:973–979. [PubMed] [Google Scholar]
- 285.Hedberg Y, Davoodi E, Ljungberg B, Roos G, Landberg G. Cyclin E and p27 protein content in human renal cell carcinoma: clinical outcome and associations with cyclin D. Int J Cancer. 2002;102:601–607. doi: 10.1002/ijc.10763. [DOI] [PubMed] [Google Scholar]
- 286.Hedberg Y, Ljungberg B, Roos G, Landberg G. Expression of cyclin D1, D3, E, and p27 in human renal cell carcinoma analysed by tissue microarray. Br J Cancer. 2003;88:1417–1423. doi: 10.1038/sj.bjc.6600922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Linehan WM, Pinto PA, Srinivasan R, et al. Identification of the genes for kidney cancer: opportunity for disease-specific targeted therapeutics. Clin Cancer Res. 2007;13:671s–679s. doi: 10.1158/1078-0432.CCR-06-1870. [DOI] [PubMed] [Google Scholar]
- 288.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 289.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 290.Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Jr, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von hippel-lindau protein. Proc Natl Acad Sci U S A. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kroog GS, Motzer RJ. Systemic therapy for metastatic renal cell carcinoma. Urol Clin North Am. 2008;35:687–701. doi: 10.1016/j.ucl.2008.07.007. ix. [DOI] [PubMed] [Google Scholar]
- 292.Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1552–1558. doi: 10.1002/cncr.23776. [DOI] [PubMed] [Google Scholar]
- 293.Bhatia S, Thompson JA. Temsirolimus in patients with advanced renal cell carcinoma: an overview. Adv Ther. 2009;26(1):55–67. doi: 10.1007/s12325-008-0138-3. [DOI] [PubMed] [Google Scholar]
- 294.Patard JJ, Pouessel D, Bensalah K, Culine S. Targeted therapy in renal cell carcinoma. World J Urol. 2008;26:135–140. doi: 10.1007/s00345-008-0237-4. [DOI] [PubMed] [Google Scholar]
- 295.Patard JJ, Pouessel D, Culine S. New therapies in renal cell carcinoma. Curr Opin Support Palliat Care. 2007;1:174–179. doi: 10.1097/SPC.0b013e3282f1238b. [DOI] [PubMed] [Google Scholar]
- 296.Patard JJ, Thuret R, Raffi A, Laguerre B, Bensalah K, Culine S. Treatment with sunitinib enabled complete resection of massive lymphadenopathy not previously amenable to excision in a patient with renal cell carcinoma. Eur Urol. 2009;55(1):237–239. doi: 10.1016/j.eururo.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 297.Baldewijns MM, van Vlodrop IJ, Vermeulen PB, Soetekouw PM, van Engeland M, de Bruine AP. VHL and HIF signalling in renal cell carcinogenesis. J Pathol. 2010;221:125–138. doi: 10.1002/path.2689. [DOI] [PubMed] [Google Scholar]
- 298.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–285. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Steffens MG, Boerman OC, de Mulder PH, et al. Phase I radio-immunotherapy of metastatic renal cell carcinoma with 131I-labeled chimeric monoclonal antibody G250. Clin Cancer Res. 1999;5:3268s–3274s. [PubMed] [Google Scholar]