Abstract
Multipotent human neural stem cells (hNSC) have traditionally been isolated directly from the central nervous system (CNS). To date, as a therapeutic tool in the treatment of neurologic disorders, the most promising results have been obtained using hNSC isolated directly from the human fetal neuroectoderm. The propagation ability of such tissue-derived hNSC is often limited, however, making it difficult to establish a large-scale culture. Following engraftment, these hNSC often show low efficiency in generating the desired neuronal cells necessary for reconstruction of the damaged host milieu and, as a result, have failed to give satisfactory results in clinical trials so far. Alternatively, human embryonic stem cells (hESC) offer a pluripotent reservoir for in vitro derivation of a rich spectrum of well-characterized neural-lineage committed stem/progenitor/precursor cells that can, theoretically, be picked at precisely their safest and most efficacious state of plasticity to meet a given clinical challenge. However, the need for ‘foreign’ biologic additives and multilineage differentiation inclination may make direct use of such cell-derived hNSC in patients problematic. The hNSC, when derived from pluripotent cells under protocols presently employed in the field, tend to display not only a low efficiency in neuronal differentiation, but also an inclination for phenotypic heterogeneity and instability and, hence, increased risk of tumorigenesis following engraftment. For hNSC derived in vitro to be used safely in therapeutic paradigms, it requires conversion of human pluripotent cells uniformly to cells that are restricted to the neural lineage in need of repair. Developing strategies for direct induction of human pluripotent cells exclusively into neural-committed progenies at a broad range of developmental stages will allow a large supply of optimal therapeutic hNSC tailor-made for safe and effective treatment of particular neurologic diseases and injuries in patients.
Keywords: cell therapy, human embryonic stem cell, human neural stem cell, multipotent, neural repair, neurologic disorder, pluripotent, transplantation
Introduction
Neural stem cells (NSC), as representative of those isolated directly from embryonic, fetal, newborn and adult tissues, have offered cures for a range of neurologic diseases because of their potential for neural cell replacement following transplantation. Although graft material could theoretically come from a number of sources, the ability of a stem cell, by definition, to self-renew makes it a potentially inexhaustible cell source for tissue and functional restoration. It is then theoretically possible to instruct such cells to differentiate into specific desired phenotypes for cell replacement therapy. However, the optimal source for stem cells with neural potential remains controversial. In human development, it is assumed that human somatic stem cells (hSSC) derive in vivo from a progressive narrowing of the potential of human embryonic stem cells (hESC) of the inner cell mass (ICM) of the blastocyst. Thus there is a continuum between embryonic and somatic stem cells. Lingering questions regarding how to judge precisely where along this continuum or what degree of lineage commitment is optimal for addressing a particular disease not only reflect fundamental gaps in our knowledge regarding the determinants of stem cell identity, but remain as obstacles to safe and effective clinical translation.
For many diseases, we might be unduly presumptuous that we know what cell type is needed and what is required to reconstitute a given region and restore function. As fundamental as these questions are, the answers are actually elusive for the majority of neurologic disorders. Neurodegeneration is becoming increasingly tied to functions of non-neuronal cells (perhaps astrocytes) as a precursor to neuronal dissolution, suggesting that one might desire a multipotent cell line that can yield both replacement glia as well as replacement neurons. Hence implanting cells pre-committed ex vivo to yield a uniform mature neural cell type may compromise the ability to accommodate varying environmental cues and provide other needed cells in an appropriate ratio within the same structure. The complexity of discerning the optimal source and state of stem cell differentiation for treating diseases, as exemplified by those of the central nervous system (CNS), is further compounded by the emerging realization that a disease may require replacement of multiple cell types and multiple proteins for the damaged milieu to be fully reconstructed, not only the neurons that have died but also the support cells that detoxify the environment, myelinate the axons and dendrites, supply ongoing trophic and matrix support and provide reservoirs for ongoing cell replenishment [1–5]. Therefore, the ideal therapeutic NSC should have not only the ability for long-term stable large-scale expansion in vitro, but also the capacity to yield an array of cell types necessary for reconstituting a heterogeneous structure not yet endowed with so much plasticity and instability as to yield inappropriate cell types or neoplasms. The choice of the stem cell source that best fulfills the criteria for a given clinical challenge requires not only examining the therapeutic aspects of a given human stem cell for a particular disease, but also generating practical means for more reliably and efficiently predicting its translational value.
Human neural stem cells (hNSC), the prototypical multipotent stem cells, have traditionally been isolated directly from the fetal or adult CNS (‘primary’ hNSC). The propagation ability of such tissue-derived hNSC is often limited, however, making it difficult to establish a large-scale culture. Their transplantation efficiency and plasticity further decline after extensive culture. Following engraftment, these hNSC often show low efficiency in generating the desired neuronal cells necessary for reconstruction of the damaged host milieu and, as a result, have failed to give satisfactory results in clinical trials so far. Alternatively, human pluripotent cells, most classically exemplified by the hESC derived from the ICM or epiblast of the blastocyst, offer a pluripotent reservoir for in vitro derivation of a rich spectrum of well-characterized neural lineage-committed stem/progenitor/precursor cells (‘secondary’ hNSC) that can, theoretically, be picked at precisely their safest and most efficacious state of plasticity targeted for a given clinical situation. However, undefined ‘foreign’ biologic supplements and/or feeders that have typically been used for the isolation, expansion and differentiation of hESC may make direct use of such cell-derived hNSC in patients problematic. Another challenge for clinical translation is how to channel the wide differentiation potential of pluripotent cells efficiently and predictably to cells that are restricted to the neural lineage in need of repair. The hNSC, when derived from pluripotent cells under protocols presently employed in the field, tend to display not only a low efficiency in neuronal differentiation but also an inclination for phenotypic heterogeneity and instability and, hence, increased risk of tumorigenesis following engraftment. For hNSC derived from pluripotent cells in vitro to be used safely in therapeutic paradigms, it requires conversion of human pluripotent cells uniformly to neural lineage-restricted cells in a process that may emulate in vivo embryonic neurogenesis. Developing strategies for direct induction of human pluripotent cells exclusively into neural-committed progenies at a broad range of developmental stages will allow a large supply of optimal therapeutic hNSC tailor-made for safe and effective treatment of particular neurologic diseases and injuries in patients.
The gold standard of NSC in neural repair
The ‘gold standard’ for what should be achievable was established with the primary NSC isolated directly from the CNS, both of animal and human origin. These primary NSC have not only the ability to engraft diffusely and become integral members of the host CNS structure, but unique tropism for pathology, such that the distribution of therapeutic genes and cells appears to be directed to the region most in need [6–8]. Therefore, they have been used to address not only diseases that are characterized by focal defects, but also those that are typified by widely disseminated lesions, such as most neurodegenerative diseases and brain tumors. In such contexts, they have been able to disseminate therapeutic gene products in models of inherited metabolic neurodegenerative diseases [9, 10], replace oligodendrocytes in models of leukodystrophies [11], deliver therapeutic agents for potentially more effective treatment of brain tumors [8] and promote locomotor recovery in models of spinal cord injury [12, 13]. ‘Replacement’ neurons have been generated from these NSC in a number of experimental models [2–5, 12–15]. Indeed, these NSC can shift their differentiation fate to yield a greater proportion of the deficient cell type [14, 15]. In models of Parkinson’s disease, a neuronal degenerative disorder associated with a loss of midbrain neurons that synthesize the neurotransmitter dopamine (DA), these primary NSC (c. 5%) have even been observed to generate neuronal cells associated with dopaminergic phenotype [4, 5].
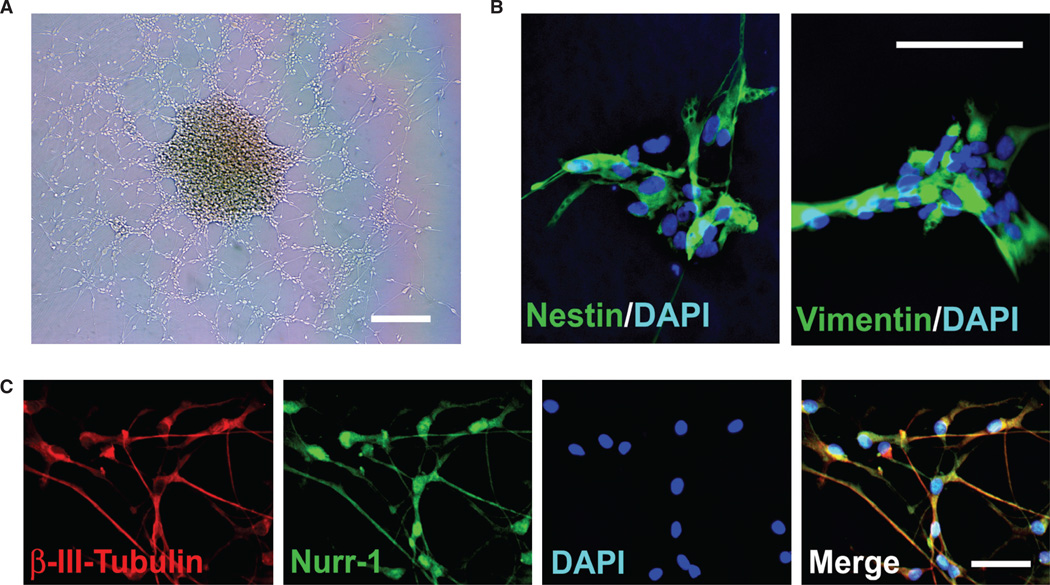
To date, as a therapeutic tool in the treatment of neurologic disorders, the most promising results have been obtained using primary hNSC isolated directly from human fetal neuroectoderm. These primary hNSC can be maintained as stable lines in serum-free, mitogen-supplemented me dium, and more than 95% of the cells express neural stem/progenitor markers (Figure 1A, B) [15, 16]. Upon removal of mitogens, c. 10–30% of these hNSC spontaneously differentiate into cells that express the early neuronal marker β-III-tubulin, and the more mature neuronal protein Map-2 by 15 days (Figure 1C) [15–18]. The capacity of these hNSC to respond to cues that might direct them toward a tyrosine hydroxylase (TH)-positive and DA-producing phenotype was established in vitro by following a well-established protocol [17, 18]. Briefly, once the cultures have begun to differentiate following mitogen removal, exposure to glial cell-derived conditioned medium (CM) and the reintroduction of basic fibroblast growth factor (bFGF) promotes the appearance and steady increase in the percentage of TH-positive neuronal cells in the culture, ultimately reaching 5–10% [17, 18]. That this TH induction is associated with aspects of a ventral mesencephelon (VM) phenotype is suggested by the expression of homeodomain transcription factors, Nurr1 (Figure 1C) and Ptx3, necessary for induction and maintenance of midbrain DA neurons [4, 5, 17, 18].
Figure 1.
Primary hNSC isolated directly from the human fetal neuroectoderm. (A) A phase image of the CNS-derived hNSC in culture. (B) The hNSC (>95%) express neural stem/progenitor marker nestin (green) and vimentin (green). All cells are revealed by DAPI staining of their nuclei (blue). (C) When provided with appropriate cues, the hNSC (c. 5–10%) differentiate into neurons [labeled for β-III-tubulin (red)] that express Nurr-1 (green), a marker associated with the midbrain dopaminergic phenotype. Scale bars: (A) 50 µm; (B, C) 5 µm.
Despite the observation that NSC have the ability to migrate and differentiate into deficient neurons, we are learning that NSC can exert important therapeutic effects by actions beyond simple cell replacement and foreign gene delivery. These NSC, even in their unmanipulated state, promote homeostasis within a disequilibrated system, often by altering host neural cells and circuitry, suggesting an unanticipated mechanism by which therapeutic outcomes might be achieved: an inherent capacity of stem cells (without genetic engineering) may help create host environments sufficiently rich in trophic and/or neuroprotective support to rescue endogenous cells [4, 5, 19–22]. A stem cell’s non-neuronal progeny produces diffusible factors that ‘chaperone’ and protect imperiled host cells (typically neurons) with an efficacy and impact that may eclipse the stem cell’s effectiveness in replacing neuronal circuitry. These NSC can restore intracellular equipoise to disordered host neurons through cell–cell contact, engage in cross-talk with host cells and induce endogenous regenerative programs, and produce trophic, angiogenic anti-scarring, anti-inflammatory and anti-apoptotic molecules [4, 5, 19]. Despite their multipotence, these primary NSC never give rise to cell types that are inappropriate to the brain, nor do they yield neoplasms [1–5].
Turning stem cells into the desired cell types necessary for restitution of damaged host structure and circuitry depends on interactions between extrinsic signals in the host and programs intrinsic to the grafted cell. Transplantation studies suggest that stem cells from different sources vary greatly in their capacity to reconstruct damaged structures. To date, as a therapeutic tool in the treatment of neurologic disorders, the most promising results have been obtained using primary hNSC isolated directly from a human fetal neuroectoderm-derived structure in the CNS (the ventricular germinal zone) [20–22]. However, like other somatic stem cells, these primary hNSC have limitations, particularly if they are not genetically modified and simply propagated with mitogens [23–25]. The propagation ability of such tissue-derived NSC is often limited, making it difficult to maintain large-scale and prolonged cultures. After extensive passaging in culture, the transplantation efficiency of such NSC often falls and their plasticity further declines, potentially restricting the tissue-derived NSC as an adequate source for graft material. As these tissue-derived NSC become more restricted in their developmental potential, their capacity to be directed by host cues in a region-specific manner to differentiate into neurons necessary for reconstruction of the damaged host milieu is down-regulated or lost with aging [20–22]. While cellular senescence can be counteracted by transducing the cells with genes such as v-myc and telomerase [2], many investigators are not eager to insert such foreign genes into cells that might ultimately be used for transplantation purposes in humans. So far, these issues have prevented such tissue-derived hNSC from giving satisfactory results in clinical trials [20–22, 26].
Pluripotent cells as an inexhaustible source for in vitro derivation of optimal therapeutic NSC
Alternatively, a more plastic undifferentiated stage of stem cells might be employed as transplants in order to achieve large-scale and prolonged propagation in culture and efficient restoration of function. Pluripotent hESC, derived from the ICM or epiblast of the blastocyst, have the capacity for long-term stable, undifferentiated growth in culture and the theoretical potential for differentiation into all somatic cell types [27]. Therefore, they have been regarded as an ideal source to provide an unlimited supply of large-scale well-characterized stem/progenitor/precursor cells for cell-based therapies. Although hNSC have traditionally been isolated directly from the CNS (‘primary’ hNSC), the field is becoming more adept at deriving hNSC in vitro from human pluripotent stem cells (‘secondary’ hNSC), most classically exemplified by the hESC and, more recently, the induced pluripotent stem cells (iPSC). The major challenge in using pluripotent cells for cell-based therapy is producing a large and uniform population of lineage-committed transplantable cells in vitro. In addition, in vivo studies, using animal models of disease, will be critical for examining the physiologic functions of engrafted hESC derivatives, evaluating their efficacy in repair and, more importantly, affirming their safety (i.e. a lack of tumorigencity and absence of inappropriate cell types).
The unconstrained capacity for self-renewal and differentiation offer hESC as an abundant source for generating a large supply of human neural stem/precursor/progenitor cells, and even mature neurons, for neural replacement therapies. So far, several groups have established protocols for selecting homogeneous populations of human neural stem/precursor/progenitor cells spontaneously differentiated from hESC for further maturation into neurons and glial cells in vitro and in vivo [28–38]. Recently, the TGF-β family signaling pathway inhibitors of SMAD (vertebrate proteins homology to Drosophila Mothers Against Decapentaplegic and C. elegans Sma), Noggin and SB431542, were used to replace stromal feeders to induce efficient neural conversion of >80% hESC maintained under mouse embryonic fibroblast (MEF)-conditioned media to early-stage intermediate cells capable of giving rise to populations of hNSC and neural crest stem (NCS) cells [39]. These hNSC derived from pluripotent cells may represent more plastic populations of human neural stem/precursor/progenitor cells capable of recreating the full cellular diversity of the developing CNS [32, 33, 38]. Although neural lineages appear at a relatively early stage in differentiation, treating hESC-differentiated embryoid bodies (EB) with retinoic acid only slightly increased the low yield of neurons (c. 3–8%) [40, 41]. Therefore a wide variety of techniques and compounds have been explored, including high-through-put screening assays, to identify conditions for pushing hESC along the neural lineage selectively toward neurons and even particular subtypes of neurons [42]. For example, the hESC-derived midbrain dopaminergic neurons can be enriched by co-culture with stromal cells or telomerase-immortalized midbrain astrocytes as well as exposure to FGF and sonic hedgehog (SHH) signaling (e.g. yielding c. 80% β-III-tubulin-positive colonies and c. 30% TH-positive colonies) [43–48]. ‘Foreign’ feeder induction followed by stimuli from retinoic acid and SHH signaling was used to increase the efficiency of spinal motoneurons from the hESC to as high as c. 30% of the cells expressing the early motor neuronal markers [49–52]. A combination of cytokines and substrates was used to push the neural progenies of hESC into striatal neurons (c. 10%) targeted for Huntington’s disease [53]. After engraftment, these hESC-derived hNSC differentiated into neurons, oligodendrocytes and astrocytes, and promoted function recovery in several models of neurologic disorders, including Parkinsonian [47, 48], spinal cord injury [51, 52], Huntington’s disease [53], and stroke [54, 55]. In addition, hESC-derived hNSC can be directed toward retinal cells that potentially may be used to treat retinal degeneration and restore vision [56, 57]. However, these pluripotent cell-derived grafts tend to display not only a low efficiency in neuronal differentiation but also multilineage inclination in culture and phenotypic heterogeneity and instability after transplantation, hence an increased risk of tumorigenesis following engraftment [48, 53, 58–60]. Recently, iPSC have shown similar differentiation potential to DA and motor neurons [61, 62], although these genetically manipulated somatic cells pose even greater oncogenic risk, compromising their clinical application for treating patients. For hNSC derived from pluripotent cells in vitro to be used safely in therapeutic paradigms, it requires the development of more efficient, controllable differentiation routes to generate a large supply of hNSC that are restricted to the neural lineage in need of repair.
The hESC can serve as an inexhaustible source for generating a rich spectrum of well-characterized neural lineage-committed stem/progenitor/precursor cells that can, theoretically, be picked at precisely their safest and most efficacious state of plasticity, if there is a metric for identifying such a state. To realize the therapeutic potential of hESC, we must better understand how a pluripotent cell becomes progressively constrained in its fate options. It is self-evident that we do not want the hESC so undifferentiated that it retains its ability to yield teratoma or give rise to cell types inappropriate to the engrafted organ; neither do we want the hESC to be so terminally differentiated that it cannot integrate in vivo (a problem to date in the dopaminergic field), respond to microenvironmental cues and yield the diversity of cross-talking cell types that might be necessary to reconstitute an organ’s function. Therefore, narrowing the pluripotence of an hESC to the multipotence of an hNSC appears to be necessary for safe and effective clinical translation. However, precisely where along this continuum of a progressive fate restriction or what degree of lineage commitment or differentiation state of a given stem cell is optimal for transplantation therapy remains unclear. It is also unknown whether the hNSC derived secondarily in vitro from pluripotent hESC in culture and isolated directly from a fetal CNS reside along the same developmental continuum with similar behavior and equivalent potential in therapeutic transplants. The search for ‘stemness genes’, a common set of transcribed genes that define the characters of all stem cells, has actually suggested that surveying gene expression alone is not sufficient to ensure or define either plasticity or lineage specification [63–66]. Overlaps in gene expression exist between cells of varying lineages, while a lack of overlap is found in phenotypes that ostensibly seem similar [67–69]. Even the expression of a lineage-defining gene within stem cells seems to require additional epigenetic cues, suggesting that epigenetic control in stem cell fate decision may hold the key to some of the pressing questions regarding the mechanisms underlying their developmental as well as therapeutic potential [70, 71]. Safe and effective clinical translation of the therapeutic potential of human pluripotent cells requires a better knowledge of the inherent cellular mechanisms maintaining plasticity and then stabilizing commitment.
Obstacles to clinical translation of the therapeutic potential of pluripotent cells for neurologic disorders
The hESC offer a pluripotent reservoir for in vitro derivation of a large supply of disease-targeted hSSC that are restricted to the lineage in need of repair. However, undefined ‘foreign’ biologic supplements and/or feeders that have typically been used for the isolation, expansion and differentiation of hESC may make direct use of such cells and their derivatives in patients problematic. The hESC lines were initially derived and maintained in co-culture with growth-arrested mouse embryonic fibroblasts (MEF) [27]. Using this mouse support system may compromise the therapeutic potential of these hESC because of the risk of transmitting xenopathogens, altering the genetic background and promoting the expression of immunogenic proteins [72]. Although several human feeder, feeder-free and even chemically defined culture systems have been suggested for hESC [73–77], the elements for sustaining the self-renewal of human pluripotent cells remain unsolved. These exogenous feeder cells and molecules help maintain the long-term stable growth of undifferentiated hESC while masking the ability of pluripotent hESC to respond to signals/molecules that induce uniformly lineage-specific commitment of pluripotent cells. Achieving uniform conversion of pluripotent cells to a particular lineage, first and at least, may require a better understanding of the elements necessary and sufficient for sustaining the self-renewal of pluripotent hESC, a platform from which controlled commitment may then directly proceed from the earliest developmental stage.
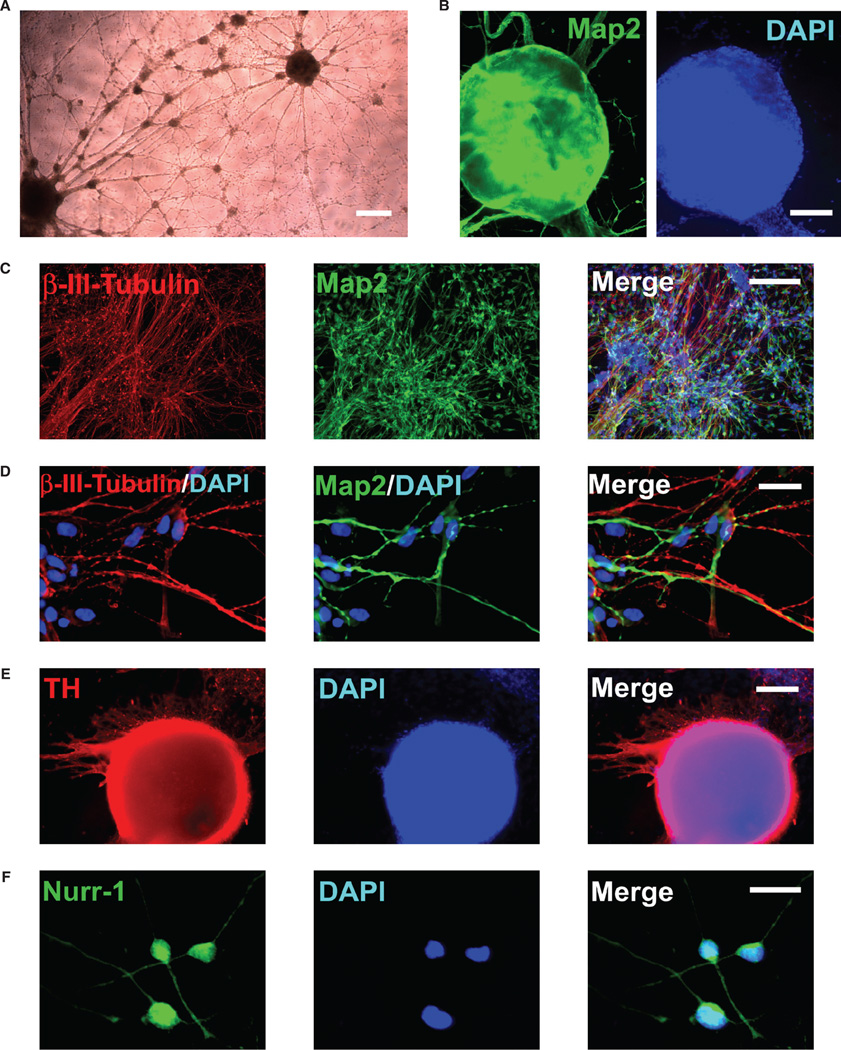
Another major challenge for clinical translation is how to channel the wide differentiation potential of human pluripotent cells efficiently and predictably to a desired phenotype. The pluripotent cells can differentiate spontaneously in vitro into cells of all germ layers by going through an intervening multilineage stage, where, however, only a small fraction pursues a given lineage. The need for ‘foreign’ biologic additives and the simultaneous emergence of widely divergent cell types often make desired differentiation down a particular route unpredictable and unreliable. Following transplantation, pluripotent hESC generally form teratomas that contain cells from all three embryonic germ layers. In culture, many different lineages and cell types have been observed from hESC by allowing aggregate formation in suspension or extended culture. However, only a small fraction of these cells progresses to display targeted differentiation characteristics. In order to enrich the populations of desired cell types, certain approaches have been applied, such as immunoselection for specific surface antigens [40, 78–80], co-culture with feeder cells [43–51], manipulation of cell density [81–83] and induction with inhibitors or signaling molecules [39–41, 50, 51, 53]. However, these approaches remain inefficient and impractical for producing a large population of uniform uncontaminated progenies from hESC that are restricted to the lineage in need of repair. Realizing the therapeutic potential of hESC for treating neurologic disorders demands the development of better differentiation strategies that permit controlled conditions for direct conversion of human pluripotent cells exclusively into neural-committed progenies that progress efficiently into neurons of the developing CNS (Figure 2).
Figure 2.
Conversion of pluripotent hESC exclusively to a neuronal lineage. (A) A phase image of neuronal cells with extensive processes and networks directly induced from pluripotent hESC. (B) The neuronal cells induced from hESC express neuronal marker Map-2 (green); DAPI staining is blue. (C, D) The hESC-derived cells pursue a neuronal fate, as indicated by co-labeling with β-III-tubulin (red) and Map-2 (green). All cells are indicated by DAPI nuclear staining (blue). (E, F) A large subpopulation of the hESC-derived neuronal cells progress to expression of TH (red, c. 60%) and Nurr-1 (green, >95%), markers associated with the midbrain dopaminergic phenotype. Scale bars: (A) 100 µm; (B, C, E) 25 µm; (D, F) 5 µm.
Current hESC differentiation strategies have largely relied on the formation of multilineage aggregates that contain cells from all three embryonic germ layers, in part because it has been assumed that tissue and organ systems arise from the endoderm, mesoderm and ectoderm. However, unlike tightly regulated ICM development in the blastocyst stage in vivo, in culture cells inside the aggregates exit the pluripotent state in a chaotic fashion, resulting in the random appearance of various phenotypes that often complicate observation. The formation of a particular tissue and organ system during embryogenesis is a multistep programming process tightly regulated by stage-specific signals/molecules. After neuronal induction during early embryogenesis, neuroectodermal cells form the neural plate that develops into the neural tube. Subsequent development, together with vesiculation within the tube, gives rise to the brain and spinal cord of the nervous system. Failure of neural tube closure is a common congenital malformation that results in morbidity and mortality, and folic acid prevents neural tube defects, possibly by stimulating cellular methylation [84]. The complexity in generating the enormous diversity of neuronal cell types is best illustrated in the development of the mammalian telencephalon. The most rostral region of the neural tube, the prosencephalon, divides into the telencephalon and diencephalon. The dorsal region of the telencephalon gives rise to the cerebral cortex, while the ventral telencephalon differentiates into the basal ganglia. The dorsoventral and rostrocaudal identities and subsequent specification of progenitors are established by diffusible morphogens, including FGF, SHH, BMP, Wnt, Nodal and Notch proteins, through regional patterning/activation of transcription factors such as Pax6, Olig2, Nkx2.1, Bmp4, Emx1/2, Ngn1/2, Noggin, Dix, Hes and Numb [85]. The key steps of neurogenesis include neuronal commitment of neural stem cells, the subtype specification of intermediate neuronal progenitors, post-mitotic precursors and mature neurons. Various growth factors, including brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), erythropoietin, glial-derived neurotrophic factor (GDNF), platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular-endothelial growth factor (VEGF), insulin-like growth factor (IGF), epithelial growth factor (EGF), and fibroblast growth factor (FGF), are involved in the survival, proliferation, differentiation and migration of these neuronal progenitors. How to direct hESC uniformly to exit the pluripotent state in a sequential manner that emulates the tightly regulated in vivo embryogenesis and organogenesis processes has been one of the major challenges for fulfilling the therapeutic promise of hESC. It requires a thorough understanding of the molecular and cellular cues that sustain pluripotence and direct neural differentiation programs. Such studies will permit to control derivation conditions in order to generate not only mature functional neurons but also a wide spectrum of intermediate neural stem/progenitor/precursor cells moldable for treating a particular neurologic disease or injury in a given patient.
Acknowledgments
Supported in part by the National Institute of General Medicine, National Institute on Aging, National Institutes of Neurologic Diseases and Stroke. X. H. P Parsons is supported by awards from the National Institute on Aging (NIHK01AG024496) and National Institute of Child Health and Human Development (NIHR21HD056530).
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Disclosure of interest: The authors declare no conflict of interest.
References
- 1.Park KI, Ourednik J, Ourednik V, Taylor RM, Aboody KA, Auguste KI, et al. Global gene and cell replacement strategies via stem cells. Gene Therapy. 2002;9:613–624. doi: 10.1038/sj.gt.3301721. [DOI] [PubMed] [Google Scholar]
- 2.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Imitola J, Snyder EY, Sidman RL. Neural stem cells rescue nervous Purkinje neurons by restoring molecular homeostasis of tissue plasminogen activator and downstream targets. J Neurosci. 2006;26:7839–7848. doi: 10.1523/JNEUROSCI.1624-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 5.Redmond DE, Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci USA. 1997;94:11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2001;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 10.Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, et al. Stem cells act through multiple mechanisms to benefit mice with neuro degenerative metabolic disease. Nat Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 11.Yandava BD, Billinghurst LL, Snyder EY. ‘Global’ cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci USA. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosario CM, Yandava BD, Kosaras B, Zurakowski D, Sidman RL, Snyder EY. Differentiation of engrafted multipotent neural progenitors towards replacement of missing granule neurons in meander tail cerebellum may help determine the locus of mutant gene action. Development. 1997;124:4213–4224. doi: 10.1242/dev.124.21.4213. [DOI] [PubMed] [Google Scholar]
- 15.Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 16.Vescovi AL, Snyder EY. Establishment and properties of neural stem cell clones: plasticity in vitro and in vivo. Brain Pathol. 1999;9:569–598. doi: 10.1111/j.1750-3639.1999.tb00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daadi MM, Weiss S. Generation of tyrosine hydroxylase-producing neurons from precursors of the embryonic adult forebrain. J Neurosci. 1999;19:4484–4497. doi: 10.1523/JNEUROSCI.19-11-04484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner J, Akerud P, Castro DS, Holm PC, Canals JM, Snyder EY, et al. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- 19.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 20.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 21.Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 22.Pluchino S, Zanotti L, Deleidi M, Martino G. Neural stem cells and their use as a therapeutic tool in neurological disorders. Brain Res Rev. 2005;48:211–219. doi: 10.1016/j.brainresrev.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3:801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci USA. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly CM, Handley OJ, Rosser AE. Human trials for neurodegenerative disease. Methods Mol Biol. 2009;549:33–47. doi: 10.1007/978-1-60327-931-4_3. [DOI] [PubMed] [Google Scholar]
- 27.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 28.Mansouri A, Fukumitsu H, Schindehuette J, Krieglstein K. Differentiation of embryonic stem cells. Curr Protoc Neurosci. 2009;47:3.6.1–3.6.21. doi: 10.1002/0471142301.ns0306s47. [DOI] [PubMed] [Google Scholar]
- 29.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 30.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 32.Koch P, Opitz T, Steinbeck JA, Ladewig J, Brüstle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci USA. 2009;106:3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, et al. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol. 2005;23:601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- 35.Barthéléry M, Jaishankar A, Salli U, Vrana KE. Reptin52 expression during in vitro neural differentiation of human embryonic stem cells. Neurosci Lett. 2009;452:47–51. doi: 10.1016/j.neulet.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Preynat-Seauve O, de Rham C, Tirefort D, Ferrari-Lacraz S, Krause KH, Villard J. Neural progenitors derived from human embryonic stem cells are targeted by allogeneic T and natural killer cells. J Cell Mol Med. 2009:13. doi: 10.1111/j.1582-4934.2009.00746.x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michibata H, Okuno T, Konishi N, Kyono K, Wakimoto K, Aoki K, et al. Human GPM6A is associated with differentiation and neuronal migration of neurons derived from human embryonic stem cells. Stem Cells Dev. 2009;18:629–639. doi: 10.1089/scd.2008.0215. [DOI] [PubMed] [Google Scholar]
- 38.Elkabetz Y, Studer L. Human ESC-derived neural rosettes and neural stem cell progression. Cold Spring Harb Symp Quant Biol. 2008;73:377–387. doi: 10.1101/sqb.2008.73.052. [DOI] [PubMed] [Google Scholar]
- 39.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 41.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS, et al. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001;913:201–205. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 42.Desbordes SC, Placantonakis DG, Ciro A, Socci ND, Lee G, Djaballah H, et al. High-throughput screening assay for the identification of compounds regulating self-renewal and differentiation in human embryonic stem cells. Cell Stem Cell. 2008;2:602–612. doi: 10.1016/j.stem.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng X, Cai J, Chen J, You ZB, Fotter E, Wang Y, et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- 45.Shimada H, Yoshimura N, Tsuji A, Kunisada T. Differentiation of dopaminergic neurons from human embryonic stem cells: modulation of differentiation by FGF-20. J Biosci Bioeng. 2009;107:447–454. doi: 10.1016/j.jbiosc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Vazin T, Chen J, Spivak CE, Amable R, Gabitzsch E, Lee CT, et al. Dopaminergic neurons derived from BG01V2, a variant of human embryonic stem cell line BG01. Restor Neurol Neurosci. 2008;26:447–458. [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 48.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 49.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 50.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 52.Hatami M, Mehrjardi NZ, Kiani S, Hemmesi K, Azizi H, Shahverdi A, et al. Human embryonic stem cell-derived neural precursor transplants in collagen scaffolds promote recovery in injured rat spinal cord. Cytotherapy. 2009;22:1–13. doi: 10.1080/14653240903005802. [DOI] [PubMed] [Google Scholar]
- 53.Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci USA. 2008;105:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, et al. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562–574. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- 56.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaudhry GR, Fecek C, Lai MM, Wu WC, Chang M, Vasquez A, et al. Fate of embryonic stem cell derivatives implanted into the vitreous of a slow retinal degenerative mouse model. Stem Cells Dev. 2009;18:247–258. doi: 10.1089/scd.2008.0057. [DOI] [PubMed] [Google Scholar]
- 58.Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 59.Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2008;18:1–12. doi: 10.1089/scd.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shamblott MJ, Axelman J, Littlefield JW, Blumenthal PD, Huggins GR, Cui Y, et al. Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc Natl Acad Sci USA. 2001;98:113–118. doi: 10.1073/pnas.021537998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. ‘Stemness’: transcriptional profiling of embryonic adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 64.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 65.Boyer A, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muller FJ, Laurent LC, Kostka D, Ulitsky I, Williams R, Lu C, et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature. 2008;455:401–405. doi: 10.1038/nature07213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parker MA, Anderson JK, Corliss DA, Abraria VE, Sidman RL, Park KI, et al. Expression profile of an operationally-defined neural stem cell clone. Exp Neurol. 2005;194:320–332. doi: 10.1016/j.expneurol.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 69.Montzka K, Lassonczyk N, Tschöke B, Neuss S, Führmann T, Franzen R, et al. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: misleading marker gene expression. BMC Neurosci. 2009;10:16. doi: 10.1186/1471-2202-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 71.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:1–5. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 73.Richards M, Fong C, Chan W, Wong P, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 74.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 75.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 76.Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically-defined conditions. Proc Natl Acad Sci USA. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 78.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 79.Sundberg M, Jansson L, Ketolainen J, Pihlajamäki H, Suuronen R, Skottman H, et al. CD marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009;2:113–124. doi: 10.1016/j.scr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Ladewig J, Koch P, Endl E, Meiners B, Opitz T, Couillard-Despres S, et al. Lineage selection of functional and cryopreservable human embryonic stem cell-derived neurons. Stem Cells. 2008;26:1705–1712. doi: 10.1634/stemcells.2008-0007. [DOI] [PubMed] [Google Scholar]
- 81.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 82.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 83.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 84.Blom HJ, Shaw GM, Hijer MD, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neuro. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]