Abstract
OBJECTIVE
To evaluate the pathologic outcome of prostate-specific antigen-screened patients with high-grade (Gleason score ≥ 8) prostate cancer limited to 1 biopsy core, without clinical evidence of disease.
METHODS
Ninety-two patients with only 1 biopsy core with cancer and treated by radical prostatectomy were divided into 4 groups according to the biopsy Gleason score: 3 + 3 = 6 (23 cases), 3 + 4 = 7 (25 cases), 4 + 3 = 7 (20 cases), and ≥8 (24 cases).
RESULTS
Cases with Gleason score ≥8 showed a significantly higher proportion of extraprostatic extension (50%), positive surgical margins (21%), and seminal vesicle invasion (12%) when compared with the other groups. Patients with Gleason score ≥8 in the biopsy had a 25-fold increased in the odds ratio for extraprostatic extension in the prostatectomy. The incidence of extraprostatic extension was higher in those with prostatic cancer involving ≥50% of one core (88%) compared with cases involving <50% (32%).
CONCLUSION
In patients with prostate cancer limited to 1 biopsy core, the presence of Gleason score ≥8 significantly increased the incidence of extraprostatic extension, positive surgical margins, and seminal vesicle invasion. The odds ratio was substantially higher in patients with ≥50% of Gleason ≥8 in the biopsy core. These data might be taken into account for proper clinical management of this set of patients.
Prostate cancer is the most common male malignancy in the United States, with an estimated 217,730 new cases and 32,050 deaths for 2010.1 About 80% of newly diagnosed patients present with localized disease (T1–2), and at this stage the 5-year survival rate is 100%.1 However, the presence of extraprostatic extension, seminal vesicle invasion, and positive surgical margins are associated with an increased risk of biochemical recurrence and prostate cancer–specific death.2–5 Patients with localized disease are treated by radical prostatectomy, radiation therapy, or active surveillance, and treatment modalities are established based on several clinicopathologic features such as clinical stage, pathologic stage, histologic Gleason grade, and serum prostate-specific antigen (PSA) levels.6,7 Various studies have demonstrated a strong association between the percentage of biopsy cores with prostate cancer, tumor stage at prostatectomy, and biochemical recurrence.8–12 In addition, the presence of high-grade prostatic carcinoma in needle biopsies has been associated with an increased risk of extraprostatic extension, seminal vesicle invasion, lymphovascular invasion, and positive surgical margins in radical prostatectomies.6,8,13–16 Nevertheless, there are limited studies to determine whether the presence of a single focus of high-grade prostatic carcinoma on a needle core biopsy carries the same aggressive implications. In this study, we evaluate the pathologic outcome at radical prostatectomy in patients with prostate cancer present in only 1 needle biopsy core.
MATERIAL AND METHODS
The study was approved by the Institutional Review Board at our hospital with HIPAA compliance.
Criteria for Selection of Patients
The surgical pathology database at the Johns Hopkins Hospital (Baltimore, MD) was searched for all in-house and outside (consult cases) prostate biopsies (1999–2008) in which prostatic adenocarcinoma was present only in 1 core, with the patient undergoing subsequent radical prostatectomy at our hospital. Ninety-two patients were found to meet the aforementioned criteria. Transrectal ultrasound-guided needle biopsy was performed after finding elevated PSA levels but without any other related findings. All microscopic slides, from both the biopsy and the radical prostatectomy, were evaluated by a senior urological pathologist from our department. Medical records were examined for clinical and laboratory data. Biopsy data on patient age, prebiopsy PSA, Gleason score, percentage of core involvement, and total number of cores per case were collected.
Prostatectomy specimens were submitted entirely for histopathological examination. Bilateral dissection of pelvic lymph nodes was performed in 91 patients. The mean number of lymph nodes per patient was 9 (range 2–28). The only patient who did not receive lymph node dissection had a Gleason score 4 + 3 = 7 at the biopsy. Prostatectomy data on Gleason score, prostate size, extraprostatic extension, surgical margin status, seminal vesicle invasion, lymphovascular invasion, and lymph node metastasis were retrieved on all cases. Tumor extension at the radical prostatectomy was categorized into 3 groups: minimal (cancer in only 1 block), extensive (cancer in more than one half of all blocks), and moderate (the remaining cases).
Cases were divided in 4 groups according to the Gleason score diagnosed at the biopsy: 3 + 3 = 6 (23 cases); 3 + 4 = 7 (25 cases); 4 + 3 = 7 (20 cases); and ≥8 (24 cases). Cases with Gleason score ≥8 were considered high-grade prostatic carcinomas. We used the updated 2007 Partin Table6 to compare the actual frequencies of extraprostatic extension, seminal vesicle involvement, and lymph node metastasis with the predicted values for the same variables.
Statistical Analysis
Continuous variables (age, PSA, number of cores/case, percentage of tumor involvement) were compared using the Kruskal–Wallis test. Categorical variables (Gleason score, extraprostatic extension, seminal vesicle involvement, positive surgical margins, lymphovascular invasion, and lymph node metastasis) were evaluated with the Fisher’s exact test. Logistic regression was used to estimate adjusted odds ratios (OR) for extraprostatic extension. Adjusted covariates included patient’s age, clinical stage, prebiopsy PSA, and percentage of tumor involvement at biopsy. Standard errors were estimated using nonparametric bootstrapping with 1000 replicates. In all cases a 2-tailed P <.05 was required for statistical significance. Data were analyzed using Stata/SE version 11.2 (StataCorp LP, College Station, TX).
RESULTS
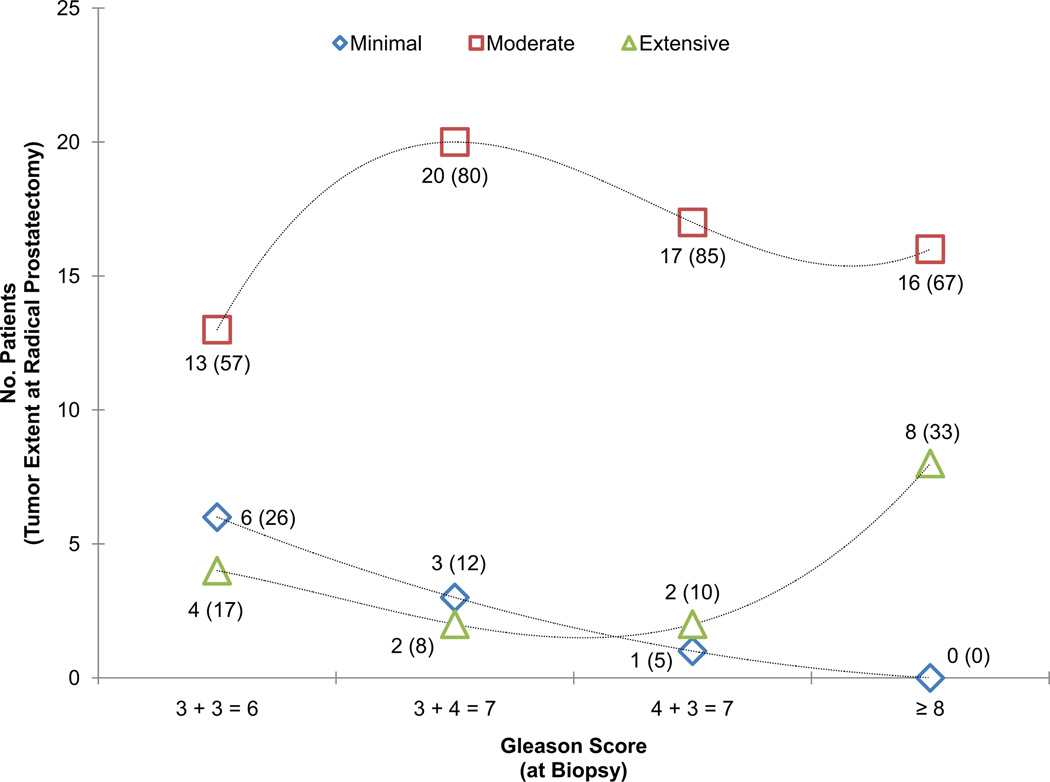
No significant differences were found in terms of patients’ age, prebiopsy PSA, number of cores per case, or prostate gland size in the prostatectomy specimen when comparing cases grouped by Gleason score (Table 1). However, the percentage of tumor involvement in the biopsy core was significantly higher in cases with Gleason score ≥8 (P = .002). Increasing Gleason scores were associated with increasing proportions of extensive tumor (Fig. 1). The presence of high-grade carcinoma in the biopsy was significantly associated with the presence of high-grade carcinoma in the prostatectomy specimen (P <.001).
Table 1.
Clinicopathologic features of cases by Gleason score at biopsy
| Gleason Score at Biopsy | |||||
|---|---|---|---|---|---|
| 3 + 3 = 6 | 3 + 4 = 7 | 4 + 3 = 7 | ≥8 | P Value | |
| Mean patient age (y) | 56.8 | 58.5 | 60.7 | 60.5 | .10 |
| Mean prebiopsy PSA (ng/mL) | 6.4 | 5.9 | 8 | 7.7 | .24 |
| Biopsy | |||||
| Mean number of cores/cases | 10.9 | 11.6 | 9.4 | 9.2 | .07 |
| Mean % core involvement | 16.1 | 24 | 19.5 | 39.2 | .002 |
| Prostatectomy | |||||
| Mean prostate size (cm) | 5.3 | 4.8 | 5.1 | 5.2 | .28 |
| Extraprostatic extension (%) | 1 (4) | 2 (8) | 6 (30) | 12 (50) | <.001 |
| Positive surgical margin (%) | 0 (0) | 0 (0) | 0 (0) | 5 (21) | .002 |
| Seminal vesicle invasion (%) | 0 (0) | 0 (0) | 0 (0) | 3 (12) | .04 |
| Lymphovascular invasion (%) | 0 (0) | 0 (0) | 0 (0) | 2 (8) | .17 |
| Lymph node metastasis (%) | 0 (0) | 0 (0) | 0 (0)* | 1 (4) | .73 |
Lymph node dissection was not done in 1 patient of this group.
Figure 1.
Tumor extension at radical prostatectomy by Gleason score groups at biopsy. Geometrical shapes represent the total number of cases for any given category of tumor extent. In parenthesis is the percentage of cases for the Gleason score group. Dotted lines represent trend lines fitted using a cubic equation. High-grade tumors showed a higher proportion of extensive tumors compared with tumors of lower Gleason score (P = .006).
Cases with Gleason score ≥8 showed a significantly higher proportion of extraprostatic extension, positive surgical margins, and seminal vesicle invasion when compared with the other groups (see Table 1). In this group, we identified extraprostatic extension in 50% of the cases, with positive surgical margins in 21% and seminal vesicle invasion in 12%. Although lymphovascular invasion and lymph node metastasis were observed only in the high-grade category, the proportions were not significantly different among the groups. In cases with Gleason score ≤7 at biopsy, we did not identify positive surgical margins, seminal vesicle invasion, lymphovascular invasion, or lymph node metastasis in the prostatectomy. Both focal and extensive extraprostatic extension were found in the specimens, with no clear association to the Gleason score at biopsy (P = .99).
The percentages of concordance between Gleason score at biopsy and in the radical prostatectomy were high in cases with Gleason score ≥8 (96%), fair in cases with Gleason score 3 + 3 = 6 (74%), and low in cases with Gleason scores 3 + 4 = 7 (32%) and 4 + 3 = 7 (35%). More than half of the cases with Gleason score 7 at biopsy were downgraded in the radical prostatectomy: 56% (14 cases) for Gleason 3 + 4 = 7, and 55% (11 cases) for Gleason 4 + 3 = 7. Only 1 case of Gleason ≥8 (4%) at biopsy was downgraded to Gleason 4 + 3 = 7 at prostatectomy. Upgrading was observed more frequently in cases with Gleason 3 + 3 = 6 (6 cases, 26%). Three cases with Gleason 3 + 4 = 7 (12%) at biopsy were upgraded to Gleason 4 + 3 = 7 at prostatectomy. Upgrading of Gleason 4 + 3 = 7 was observed in 2 cases (10%).
There was a significant tendency in the proportion of extraprostatic involvement and higher grades (Table 2). Patients with Gleason score ≥8 in the biopsy presented a 25-fold increased in the odds ratio for extraprostatic extension in the prostatectomy when compared with patients with Gleason score 3 + 3 = 6, independent of patient’s age, clinical stage, prebiopsy PSA or percentage of tumor involvement (see Table 2, model 1). The odds ratios of patients with Gleason scores 3 + 4 = 7 and 4 + 3 = 7 were 2 and 15 times the odds ratio of patients with Gleason score 3 + 3 = 6. When cases in the high-grade group were stratified by the percentage of tumor involvement using 50% as a cutoff point (see Table 2, model 2), the incidence of extraprostatic extension was higher in those with prostatic cancer involving ≥50% of one core (88%) compared with cases with cancer involving <50% (32%). Patients in the former category had a 46-fold increased in the odds ratio for extraprostatic extension in the prostatectomy specimen compared with those with Gleason score ≤7, independent of patient’s age, clinical stage, or prebiopsy PSA. Compared with the latter, patients with Gleason score ≥8 in <50% of the biopsy core had an odds ratio 3 times higher.
Table 2.
Odds ratios for extraprostatic extension in prostatectomy specimens
| Gleason Score at Biopsy |
No. Cases with Extraprostatic Extension (%) |
Odds Ratio (95% CI) |
|---|---|---|
| Model 1* | ||
| 3 + 3 = 6 | 1/23 (4) | 1.0 |
| 3 + 4 = 7 | 2/25 (8) | 2.1 (0.4, 12.1) |
| 4 + 3 = 7 | 6/20 (30) | 14.6 (2.2, 96.5) |
| ≥8 | 12/24 (50) | 24.8 (4.1, 148.8) |
| Model 2† | ||
| 6 and 7 | 9/68 (13) | 1.0 |
| ≥8 in <50% of the core | 5/16 (32) | 3.2 (0.6, 16.3) |
| ≥8 in ≥50% of the core | 7/8 (88) | 45.8 (7.1, 295) |
Adjusted for patient’s age, prebiopsy PSA, clinical stage, and percentage of tumor involvement at biopsy.
Adjusted for patient’s age, prebiopsy PSA, and clinical stage.
The actual and predicted values using the Partin tables for extraprostatic extension, seminal vesicle invasion, and lymph node metastasis are shown in Table 3. For patients with clinical stage T1c disease the 95% CI overlapped in all the Gleason score distributions, indicating that the actual values were not significantly different from the predicted ones. The same was true for patients with clinical stage T2a disease and Gleason score ≤7. However, in patients with clinical stage T2a disease and Gleason score ≥8, the actual values for extraprostatic extension, seminal vesicle invasion, and lymph node metastasis deviated from the predicted ones, with higher values, falling outside the 95% CI of the latter. Only 1 patient had clinical T2b stage disease, with values below the predicted ones.
Table 3.
Comparison of percentages of extraprostatic extension, seminal vesicle invasion, and lymph node metastasis to the predicted values using the updated 2007 Partin tables, by Gleason score
| Gleason Score at Biopsy |
No. Cases |
Mean PSA | Extraprostatic Extension | Seminal Vesicle Invasion |
Lymph Node Metastasis |
|||
|---|---|---|---|---|---|---|---|---|
| Current* | Predicted† | Current* | Predicted† | Current* | Predicted† | |||
| T1c stage | ||||||||
| 3 + 3 = 6 | 22 | 6.6 (4.6, 8.6) | 5 (0, 23) | 18 (16, 19) | 0 (0, 15) | 1 (1, 2) | 0 (0, 15) | 0 (0, 0) |
| 3 + 4 = 7 | 23 | 5.9 (3.7, 8.2) | 9 (1, 28) | 30 (26, 33) | 0 (0, 15) | 6 (4, 8) | 0 (0, 15) | 2 (1, 3) |
| 4 + 3 = 7 | 20 | 8 (4.7, 11.3) | 30 (12, 54) | 42 (36, 47) | 0 (0, 17) | 8 (5, 12) | 0 (0, 17) | 3 (1, 5) |
| ≥8 | 16 | 8.6 (6.1, 11.1) | 38 (15, 64) | 34 (26, 42) | 0 (0, 21) | 12 (8, 19) | 0 (0, 21) | 3 (1, 5) |
| T2a stage‡ | ||||||||
| 3 + 3 = 6 | 1 | 1.6 | 0 (0, 98) | 12 (9, 15) | 0 (0, 98) | 0 (0, 1) | 0 (0, 98) | 0 (0, 1) |
| 3 + 4 = 7 | 2 | 5.7 (0.6, 10.8) | 0 (0, 84) | 44 (39, 49) | 0 (0, 84) | 7 (4, 10) | 0 (0, 84) | 2 (1, 4) |
| ≥8 | 7 | 6.8 (3.6, 9.9) | 86 (42, 99) | 47 (37, 56) | 43 (10, 82) | 13 (8, 20) | 14 (0, 58) | 4 (1, 8) |
| T2b stage§ | ||||||||
| ≥8 | 1 | 1.2 | 0 (0, 98) | 24 (14, 36) | 0 (0, 98) | 8 (0, 21) | 0 (0, 98) | 17 (3, 42) |
Values correspond to percentage of cases (95% CI).
Values correspond to estimated probability of occurrence (95% CI).
No cases with Gleason score 4 + 3 + 7.
No case with Gleason score ≤ 7.
COMMENT
Although previous studies have shown that small volumes of high-grade prostate cancer on needle-core biopsies may be associated with more extensive high-grade and non–organ-confined disease in the corresponding radical prostatectomy,17,18 ours is the first one to demonstrate that even a single biopsy core with Gleason score ≥8 prostatic adenocarcinoma is associated with higher odds ratio of extraprostatic extension, seminal vesicle invasion, and positive surgical margins when compared with cases with a single core biopsy of Gleason score ≤7 prostatic carcinoma. In PSA-screened patients with clinically unapparent or localized disease and carcinoma in only 1 biopsy core, the mere presence of Gleason score ≥8 was associated with a 25-fold increase in the odds ratio for extraprostatic extension when compared with patients with Gleason score 3 + 3 = 6. Significant differences were also noted in Gleason sum 7 cases when compared with Gleason score 3 + 3 = 6: patients with Gleason score 3 + 4 = 7 presented twice the odds ratio for extraprostatic extension, whereas the odds ratio increased 15 times in patients with Gleason score 4 + 3 = 7. These findings, although expected considering the well-established impact that Gleason scoring has for prognosis, highlight the necessity of proper grading for accurate estimation of extraprostatic extension, and the importance of distinguishing between Gleason scores 3 + 4 = 7 and 4 + 3 = 7, even in single foci. Interobserver variability in Gleason scoring continues to be present in biopsy and prostatectomy specimens, especially among general pathologists.19,20 In difficult cases, the request for a second opinion by a urological pathologist could be helpful.21,22 The differences in pathologic outcome between patients with Gleason scores 3 + 4 = 7 and 4 + 3 = 7 has been previously recognized,23 although in a recent study these differences were not significant regarding prostate cancer mortality.4
Comparing the actual values of extraprostatic extension, seminal vesicle invasion, and lymph node metastasis with the predicted ones for matched prebiopsy PSA, Gleason score at biopsy, and clinical stage (see Table 3) we observed no significant differences between actual percentages and predicted percentages, except for the high-grade T2a tumors, with higher percentages than expected according to the Partin tables. Considering the small number of cases in this category (7 patients) these results require further confirmation by larger series. Even though the actual percentages were within the expected values given by the Partin tables, it might also be necessary to consider the percentage of tumor involvement in the biopsy core. Previous studies have shown that the percentage of positive cores in prostate needle biopsy specimens is a strong predictor of tumor stage at radical prostatectomy.8 The significance of the percentage involved by high-grade carcinoma became more evident when the Gleason score ≥8 group was stratified using 50% of tumor involvement as a cutoff point. In those cases with carcinoma involving ≥50% of the core, extraprostatic extension was identified in 7/8 (88%) patients, a value drastically higher than that predicted by the Partin tables, with a 46-fold increase in the odds ratio compared with cases with Gleason score ≤7 (see Table 2, model 2). Even in cases with Gleason score ≥8 involving <50% of one core, the odds ratio for extraprostatic extension on radical prostatectomy was 3 times higher compared with cases with a single core biopsy of prostatic adenocarcinoma with a Gleason score of ≤7.
The presence of seminal vesicle invasion and positive surgical margins are also significant for predicting the outcome of patients with prostate cancer. Several studies have demonstrated that pathologic stage at prostatectomy and positive surgical margins are associated with an increased risk of biochemical recurrence and prostate cancer death.2,3 In a recently published series, including more than 20,000 cancer patients treated by radical prostatectomy, Eggener et al4 found that seminal vesicle invasion and Gleason score ≥8 were the strongest factors associated with prostate cancer death. In patients presenting these features, the risk of dying of the disease was substantially higher than in those without them. In another recently published study, including more than 65,000 patients, Wright et al3 found that the status of the surgical margins was an independent predictor of prostate cancer mortality, highlighting the importance of achieving adequate margins during prostatectomy. In our series, positive surgical margins were identified in 5 cases, all of them patients with Gleason score ≥8 at biopsy. Although this represents only about 20% of all individuals with Gleason score ≥8, we did not observe positive surgical margins in cases with Gleason score ≤7, suggesting the ominous influence of Gleason score ≥8. Even for cases showing cancer in only 1 biopsy core, the presence of Gleason score ≥8 should alert the physician about a higher risk for positive surgical margins. In this context, the identification of the exact site of the biopsy with cancer could help plan therapy and reduce the chances of positive surgical margins during prostatectomy. For those patients undergoing definitive management with radiation therapy, the knowledge that even a single core of Gleason ≥8 disease predicts for a high risk of extraprostatic extension and seminal vesicle invasion should guide the use of combined modality therapy, typically with androgen suppression and radiation, as well as assist in the planning of the radiation therapy fields. Finally, for patients primarily managed by radical prostatectomy, this knowledge should be used to inform patients that adjuvant radiation therapy may be required.
Another noteworthy finding was the high concordance (>95%) in grading between biopsy and prostatectomy in cases with Gleason score ≥8. Only 1 case was downgraded to Gleason 4 + 3 = 7. The concordance was also fair for cases with Gleason 3 + 3 = 6 (74%), but it was poor for cases with 3 + 4 = 7 and 4 + 3 = 7 (≈30%). Considering the distinctive prostatectomy outcomes of patients with Gleason score ≥8, our results suggest that pathologists should be extremely cautious in identifying even a minor component of Gleason pattern 3 that will classify the biopsy core as Gleason score 4 + 3 = 7 instead of Gleason 4 + 4 = 8. In this regard, a Gleason pattern 3 component constituting >% of the tumor should be incorporated as a secondary pattern, as recommended by the revised 2005 ISUP Consensus Conference of Gleason grading of prostatic carcinoma.24
One possible shortcoming of this series is the relatively small number of cases, limiting the extrapolation of our results to the population of patients with similar characteristics (Gleason ≥8 identified in only 1 biopsy core). However, given the rarity of this situation, we believe that our results show a clear tendency toward an adverse pathologic outcome. Nevertheless, confirmation by other series would be necessary. Another possible limitation could be the variability in the number of cores per case. However, at least 6 biopsy cores were taken per patient; the mean ranged between 9 and 12 biopsy cores per Gleason group, and we observed no significant differences in the number of cores per case when stratified by Gleason score (see Table 1). This indicates that the Gleason groups were equally represented and the population was homogeneous regarding the number of biopsy cores per case. Moreover, only 12% of the patients had 6 core biopsies, whereas 70% of them had 12 or more biopsy cores, ensuring an adequate representation of the prostate gland. Finally, prostate size was similar in all groups. Thus, high-grade Gleason score in a single biopsy core was associated with aggressive behavior, independent of gland size or the number of biopsy cores that were taken. This suggests that, in patients with single positive cores, no additional biopsies are required to plan treatment, if at least 6 cores are taken.
CONCLUSIONS
In patients with prostate cancer limited to 1 biopsy core, the presence of Gleason score ≥8 significantly increased the odds ratios for extraprostatic extension, positive surgical margins, and seminal vesicle invasion. The risk was substantially higher in patients with >50% of Gleason ≥8 in the biopsy core. In this context, the actual percentages of extraprostatic extension deviated radically from the ones predicted by the Partin tables. The management of patients with Gleason ≥8 limited to 1 biopsy core should consider the impact that seminal vesicle invasion and positive surgical margins have on the mortality rate of patients with prostate cancer.
Acknowledgments
Funding Support: This study was partially supported by the Johns Hopkins Medicine–Patana Fund for Research.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Pettus JA, Weight CJ, Thompson CJ, et al. Biochemical failure in men following radical retropubic prostatectomy: impact of surgical margin status and location. J Urol. 2004;172:129–132. doi: 10.1097/01.ju.0000132160.68779.96. [DOI] [PubMed] [Google Scholar]
- 3.Wright JL, Dalkin BL, True LD, et al. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. 2010;183:2213–2218. doi: 10.1016/j.juro.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–875. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan SM, Garcia FJ, Chin JL, et al. The clinical significance of in-depth pathological assessment of extraprostatic extension and margin status in radical prostatectomies for prostate cancer. Prostate Cancer Prostatic Dis. 2011 doi: 10.1038/pcan.2011.15. [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Makarov DV, Trock BJ, Humphreys EB, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715–717. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebo TJ, Bock BJ, Cheville JC, et al. The percent of cores positive for cancer in prostate needle biopsy specimens is strongly predictive of tumor stage and volume at radical prostatectomy. J Urol. 2000;163:174–178. [PubMed] [Google Scholar]
- 9.Grossfeld GD, Latini DM, Lubeck DP, et al. Predicting disease recurrence in intermediate and high-risk patients undergoing radical prostatectomy using percent positive biopsies: results from CaPSURE. Urology. 2002;59:560–565. doi: 10.1016/s0090-4295(01)01658-2. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico AV, Whittington R, Malkowicz SB, et al. Clinical utility of the percentage of positive prostate biopsies in defining biochemical outcome after radical prostatectomy for patients with clinically localized prostate cancer. J Clin Oncol. 2000;18:1164–1172. doi: 10.1200/JCO.2000.18.6.1164. [DOI] [PubMed] [Google Scholar]
- 11.Gancarczyk KJ, Wu H, McLeod DG, et al. Using the percentage of biopsy cores positive for cancer, pretreatment PSA, highest biopsy Gleason sum to predict pathologic stage after radical prostatectomy: the Center for Prostate Disease Research nomograms. Urology. 2003;61:589–595. doi: 10.1016/s0090-4295(02)02287-2. [DOI] [PubMed] [Google Scholar]
- 12.Freedland SJ, Aronson WJ, Terris MK, et al. Percent of prostate needle biopsy cores with cancer is significant independent predictor of prostate specific antigen recurrence following radical prostatectomy: results from SEARCH database. J Urol. 2003;169:2136–2141. doi: 10.1097/01.ju.0000065588.82511.06. [DOI] [PubMed] [Google Scholar]
- 13.Paulson DF, Piserchia PV, Gardner W. Predictors of lymphatic spread in prostatic adenocarcinoma: uro-oncology research group study. J Urol. 1980;123:697–699. doi: 10.1016/s0022-5347(17)56095-4. [DOI] [PubMed] [Google Scholar]
- 14.Olsson CA. Staging lymphadenectomy should be an antecedent to treatment in localized prostatic carcinoma. Urology. 1985;25:4–6. [PubMed] [Google Scholar]
- 15.Ravery V, Schmid HP, Toublanc M, et al. Is the percentage of cancer in biopsy cores predictive of extracapsular disease in T1–T2 prostate carcinoma? Cancer. 1996;78:1079–1084. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1079::AID-CNCR18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Gilliland FD, Hoffman RM, Hamilton A, et al. Predicting extracapsular extension of prostate cancer in men treated with radical prostatectomy: results from the population based prostate cancer outcomes study. J Urol. 1999;162:1341–1345. [PubMed] [Google Scholar]
- 17.Noguchi M, Stamey TA, McNeal JE, et al. Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with nonpalpable prostate cancer. J Urol. 2001;166:104–109. [Discussion:109–110]. [PubMed] [Google Scholar]
- 18.Yang XJ, Lecksell K, Potter SR, et al. Significance of small foci of Gleason score 7 or greater prostate cancer on needle biopsy. Urology. 1999;54:528–532. doi: 10.1016/s0090-4295(99)00166-1. [DOI] [PubMed] [Google Scholar]
- 19.Netto GJ, Eisenberger M, Epstein JI, et al. Interobserver Variability in Histologic Evaluation of Radical Prostatectomy between Central and Local Pathologists: Findings of TAX 3501 Multinational Clinical Trial. Urology. 2011;77:1155–1160. doi: 10.1016/j.urology.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allsbrook WC, Jr, Mangold KA, Johnson MH, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum Pathol. 2001;32:81–88. doi: 10.1053/hupa.2001.21135. [DOI] [PubMed] [Google Scholar]
- 21.Brimo F, Schultz L, Epstein JI. The value of mandatory second opinion pathology review of prostate needle biopsy interpretation before radical prostatectomy. J Urol. 2010;184:126–130. doi: 10.1016/j.juro.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Allsbrook WC, Jr, Mangold KA, Johnson MH, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: urologic pathologists. Hum Pathol. 2001;32:74–80. doi: 10.1053/hupa.2001.21134. [DOI] [PubMed] [Google Scholar]
- 23.Chan TY, Partin AW, Walsh PC, et al. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823–827. doi: 10.1016/s0090-4295(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 24.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]