Abstract
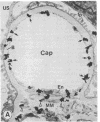
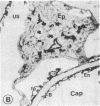
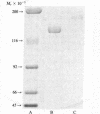
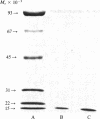
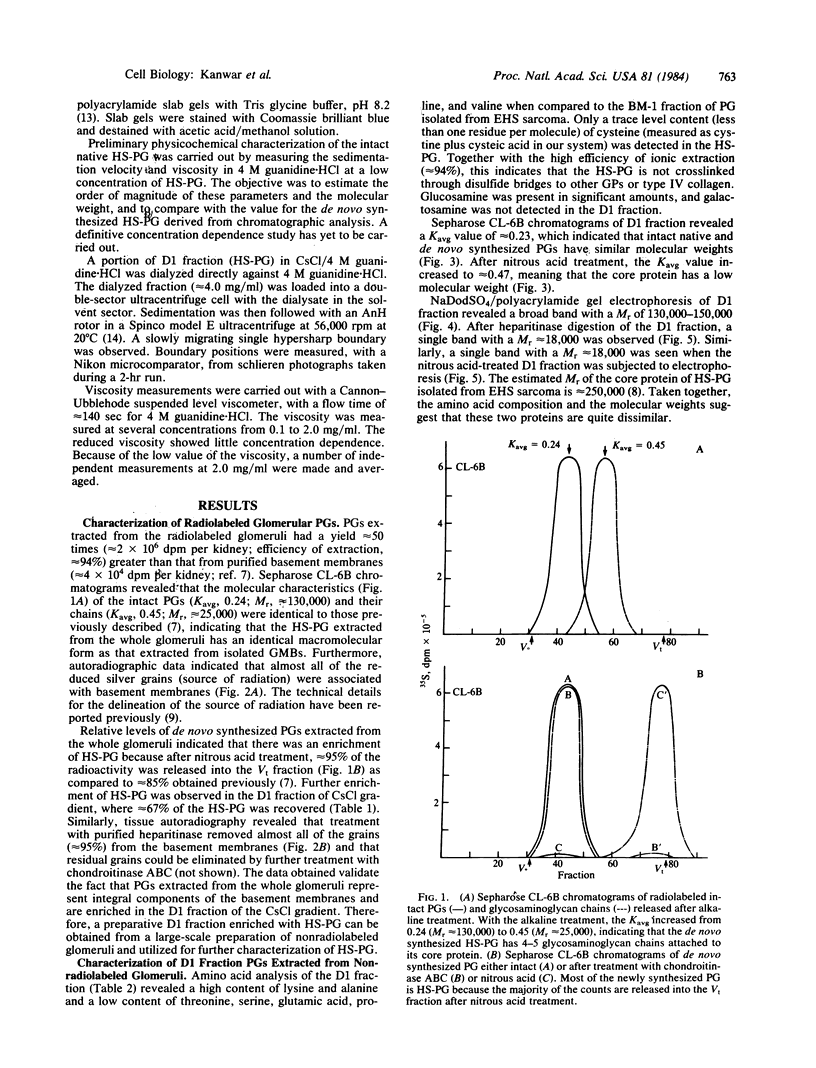
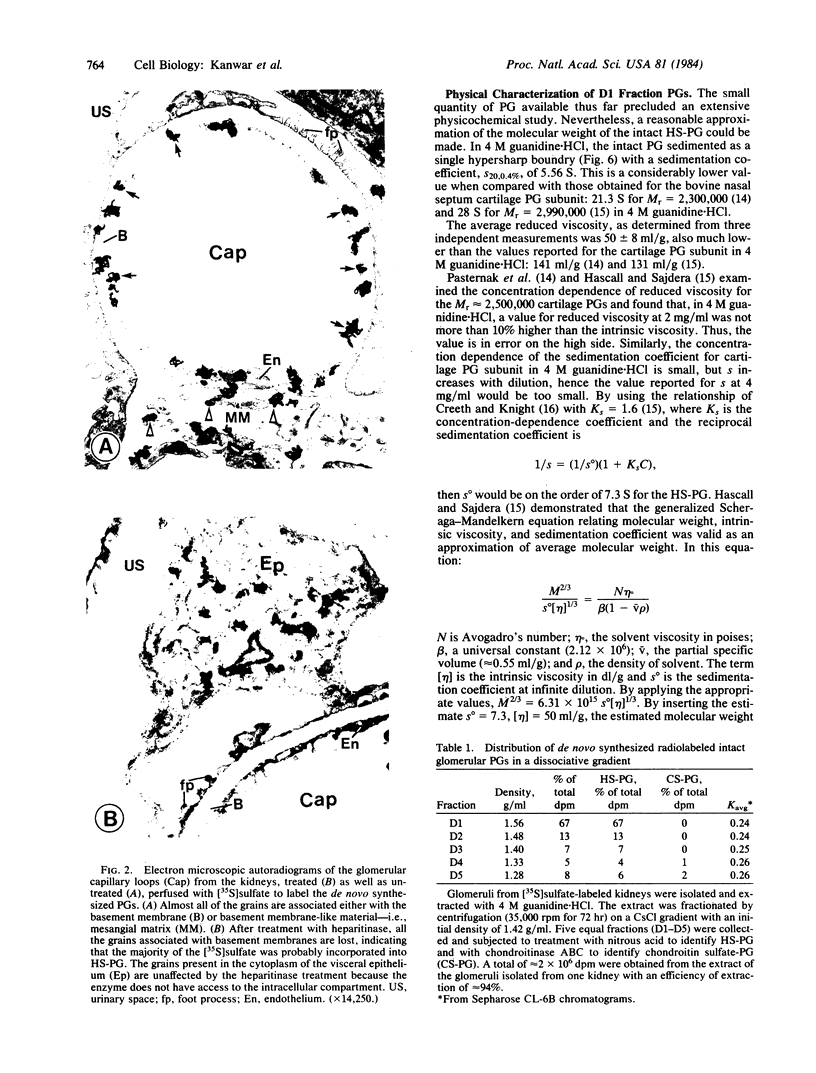
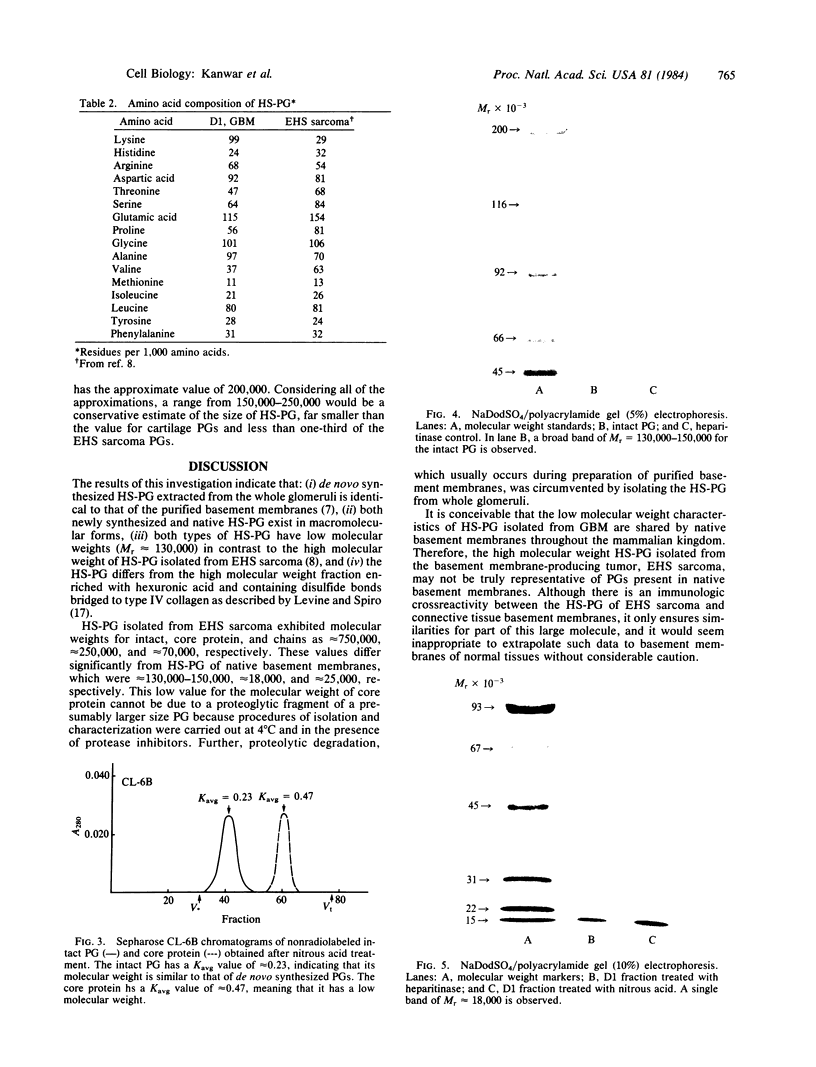
Native and de novo synthesized heparan sulfate-proteoglycan (HS-PG) of basement membranes from isolated whole glomeruli were characterized. Sepharose CL-6B chromatograms of [35S]sulfate-labeled de novo synthesized HS-PG extracted from whole glomeruli indicated identical molecular weight characteristics to that isolated from purified basement membranes (Mr of intact HS-PG approximately equal to 130,000; Mr of chains approximately equal to 25,000). Electron microscopic autoradiography showed that almost all radioactive grains were localized to the basement membranes proper. The estimated Mr of core protein approximately equal to 18,000. The sedimentation coefficient of native intact HS-PG was 5.56 S, corresponding to a Mr between 150,000-250,000, a value in accord with gel filtration data on newly synthesized HS-PG. Physicochemical characteristics of HS-PG of native functional basement membranes differed remarkably from that isolated from the basement membrane-producing tumor, Engelbreth-Holm-Swarm sarcoma.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B. M., Hostetter T. H., Humes H. D. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978 Apr 13;298(15):826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Knight C. G. On the estimation of the shape of macromolecules from sedimentation and viscosity measurements. Biochim Biophys Acta. 1965 Jul 22;102(2):549–558. doi: 10.1016/0926-6585(65)90145-7. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4493–4497. doi: 10.1073/pnas.76.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Hascall V. C., Farquhar M. G. Partial characterization of newly synthesized proteoglycans isolated from the glomerular basement membrane. J Cell Biol. 1981 Aug;90(2):527–532. doi: 10.1083/jcb.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J. Clogging of the glomerular basement membrane. J Cell Biol. 1982 May;93(2):489–494. doi: 10.1083/jcb.93.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J., Linker A., Jakubowski M. L. Decreased de novo synthesis of glomerular proteoglycans in diabetes: biochemical and autoradiographic evidence. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2272–2275. doi: 10.1073/pnas.80.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Pettersson I., Hök M. Cell-surface heparan sulfate: an intercalated membrane proteoglycan. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5371–5375. doi: 10.1073/pnas.78.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Spiro R. G. Isolation from glomerular basement membrane of a glycopeptide containing both asparagine-linked and hydroxylysine-linked carbohydrate units. J Biol Chem. 1979 Sep 10;254(17):8121–8124. [PubMed] [Google Scholar]
- Linker A., Hovingh P., Kanwar Y. S., Farquhar M. G. Characterization of heparan sulfate isolated from drug glomerular basement membranes. Lab Invest. 1981 Jun;44(6):560–565. [PubMed] [Google Scholar]
- Meezan E., Hjelle J. T., Brendel K., Carlson E. C. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975 Dec 1;17(11):1721–1732. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Kjellén L., Hök M. Cell-surface heparan sulfate. Isolation and characterization of a proteoglycan from rat liver membranes. J Biol Chem. 1979 Sep 10;254(17):8505–8510. [PubMed] [Google Scholar]
- Pasternack S. G., Veis A., Breen M. Solvent-dependent changes in proteoglycan subunit conformation in aqueous guanidine hydrochloride solutions. J Biol Chem. 1974 Apr 10;249(7):2206–2211. [PubMed] [Google Scholar]
- Rapraeger A. C., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. A putative membrane proteoglycan associates quantitatively with lipid vesicles. J Biol Chem. 1983 Mar 25;258(6):3632–3636. [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]