Abstract
Trichinella spiralis larvae establish chronic infections in skeletal muscles of immunocompetent hosts. Muscle infection is crucial to transmission and survival of the parasite in nature. Chronic infections by this highly immunogenic parasite are associated with modulation or escape from potentially destructive immune responses. This review summarizes our current knowledge of immunity to muscle infection with T. spiralis.
Keywords: Trichinella, immunity, regulation, muscle
1. Introduction
Trichinella spiralis is a zoonotic pathogen that has evolved to infect a wide range of mammalian hosts. The nematode is unusual in two ways that have significance for the host immune response: first, the life-cycle is completed in a single host, and second, the parasite resides in two distinct intracellular habitats. Transmission occurs when muscle contaminated with infective, first-stage larvae is consumed. After the parasite is released from the muscle, it invades the epithelium of the small intestine where it matures and reproduces. The immunodominant antigens in larvae are distinct from those in adult worms (Philipp et al., 1980). This antigenic variation, together with the brief period of time required for larvae to mature to adulthood (36–48 hours) and develop into fecund adults (4 to 5 days), allow intestinal worms to escape the immune response until they have reproduced. Eggs hatch in utero and female worms release newborn larvae (NBL) which enter mesenteric venules and disseminate throughout the host (Wang and Bell, 1986a, b). Larvae initiate the muscle phase of infection when they invade individual, terminally-differentiated myotubes (Despommier et al., 1975). Over a period of 20 days (Despommier et al., 1975), the infected myotube re-enters the cell cycle (Jasmer, 1993), remodels the cytoplasmic matrix (Despommier, 1975), synthesizes a collagen capsule (Ritterson, 1966), and induces the formation of a capillary rete around the cell (Humes and Akers, 1952). These changes correlate with profound alterations in host gene expression. Transcription of muscle-specific genes falls dramatically (Jasmer, 1993), while synthesis of syndecan-1 is induced (Beiting et al., 2006), vascular endothelial growth factor (VEGF) genes are activated (Capo et al., 1998) and collagen transcripts are increased (Polvere et al., 1997). These changes correlate with the formation of a structure known as the nurse cell (Purkerson, 1974). Once the parasite completes development in the muscle, it remains infectious for months to years (Froscher et al., 1988).
Mechanisms of immune regulation during this time must be potent in order to preserve the integrity of nurse cells occupied by larvae as large and immunogenic as T. spiralis. While immunity to the intestinal phase of T. spiralis infection has been studied intensively, immunity during the muscle phase has received considerably less attention.
2. Immune responses to T. spiralis muscle infection
2.1. Antibody responses
The circulating antibody responses to T. spiralis infection have been studied extensively in orally-infected mice. Parasite-specific IgG1, IgG2 (Almond and Parkhouse, 1986) and IgE (Zakroff et al., 1989) increase significantly during chronic muscle infection. Eighty percent of IgG1 specific for larval antigens recognize a single shared epitope (Denkers et al., 1990), now known to be the highly immunogenic sugar, tyvelose (Reason et al., 1994). The dominance of parasite-specific IgG1 and IgE during chronic infection is associated with a strong TH2 response in cervical lymph nodes (Beiting et al., 2007).
2.2. Cellular responses
Inflammatory infiltrates fail to form around nurse cells in T lymphocyte deficient mice, identifying these cells as the coordinators of the cellular response to muscle infection (Walls et al., 1973). The mechanisms by which T cells regulate inflammation during muscle infection have begun to be elucidated. Direct injection of NBL into thigh muscles of BALB/c mice initiates muscle infection and activates popliteal lymph node cells that produce IL-4 when stimulated with the mitogen concanavalin A (Li and Ko, 2001). Furthermore, the number of IL-4 transcribing cells increases dramatically in diaphragms of mice during chronic infection (Table 1) (Beiting and Appleton, unpublished observations). Cells recovered from cervical lymph nodes of C57BL/10 or C57BL/6 mice bearing muscle larvae produce IL-5, IL-10, IL-13 and IFN-γ after stimulation with somatic larval antigens (Beiting et al., 2007; Beiting et al., 2006). Blood mononuclear cells recovered from human subjects over a year after an outbreak of trichinosis produced significant quantities of IFN-γ, IL-10 and IL-5, and retained the ability to proliferate in response to larval antigens for as long as three years after infection (Morales et al., 2002). Collectively, these data suggest that T. spiralis induces a mixed cytokine response, but a more thorough examination of the role of T cells in muscle infection is needed.
Table 1.
Representation of IL-4+ cells in diaphragm and cervical lymph nodes (CLN) of 4get (Mohrs et al. 2001) mice prior to infection or 39 days post-intravenous infection with 25,000 T. spiralis newborn larvae.
| % IL-4+ cells* |
||||
|---|---|---|---|---|
| Uninfected |
39 days post-infection |
|||
| Lymphocytes | CD4+ | Lymphocytes | CD4+ | |
| CLN | 0.28±0.02# | 0.53 ± 0.03 | 0.66 ± 0.05 | 1.57 ± 0.15 |
| Diaphragm | 0.45 ± 0.027 | 8.2 ± 3.3 | 3.7 ± 0.56 | 22.5 ± 1.6 |
Percentages of total lymphocytes or CD4+ lymphocytes in each compartment that were GFP+ when cells were analyzed by flow cytometry. Cells were prepared and analyzed as described in Beiting et al. 2004.
mean ± SD; n = 4 per group.
Despite a potent and persistent B cell response during muscle infection, the local inflammatory response to the T. spiralis nurse cell is limited suggesting that suppressive parasite or host factors are at work. Trichinella spiralis infection of host muscle affords a relevant model to study the mechanisms by which nematodes modulate the host cellular response during chronic infection.
Early histologic studies of infected muscle revealed a focus of inflammation surrounding individual infected muscle cells (Walls et al., 1973). Similar to a granuloma, the focal infiltrate surrounding individual nurse cells is rich in macrophages (Beiting et al., 2004; Karmanska et al., 1997a). Macrophages are also observed in the cytoplasm of the nurse cell (Karmanska et al., 1997b) and these cells can be detected with antibody specific for CD11b (Beiting and Appleton, unpublished observations).
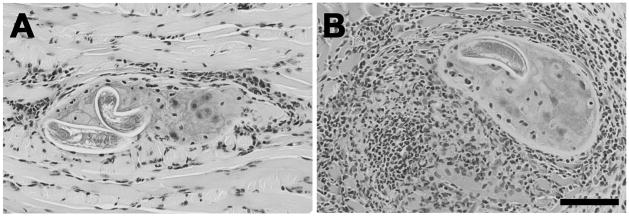
We have tested the hypothesis that the anti-inflammatory cytokine IL-10 moderates cellular responses to muscle stage parasites (Beiting et al., 2004). In order to study the immune response induced and maintained by muscle infection, we infected mice by injecting NBL intravenously. This method establishes a synchronous infection that is not complicated by the potent immune response induced by the intestinal phase. Cells infiltrating sites of infection were predominantly macrophages but included numerous CD4+ T cells, fewer CD8+ T cells, and rare B lymphocytes. The effect of IL-10 was most evident 20 days after muscle infection, when the intensity of the focal response in IL-10 deficient mice was dramatically increased compared to C57BL/6 wild-type mice (Figure 1). While the numbers of cells increased in the absence of IL-10, the composition of muscle infiltrates was unchanged. Mice that are infected by the natural route such that they experience intestinal infection prior to muscle infection develop stronger TH2 responses in cervical lymph nodes than do mice given synchronous muscle infections (Fabre and Appleton, unpublished observations). Nevertheless, the influence of IL-10 on myositis is similar in infections initiated by either route (Fabre and Appleton, unpublished observations).
Fig. 1.
Influence of IL-10 on cellular responses to T. spiralis muscle infection. Cellular infiltrates around nurse cells in (A) C57BL/10 or (B) IL-10 deficient mice, 21 days post-intravenous infection. H&E stained sections of tongues collected from C57BL/10 or IL-10 deficient mice. Scale bar, 100 μm.
Enhanced myositis in IL-10 deficient mice did not affect parasite establishment or survival (Beiting et al., 2004). Thus, in the context of a primary muscle infection, IL-10 functions largely to protect the host against injury caused by inflammation.
Between 20 and 50 days post-infection the inflammatory response attenuates in both wild-type and IL-10 deficient mice, suggesting that chronic inflammation is controlled independently of IL-10. Muscle infection elicits an antibody response of mixed isotypes directed at somatic larval antigens that changes to an IgG1 dominated response directed at tyvelose-bearing ES antigens (Beiting et al., 2004). The emergence of IgG1 coincides with the IL-10 independent phase of the cellular response. Thus, IL-10 limits the local inflammatory response during the early stages of muscle infection while control of chronic inflammation is IL-10-independent and is coincident with the emergence of a potent TH2 response.
Results of cell depletion and adoptive transfer experiments implicate CD4+ CD25− T cells rather than CD4+ CD25+ T regulatory cells as pivotal to IL-10 modulation of acute inflammation (Beiting et al., 2007). In addition to IL-10, TGF-β is known to mediate T cell suppression (Zeller et al., 1999). Treatment of wild-type mice with antibodies to TGF-β promotes myositis; however, the combined deficiency in TGF-β and IL-10 leads to a much more severe inflammatory response in muscles. The depletion of TGF-β alone did not affect parasite survival but the combined deficiency of TGF-β and IL-10 was associated with parasite death in muscle (Beiting et al., 2007). Thus, TGF-β cooperates with IL-10 in the control of local inflammation. In the absence of IL-10, TGF-β promotes parasite survival in muscles.
3. Conclusions
Muscle larvae are important causes of morbidity during T. spiralis infection. IL-10 is an influential regulator of the inflammation caused by this life stage. IL-10 limits the inflammatory responses to the developing larva during the acute phase of muscle infection, while the TH2 response induced during chronic infection may serve to protect the mature, infectious parasite. The source(s) of IL-10, as well as the identities of other regulatory influences need to be defined in order to more completely understand the interaction between the successful parasite and its host.
Acknowledgments
We acknowledge the support of these grants from the National Institutes of Health: AI14490 (JAA), T32-AI07643 (DPB), T32- CA09682 and DK67290 (SPK).
Footnotes
Conflict of interest statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almond NM, Parkhouse RM. The Ig class distribution of anti-phosphoryl choline responses in mice infected with parasitic nematodes. Immunology. 1986;59:633–635. [PMC free article] [PubMed] [Google Scholar]
- Beiting DP, Bliss SK, Schlafer DH, Roberts VL, Appleton JA. Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infection and immunity. 2004;72:3129–3137. doi: 10.1128/IAI.72.6.3129-3137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. Journal of immunology (Baltimore, Md: 1950) 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- Beiting DP, Park PW, Appleton JA. Synthesis of syndecan-1 by skeletal muscle cells is an early response to infection with Trichinella spiralis but is not essential for nurse cell development. Infect Immun. 2006;74:1941–1943. doi: 10.1128/IAI.74.3.1941-1943.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo VA, Despommier DD, Polvere RI. Trichinella spiralis: vascular endothelial growth factor is up-regulated within the nurse cell during the early phase of its formation. The Journal of parasitology. 1998;84:209–214. [PubMed] [Google Scholar]
- Denkers EY, Wassom DL, Krco CJ, Hayes CE. The mouse antibody response to Trichinella spiralis defines a single, immunodominant epitope shared by multiple antigens. Journal of immunology (Baltimore, Md: 1950) 1990;144:3152–3159. [PubMed] [Google Scholar]
- Despommier D. Adaptive changes in muscle fibers infected with Trichinella spiralis. The American journal of pathology. 1975;78:477–496. [PMC free article] [PubMed] [Google Scholar]
- Despommier D, Aron L, Turgeon L. Trichinella spiralis: growth of the intracellular (muscle) larva. Experimental parasitology. 1975;37:108–116. doi: 10.1016/0014-4894(75)90058-2. [DOI] [PubMed] [Google Scholar]
- Froscher W, Gullotta F, Saathoff M, Tackmann W. Chronic trichinosis. Clinical, bioptic, serological and electromyographic observations. European neurology. 1988;28:221–226. doi: 10.1159/000116271. [DOI] [PubMed] [Google Scholar]
- Humes AG, Akers RP. Vascular changes in the cheek pouch of the golden hamster during infection with Trichinella spiralis larvae. The Anatomical Record. 1952;114:103–113. doi: 10.1002/ar.1091140108. [DOI] [PubMed] [Google Scholar]
- Jasmer DP. Trichinella spiralis infected skeletal muscle cells arrest in G2/M and cease muscle gene expression. The Journal of cell biology. 1993;121:785–793. doi: 10.1083/jcb.121.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmanska K, Houszka M, Widyma A, Stefaniak E. Macrophages during infection with Trichinella spiralis in mice. Wiadomosci parazytologiczne. 1997a;43:245–249. [PubMed] [Google Scholar]
- Karmanska K, Houszka M, Widyma A, Stefaniak E. The cells observed inside capsules of larvae in the course of experimental trichinellosis in mice. Wiadomosci parazytologiczne. 1997b;43:251–256. [PubMed] [Google Scholar]
- Li CK, Ko RC. Inflammatory response during the muscle phase of Trichinella spiralis and T. pseudospiralis infections. Parasitology research. 2001;87:708–714. doi: 10.1007/s004360100420. [DOI] [PubMed] [Google Scholar]
- Morales MA, Mele R, Sanchez M, Sacchini D, De Giacomo M, Pozio E. Increased CD8(+)-T-cell expression and a type 2 cytokine pattern during the muscular phase of Trichinella infection in humans. Infection and immunity. 2002;70:233–239. doi: 10.1128/IAI.70.1.233-239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M, Parkhouse RM, Ogilvie BM. Changing proteins on the surface of a parasitic nematode. Nature. 1980;287:538–540. doi: 10.1038/287538a0. [DOI] [PubMed] [Google Scholar]
- Polvere RI, Kabbash CA, Capo VA, Kadan I, Despommier DD. Trichinella spiralis: synthesis of type IV and type VI collagen during nurse cell formation. Experimental parasitology. 1997;86:191–199. doi: 10.1006/expr.1997.4180. [DOI] [PubMed] [Google Scholar]
- Purkerson M, Despommier DD. Trichinellosis. Intext Educational Publishers; New York: 1974. Fine structure of the muscle phase of Trichinella spiralis in the mouse; pp. 7–23. [Google Scholar]
- Reason AJ, Ellis LA, Appleton JA, Wisnewski N, Grieve RB, McNeil M, Wassom DL, Morris HR, Dell A. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology. 1994;4:593–603. doi: 10.1093/glycob/4.5.593. [DOI] [PubMed] [Google Scholar]
- Ritterson AL. Nature of the cyst of Trichinella spiralis. The Journal of parasitology. 1966;52:157–161. [PubMed] [Google Scholar]
- Walls RS, Carter RL, Leuchars E, Davies AJ. The immunopathology of trichiniasis in T-cell deficient mice. Clinical and experimental immunology. 1973;13:231–242. [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Bell RG. Trichinella spiralis: newborn larval migration route in rats reexamined. Experimental parasitology. 1986a;61:76–85. doi: 10.1016/0014-4894(86)90137-2. [DOI] [PubMed] [Google Scholar]
- Wang CH, Bell RG. Trichinella spiralis: vascular recirculation and organ retention of newborn larvae in rats. Experimental parasitology. 1986b;62:430–441. doi: 10.1016/0014-4894(86)90052-4. [DOI] [PubMed] [Google Scholar]
- Zakroff SG, Beck L, Platzer EG, Spiegelberg HL. The IgE and IgG subclass responses of mice to four helminth parasites. Cellular immunology. 1989;119:193–201. doi: 10.1016/0008-8749(89)90235-9. [DOI] [PubMed] [Google Scholar]
- Zeller JC, Panoskaltsis-Mortari A, Murphy WJ, Ruscetti FW, Narula S, Roncarolo MG, Blazar BR. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. Journal of immunology Baltimore, Md. 1999;163:3684–3691. 1950. [PubMed] [Google Scholar]