Abstract
Cardiomyocytes express one or more subtypes of P2 receptors for extracellular nucleotides. P2 purinoceptors, which are activated by nucleotides, are classified as P2X or P2Y: P2X receptors are ligand-gated intrinsic ion channels, and P2Y receptors are G protein-coupled receptors. Extracellular pyrimidine and purine nucleotides are released from the heart during hypoxia. Although the cardioprotective effects of purines acting via purinoceptors were studied intensively, the physiological role of uracil nucleotide-responsive P2Y2, P2Y4, P2Y6, and P2Y14 receptors is still unclear, especially in the cardiovascular system. This study revealed that uridine-5′-triphosphate (UTP) protected cultured rat cardiomyocytes during hypoxia and explored the UTP signaling pathway leading to this cardioprotection. We found that UTP, but not UDP or uridine, significantly reduced cardiomyocyte death induced by hypoxia. Incubation with UTP for 1 h, before exposure to hypoxic conditions, protected the cells 24 h later. The cardioprotective effect of UTP was reduced in the presence of the P2 antagonist suramin. In addition, UTP caused a transient increase of [Ca2+]i in cardiomyocytes. Pyridoxal-5′-phosphate-6-azophenyl-2,4-disulfonate (PPADS) or Reactive blue 2 (RB-2), other antagonists of P2 receptors, abolished the [Ca2+]i elevation caused by UTP. We used various inhibitors of the Ca2+ signaling pathway to show that UTP elevated levels of [Ca2+]i, originating from intracellular sources, via activation of phospholipase C and the IP3 receptor. Interestingly, these inhibitors of the Ca2+ signaling pathway did not prevent the immediate protective effect caused by UTP. Although mitochondrial KATP channels are involved in other preconditioning mediator pathways, the involvement of these channels in the cardioprotective effect induced by UTP was ruled out, because 5-hydroxydecanoic acid (5-HD), a specific inhibitor of these channels, did not prevent the protection.
Keywords: P2Y2 nucleotide receptor, G protein-coupled receptor, Pyrimidines, Cardioprotection, Ischemia, Preconditioning
1. Introduction
Myocardial infarction and heart failure are leading causes of morbidity and mortality in humans. Considerable effort has been devoted to improving functional recovery and reducing the extent of infarction after ischemic episodes. A step in this direction was the discovery that the heart is significantly protected against ischemic injury when first preconditioned by a brief ischemia [1]. Adenosine, a metabolite of adenine nucleotides, simulates the protection afforded by preconditioning (PC) via A1 and A3 receptors. However, accumulating evidence suggests that adenosine receptors are not the only mediators of PC [2-4], but that adrenoceptors [5], bradykinin receptors [6], opioid receptors [7], and ATP receptors [8] may also contribute to PC.
Purine and pyrimidine nucleotides have widespread and specific extracellular signaling actions in the regulation of a variety of functions in many tissues. In 1978 Burnstock proposed that specific receptors for extracellular nucleosides (P1) or nucleotides (P2) mediate the physiological effects of adenosine and ATP, respectively [9]. According to pharmacological evidence, mechanisms of signal transduction, and molecular structure, receptors that mediate the extracellular actions of purine and pyrimidine nucleotides are divided into two major families: ligand-gated receptors (P2X) and G protein-coupled receptors (P2Y). In contrast to P2X receptors, the class of P2Y receptors is activated not only by ATP and its derivatives, but also by other naturally occurring nucleotides. The P2Y2 and P2Y4 receptors are activated by the uracil nucleotide UTP (uridine-5′-triphosphate) acting as an autocrine regulator [10,11]. These receptors are preferentially coupled to Gq and therefore activate phospholipase Cβ, leading to the formation of inositol trisphosphate, Ca2+ elevation, and production of diacylglycerol, which activates protein kinase C (PKC) and mitogen-activated protein kinases [12,13]. Thus, activation of P2Y2 and P2Y4 receptors and subsequently kinases can have long-term, tropic effects on cardiomyocyte activity.
Whereas the effects of purine nucleosides and nucleotides in myocardial infarction and ischemia have been intensively studied [14], the role of pyrimidine nucleotides under hypoxic conditions has not been well explored. Our principal aim was to elucidate the protective effects of UTP and pyrimidinergic receptor activation against detrimental factors of ischemia/hypoxia. For this reason we sought to explore whether P2Y2 and P2Y4 receptors are present on the surface of cultured newborn rat cardiomyocytes and the expression levels of these receptors. Receptor functionality was demonstrated by evaluation of the calcium response following receptor activation and by the initiation of signal transduction pathways, which resulted in attenuating cardiomyocyte injury in hypoxia. For this purpose we studied the expression and localization of P2Y2 and P2Y4 receptor proteins in cultured cardiomyocytes through immunohistochemistry and Western blotting. Changes in intracellular Ca2+ concentration ([Ca2+]i) after the application of nucleotides were studied by indo-1 cytofluorometry. Morphological evaluation of cultured cells, measurement of lactate dehydrogenase (LDH) release to the culture medium, and determination of ATP content were used to characterize the protection from hypoxic damage upon UTP receptor activation.
The present study provides evidence for the first time that the extracellular pyrimidine nucleotide, UTP, could play a substantial role in mediating cardioprotection from hypoxic damage through specific receptors.
2. Experimental procedures
2.1. Cell culture
Rat hearts (2–3 d old) were removed under sterile conditions and washed three times in PBS to remove excess blood cells. The hearts were minced and then gently agitated in RDB, a solution of proteolytic enzymes (Life Science Research Inst. [15]), prepared from a fig tree extract. After treatment with RDB, cell viability was found to be 98%, in contrast to 85% in trypsin-treated monolayer cultures. The RDB was diluted 1:100 in Ca2+- and Mg2+-free PBSat 25 °C for a fewcycles of 10 min each, as described previously [16]. Dulbecco’s modified Eagle’s medium, supplemented with inactivated 10% horse serum (Biological Industries) and 0.5% chick embryo extract, was added to supernatant suspensions containing dissociated cells. The mixture was centrifuged at 300 × g for 5 min. The supernatant phase was discarded, and the cells were resuspended in the same medium. The suspension of the cells was diluted to 1.0 × 106 cells/ml, and 1.5 ml of the suspension was placed in 35-mm plastic culture dishes on collagen/gelatin-coated coverglasses. The cultures were incubated in a humidified atmosphere of 5% CO2, 95% air at 37 °C. Confluent monolayers exhibiting spontaneous contractions were developed in culture within 2 d. All experiments were performed between days 5 and 7 in culture.
2.2. [Ca2+]i measurements
Intracellular free calcium ion concentration was estimated from indo-1 fluorescence with the ratio method (the SAMPLE program) described elsewhere [17,18].
2.3. Hypoxic conditions
Myocyte cultures 5–7 d old were washed from the medium with glucose-free PBS containing 5 μM HEPES at pH 7.4 before exposing the myocytes to the various conditions at 37 °C. The hypoxic condition consisted of 120 min in a hypoxic incubator in which the atmosphere was replaced by the inert gas argon (100%) in glucose-free media [16,19]. The hypoxic damage was characterized at the end of the hypoxic period by morphological and biochemical evaluation. Continuous monitoring of [Ca2+]i during hypoxia was realized in a special barrier well, where cells were protected from oxygen by a laminar counterflowing layer of argon gas as previously described [20]. The coverglasses with cultured cells were placed at the bottom of the well. This chamber was mounted on a specially modified Zeiss inverted epifluorescence microscope.
2.4. Assays of intracellular ATP level
After hypoxia, control and experimental cells were harvested in 1 ml cold 5% trichloroacetic acid. The cell extract was used for the measurement of ATP content with the luciferin–luciferase bioluminescence kit (CLSII, Boehringer) following the manufacturer’s protocol. The values are expressed as nmol/mg of protein [17].
2.5. Experiments with purinergic and pyrimidinergic ligands
UTP at concentrations of 3–50 μM was applied to the cell cultures for 15 min following a 15-min preincubation with various antagonists (Sigma): pyridoxal-5′-phosphate- 6-azophenyl-2,4-disulfonate (PPADS), suramin, or Reactive blue 2 (RB-2). The cell cultures were washed with PBS prior to the experimental treatment.
2.6. Release of LDH
Protein content and LDH activity were determined according to El-Ani et al. [21]. Briefly, 25 μl supernatant was transferred into a 96-well dish, and the LDH activities were determined with an LDH-L kit (Sigma), as described by the manufacturer. The product of the enzyme was measured spectrometrically at 30 °C at a wavelength of 340 nm as described previously [19]. The results were expressed relative to the control (X-fold) in the same experiment. Each experiment was done in triplicate and was repeated at least three times.
2.7. Staining
2.7.1. Propidium iodide
The assay is based on vital binding of propidium iodide (5 μg/ml) to nuclei of cells whose plasma membranes have become permeable because of cell damage. The assay was performed according to Nieminen et al. [22]. For counter-staining we used Hoechst 33342 (10 μM), which stains the nuclei of all cells.
2.7.2. Lysosome staining by neutral red
Neutral red is a widely used marker of membrane-bound compartments with an acidic lumen (lysosomes and several other acidic compartments). Live cardiomyocytes were incubated for 30 min in PBS containing 4 μg/ml neutral red (Molecular Probes).
2.7.3. Immunostaining
Cells were fixed in absolute methanol for 10 min, permeabilized with 0.1% Triton X-100 for 5 min, and blocked in 2% normal goat serum for 10 min. Anti-P2Y2 or anti-P2Y4 antibodies (Alomone Labs) were diluted in PBS to a final concentration of 1:100 and then incubated overnight at room temperature with the cells. As a reporter we used goat antirabbit fluorescein isothiocyanate (FITC) that was incubated for 30 min at room temperature.
2.7.4. Western blotting
Aliquots (~70 μg) from each protein sample were loaded on 11% SDS polyacrylamide gels and blotted onto nitrocellulose membranes. Filters were saturated with Tris-buffered saline in 10% milk for 1 h at room temperature to avoid non-specific binding of the primary antibody and incubated overnight at 4 °C with anti-P2Y2 (1:200) and anti-P2Y4 (1:300) antibodies. Each P2Y receptor was also tested in the presence of neutralizing peptides (ratio 1:1 between peptide and antiserum). Blots were then washed in Tris-buffered saline (1 mM Tris–HCl, pH 8.0, 15 mM NaCl, 0.1% Tween X-100, final concentration), incubated for 1 h with antirabbit IgG coupled to FITC. Immunoreactions were analyzed with enhanced chemiluminescence.
2.8. Statistics
Results were expressed as mean ± S.D. Data were analyzed by analysis of variance with application of a post hoc Tukey-Kremer test. Statistical significance was determined as *P < 0.01 and **P < 0.05 in different experiments.
3. Results
3.1. Cardiomyocytes express UTP-responsive P2Y receptors
The presence of P2Y2 and P2Y4 receptors in cultured rat cardiomyocytes was demonstrated with immunofluorescent staining and Western immunoblot assay. Newborn rat cardiomyocytes exhibited P2Y2 and P2Y4 receptors on the cell surface, as seen from the fluorescence intensity level. The specificity of receptor immunostaining was indicated by lack of staining in the presence of competitive peptide antigens, derived from the receptor sequence. Anti-P2Y2 or -P2Y4 antibodies stained the whole cardiomyocyte membrane, but no staining was observed in fibroblasts that were present in the same culture dish (Fig. 1A and B). An immunoblot assay showed that anti-P2Y2 antibodies detected four major protein bands of the predicted molecular weight at 42, 55, 68, and 84 kDa, and P2Y4 receptors were identified by two protein bands, which were ~50 and ~80 kDa. These bands disappeared following preincubation of the antibodies with their competitive peptide antigens (Fig. 1C). These results demonstrated the existence of P2Y2 and P2Y4 receptors in neonatal cardiomyocytes.
Fig. 1.
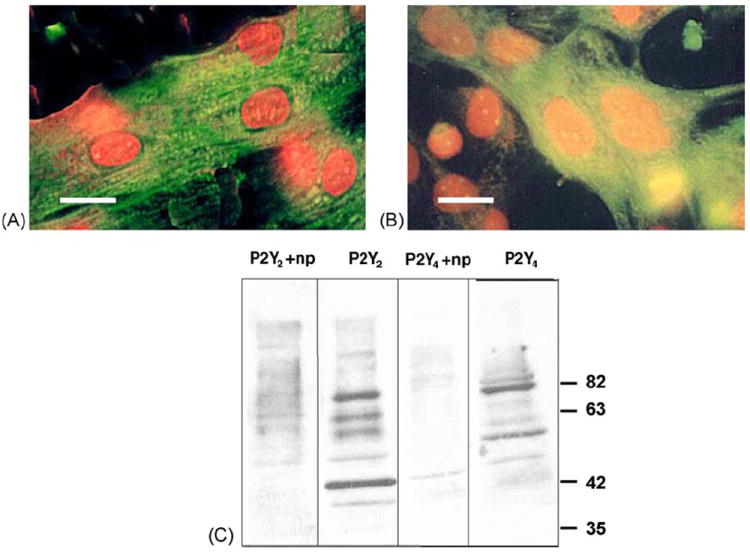
Determination of P2Y receptors in cardiomyocytes by immunofluorescent staining and immunoblotting. Cardiomyocytes were fixed in methanol, permeabilized with 1% Triton X-100, blocked and incubated in the presence of antibodies against P2Y2 (A) or P2Y4 (B) receptors. For detection of the bound antibodies, cells were incubated with goat antirabbit FITC (green) following counterstaining with propidium iodide (red). Bars = 10 μm. Proteins isolated from rat cardiomyocyte homogenates were subjected to SDS-PAGE on an 11% acrylamide gel and transferred onto a nitrocellulose membrane. Filters were probed with the indicated P2Y antibodies simultaneously with the neutralizing peptide (np). Protein bands were detected with a secondary antibody coupled to peroxidase by enhanced chemiluminescence (C).
3.2. Effect of UTP on cardiomyocytes subjected to hypoxia
To investigate the role of UTP in attenuation of myocyte injury during prolonged (120 min) hypoxia, cultured cardiomyocytes were incubated with various concentrations of UTP (3–50 μM) for 15 min prior to hypoxia. The pyrimidine (10 μM) did not cause any cell injury but elicited a significant attenuation of the cellular injury produced by the subsequent 120-min hypoxia, as measured by the level of LDH released from the cells (Fig. 2A). The decrease in LDH release suggested that UTP receptor activation delayed acute hypoxia-induced cardiomyocyte injury. For investigation of the time dependence of UTP in protecting the cardiocytes, the cells were pretreated with 50 μM UTP for 1 h and then exposed to 2 h hypoxia after incubation in full medium for 15 min to 72 h. UTP protected the cells from hypoxia even when treated 48 h before hypoxia, as revealed by LDH release (Fig. 2B). These results suggest that UTP activates a long, persistent mechanism of protection (a second window of protection). On the other hand, the related derivatives uridine and UDP (50 μM) did not exhibit a protective effect on cardiomyocytes against hypoxia (Table 1). This ruled out the P2Y6 receptor as the site of action of protective uracil nucleotides.
Fig. 2.
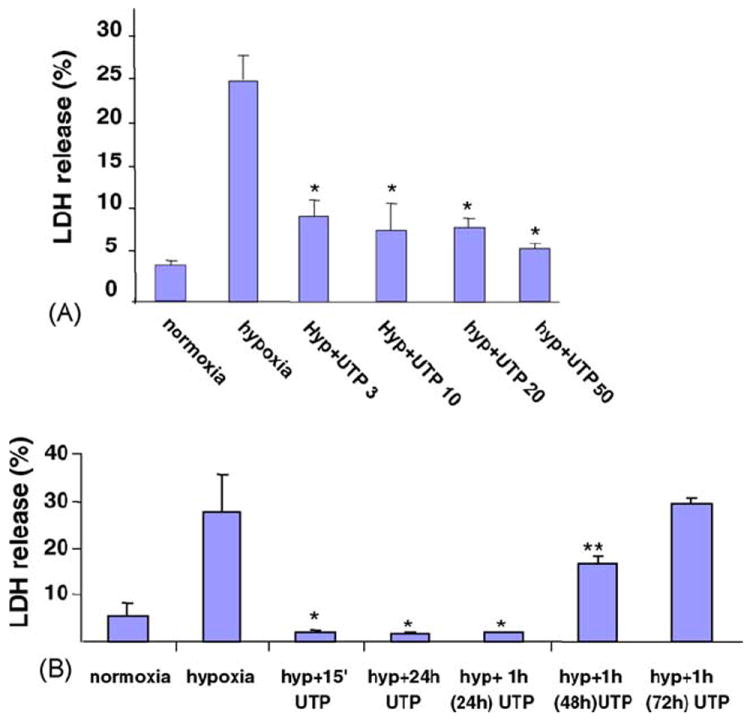
Concentration- and time-dependent effect of UTP on cardiomyocytes subjected to hypoxia. Six-day-old cardiomyocytes were treated for 15 min with various concentrations of UTP (3–50 μM). The cells were then washed twice and subjected to hypoxia for 2 h in glucose-free PBS, at 37 °C. The amount of LDH released to the medium was determined and compared to the total activity of control homogenate (100%; A). Cardiomyocytes were treated with 50 μM UTP for 15 min or 24 h before hypoxia. Other groups of cells were incubated with 50 μM UTP for 1 h, washed and replaced with normal medium and then subjected to hypoxia 24, 48, or 72 h subsequent to the treatment with UTP (B). Data are means of at least three replicates in five separate experiments ± S.D. *P < 0.01, **P < 0.05 compared with hypoxia.
Table 1.
Effect of uracil derivatives or P2 receptors antagonists on cardiomyocytes subjected to hypoxia
| Treatment | LDH release (%) |
|---|---|
| Normoxia | 4.2 ± 0.6 |
| Hyp | 40 ± 2.4 |
| Hyp + UTP | 10 ± 1.6* |
| Hyp + uridine | 29 ± 2.5 |
| Hyp + UDP | 36 ± 2.6 |
| Hyp + 5-HD | 37 ± 0.6 |
| Hyp + UTP + 5-HD | 12 ± 1.6* |
| Hyp + UTP + PPADS | 32 ± 3.6 |
| Hyp + UTP + suramin | 25 ± 3.5 |
| Hyp + UTP + RB-2 | 11 ± 0.8* |
Cardiomyocytes were treated with 50 μM uridine, UDP, or UTP or with P2 receptor antagonist PPADS (10 μM), RB-2 (50 μM), or suramin (300 μM) or with 5-HD, a mitochondrial KATP channel inhibitor (300 μM), for 15 min before hypoxia. The cells were then washed twice and subjected to hypoxia for 2 h in glucose-free PBS, at 37 °C. The amount of LDH released to the medium was determined and compared to the total activity of control homogenate (100%). Data are means of at least three replicates in three separate experiments ± S.D.
P < 0.01 compared with hypoxia.
To verify whether the protective effect caused by UTP is mediated via P2 receptors, cardiomyocytes were treated with a P2 receptor antagonist, e.g. RB-2, PPADS, or suramin, before exposing them to 50 μM UTP and hypoxia. Treatment with suramin or PPADS, but not RB-2, inhibited the protective effect of UTP, as determined by LDH released to the medium (Table 1), indicating the involvement of P2Y2 receptors and at least partly of P2Y4 receptors in UTP-induced protection of cultured cardiomyocytes.
3.3. Effect of P2 agonists/antagonists on intracellular ATP levels during hypoxia
The protective effect caused by extracellular UTP was also demonstrated by protection of total ATP content after hypoxia. The results showed that the ATP level decreased dramatically after 2 h exposure to hypoxia (2.92 ± 0.17 nmol/mg protein compared with 20.12 ± 2.31 nmol/mg protein in normoxic cultures; Fig. 3). Pretreatment of cardiomyocytes with 50 μM UTP prevented the dramatic decrease of ATP levels (16.62 ± 4.62 nmol/mg protein) in cultures subjected to hypoxia. However, protection against ATP depletion did not occur when cells were pretreated with P2 receptor antagonist PPADS (1.84 ± 1.31 nmol/mg protein) or suramin (2.56 ± 0.87 nmol/mg protein) before exposure to UTP, indicating that the protective effect of UTP is mediated via P2 receptors.
Fig. 3.
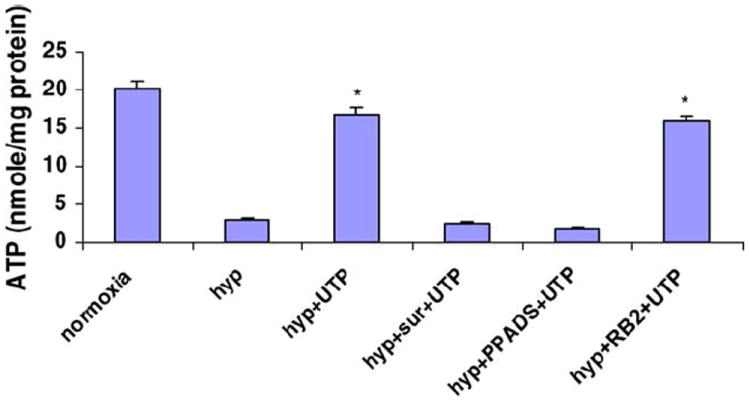
Effect of P2 agonists/antagonists on intracellular ATP levels after hypoxia. Cardiomyocytes were treated for 15 min with P2 antagonist PPADS (10 μM), RB-2 (50 μM), or suramin (300 μM) before application of UTP (50 μM). Intracellular ATP levels after 2 h hypoxia were measured as described. Data are means of at least three replicates in six separate experiments ± S.D. *P < 0.01 compared with hypoxia.
The protective effect of UTP was also demonstrated through cardiomyocyte morphology. Following hypoxia the cells were fixed and stained with hematoxylin and eosin, which showed typical irreversible oncotic damage of cardiomyocytes: vacuoles and disorder of the myofilaments, pyknotic nuclei, and edematous areas in the cytoplasm and around the nucleus. In addition, hypoxic conditions increased the accumulation of the lysosomotropic vital dye neutral red in cytoplasmic granules and around the nucleus and increased the size of the lysosomes and the intensity of their color (Fig. 4). The damage to culture cells treated with UTP before hypoxia was greatly reduced. Cell loss (percentage of cell death) was presented as the number of dead cells (propidium iodide stained) as a percentage of the total number of cells (Hoechst 33341 stained). After 120 min of hypoxia cell loss was 40% ± 5%. Activation of the UTP receptors attenuated cell loss caused by hypoxia to 18% ± 2%; control untreated cells had 3.1% ± 0.2% cell loss.
Fig. 4.
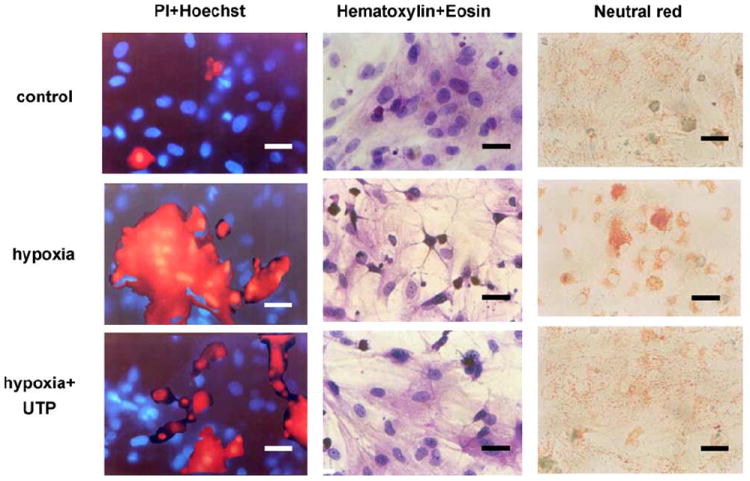
Effect of UTP on cardiomyocyte morphology in hypoxic conditions. Six-day-old cultured cardiomyocytes under normoxic conditions were subjected to 2 h hypoxic environment or pretreated with 50 μM UTP and then subjected to hypoxia. One group of these cells was stained with propidium iodide (red), which marks damaged cells, and with Hoechst 33342 (blue), which stains live-cell nuclei (first column). A second group of cells was stained with hematoxylin and eosin (second column). A third group of cells was stained with neutral red, which stains lysosomes (third column). The results shown are representative of six experiments. Bars = 10 μm.
The contribution of the mitochondrial KATP channel to the protective effects of UTP was examined by application of a mKATP channel blocker, 5-hydroxydecanoic acid (5-HD; 200 μM), 15 min before treatment with UTP and hypoxia. The presence of 5-HD did not alter the protective effects of UTP, as revealed by LDH release to the medium (Table 1). These findings indicate that UTP exerts its protective effect via a pathway bypassing the mitochondrial KATP channel.
3.4. Effect of UTP receptor activation on intracellular [Ca2+]i
[Ca2+]i levels were determined by using indo-1 as a fluorescent probe. Treatment with 10 μM UTP induced transient accelerations of the beating rate and a transient elevation of [Ca2+]i baseline, which lasted 30–40 s, and finally restored the normal beating activity (Fig. 5A). Application of PPADS (10 μM) or RB-2 (50 μM) prevented [Ca2+]i elevation (Fig. 5B and C). Suramin (300 μM), which acts as an antagonist of P2Y2 receptors among other P2 subtypes, did not prevent [Ca2+]i elevation following activation of the UTP receptors (Fig. 5D). A possible pathway of UTP receptor signaling leading to an increase in [Ca2+]i is activation of phospholipase C (PLC), which mediates inositol 1,4,5-trisphosphate (IP3) production and calcium release from IP3 receptors [23]. Application of (1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione) (U73122; 2 μM an inhibitor of PLC activity) or 2-aminoethoxydiphenylborane (2APB), an IP3 receptor inhibitor (50 μM) abolished Ca2+ elevation after activation of UTP receptors (Fig. 6).
Fig. 5.
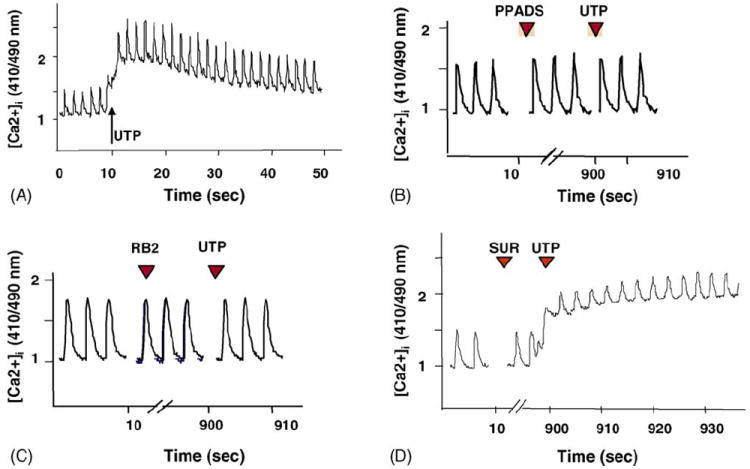
Effect of antagonists on the [Ca2+]i response. Indo-1-loaded cardiomyocytes were pretreated with UTP (A) or with P2 antagonist PPADS (10 μM, B), RB-2 (50 μM, C), or suramin (300 μM, D) before application of UTP (50 μM). The fluorescence ratio of 410:490 nm, which is proportional to changes in Ca2+ levels, is demonstrated. One experiment shown is representative of seven.
Fig. 6.
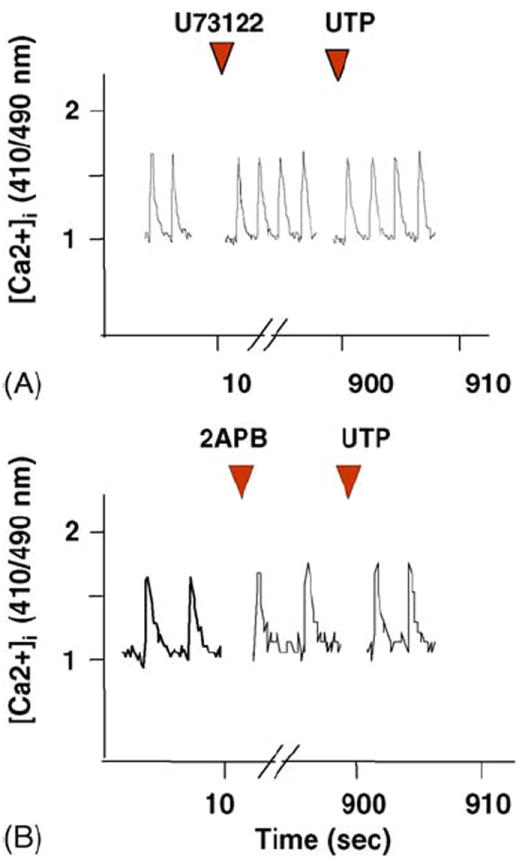
Effect of UTP on [Ca2+]i elevation in Ca2+-free medium or in the presence of PLC inhibitor. Indo-1-loaded cardiomyocytes were exposed to 2 μM U73122, a PLC inhibitor (A), or to 2APB an IP3 receptor inhibitor (50 μM) (B) 15 min before UTP (50 μM) application. The changes in Ca2+ levels are demonstrated. One experiment shown is representative of five.
3.5. Effect of UTP on [Ca2+]i level during hypoxia
Continuous monitoring of [Ca2+]i during hypoxia was accomplished with a specially designed chamber, where cells were protected from oxygen by a laminar counterflowing layer of the inert gas argon. The level of [Ca2+]i began to be elevated ~10–12 min after the initiation of hypoxia, with the cessation of beating activity (Fig. 7A). When the cardiomyocytes were pretreated with UTP (50 μM) for 15 min and then subjected to hypoxia, a delay in [Ca2+]i elevation was observed, and spontaneous contractions lasted for 30–40 min after the initiation of hypoxia (Fig. 7B). Pretreatment with UTP 24 h before the hypoxia resulted in maintenance of spontaneous contractility even after 40 min of hypoxia (Fig. 7C).
Fig. 7.
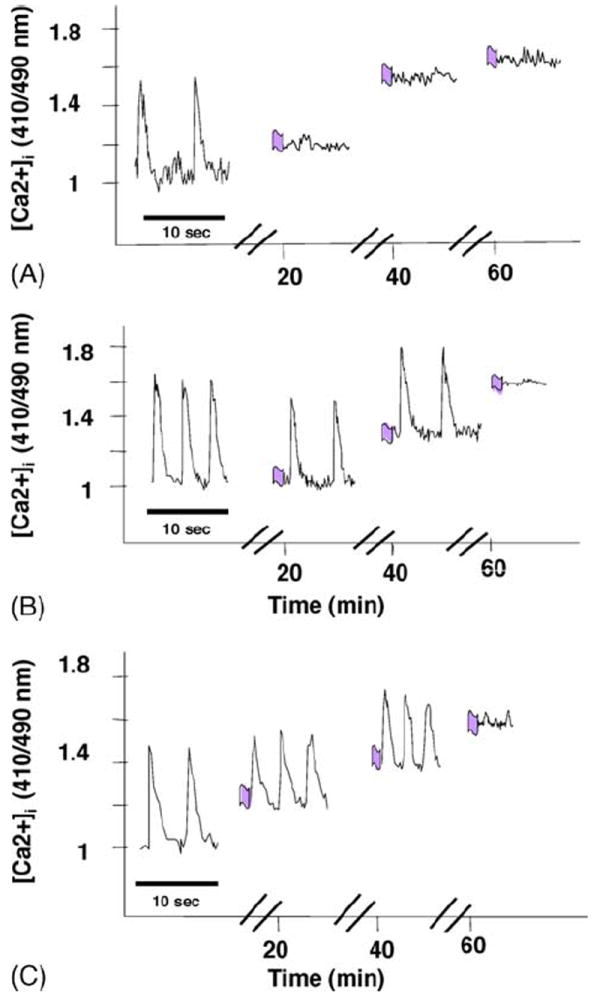
Effect of UTP on [Ca2+]i accumulation during hypoxia. Cardiomyocytes received UTP for 15 min just before hypoxia (B) or 24 h before hypoxia (C). Calcium was monitored during hypoxia with indo-1. The fluorescence ratio of 410:490 is demonstrated. Each recording was done for 10 s at times 0, 20, 40, and 60 min after the initiation of the hypoxia. The effect of hypoxia alone is also shown (A). One experiment shown is representative of five.
4. Discussion
In this work we demonstrate that short-term activation of UTP receptors protects newborn rat cardiomyocytes against hypoxic damage and significantly reduces the cell death caused by hypoxia via activation of P2Y2 and P2Y4 receptors.
It was shown in rat cardiomyocytes that UTP, an alternate agonist to ATP at P2Y2 purinergic receptors, is as effective as ATP in stimulating PLC, and – as with ATP – the response is Gq mediated [23,24]. The uridine nucleotide-responding receptor P2Y4, which when cloned from rat heart [25] is equipotently activated by ATP and UTP, is coupled, in part, to Gi/o [24,26]. The presence of P2Y2 and P2Y4 receptors was demonstrated in the human fetal heart [27], but in adult myocytes, P2Y4 receptors could hardly be detected [28]. Therefore, it is important to establish the expression of P2Y2 and P2Y4 receptors in newborn rat cardiomyocytes by immunohistochemical methods and immunoblotting. As was found in this study, rat newborn cardiomyocytes abundantly express P2Y2 receptors, and anti-P2Y2 antibodies detected four major proteins, indicating heterogeneity of these receptors. Higher MW bands may reflect receptor dimerization. Expression of P2Y4 receptors also was found in newborn rat cardiomyocytes, although less abundant and represented by two protein bands.
The mechanism of action by which UTP induces cardioprotection is not known. Recent studies on UTP signaling showed that these receptors are coupled to the IP3 pathway, which causes Ca2+ elevation [29]. As our study shows, the application of UTP induces an immediate increase in [Ca2+]i level. This increase is not abolished by suramin, antagonist to P2Y2 but not to P2Y4 receptors [30], indicating that the immediate Ca2+ response to UTP is mediated through P2Y4 receptors. The observed Ca2+ response may be a mechanism of UTP-mediated contraction (i.e., P2Y4 receptor-mediated contraction), but evidently this pathway of [Ca2+]i elevation is not involved in acute myocyte protection from hypoxia, because other uridine derivatives (UDP, uridine), although causing a transient elevation of intracellular Ca2+, do not protect the cells from hypoxia. These data are consistent with known effects of uridine derivatives on heart performance [12]. These results imply that in spite of its central physiological role in cardiac cells, [Ca2+]i elevation is not one of the critical elements in the cell-signaling pathway required for UTP-mediated classical cardioprotection (first window of protection).
This study shows that activation of UTP receptors protects cultured newborn rat cardiomyocytes from damage induced by hypoxia. It appears that the P2Y2 receptor triggers a cardioprotective response, although we cannot exclude a role of the P2Y4 receptor, because selective antagonists for this receptor are still not available. P2Y4 receptors have been reported to couple via pertussis toxin-sensitive G proteins, and as Oldenburg et al. wrote, “Virtually all Gi-coupled receptors present on the cardiomyocyte are capable of preconditioning the myocardium, even if their ligands (exogenous agonists) are not released during a PC protocol” [13].
The present study found that the mitochondrial ATP-sensitive potassium channel, the opening of which is considered as an important step in the anti-infarct effect of ischemic preconditioning, plays no role in protection induced through UTP receptor activation.
Arthur et al. showed that ATP causes a robust activation of PLC via activation of Gq in newborn rat cardiomyocytes [31] and would be expected to cause hypertrophic responses like those caused by other factors that activate Gq-coupled receptors, such as α-adrenergic agonists, endothelin, and angiotensin II during long-term exposure [24]. However, ATP was reported to inhibit hypertrophic responses [32]. On the contrary, UTP is as effective as ATP in stimulating PLC [33] and causes hypertrophic responses in rat cardiomyocytes [24]. As the present study shows, short-term exposure to UTP stimulates both classical protection and a long-term, persistent mechanism of protection (second window of protection). It was reported that activation of phosphoinositide-3-kinase (PI3K) leads to cell survival, cytoskeletal rearrangement, and Ca2+ signaling. Cardiac cells express several PI3Ks, and their activity, together with the tyrosine kinase, is required to mediate Ca2+ spiking of neonatal rat cardiomyocytes [34,35]. PI3Ks phosphorylate phosphatidylinositol 4,5-bisphosphate, which after hydrolysis by PLCs produces IP3.
It was recently shown that P2Y receptor activation leads to IP3 generation, which induces mitochondrial Ca2+ loading and indirectly depletes Ca2+ from the sarcoplasmic reticulum [34,35]. The mitochondria can accumulate and release large amounts of Ca2+. Increased mitochondrial Ca2+ activates respiratory enzymes and increased ATP production, but pathological states such as ischemia cause a significant decrease of the ATP-ADP ratio and massive Ca2+ entry into the mitochondrial matrix, leading to cell death. As this study shows, short-term activation of UTP receptors protects mitochondria from Ca2+ overload and maintains increased levels of ATP production. These data complemented recent findings of Belous et al. [36], which described mitochondrial P2Y2-like receptors linking cytosolic UTP to mitochondrial calcium uptake. It was found that UTP strongly inhibits mitochondrial Ca2+ uptake, which may promote respiratory chain activity and ATP production.
The involvement of UTP receptors in mediating cardioprotection reflects biological redundancy in the life-saving signals of P2Y2 and P2Y4 pyrimidinoceptors. Therapeutic targeting of pyrimidinergic receptors for protection against ischemic myocardial damage may be more effective than targeting the purinergic receptors. The breakdown product of UTP, uridine, unlike adenosine, has limited ancillary pharmacologic effects [37]. ATP is degraded to adenine nucleotides and nucleosides, so that the response of cardiomyocytes should be the mixture of various effects of these multiple compounds [38]. Thus, UTP may be used in preference to ATP as a P2Y2 and P2Y4 receptor agonist, because it does not form cardiovascularly active metabolites such as adenosine.
Acknowledgments
This research was partially supported by the Horowitz Foundation at Bar-Ilan University and the Israel Ministry of Health. We are indebted to Ms. Avrille Goldreich for helping to prepare this manuscript and to Tova Zinman and Ahuva Isaac for their valuable technical assistance.
Abbreviations
- 2APB
2-aminoethoxydiphenylborane
- ATP
adenosine 5′-triphosphate
- FITC
fluorescein isothiocyanate
- 5-HD
5-hydroxydecanoic acid
- IP3
inositol 1,4,5-trisphosphate
- LDH
lactate dehydrogenase
- PBS
phosphate buffered saline
- PC
preconditioning
- PI3K
phosphoinositide-3-kinase
- PLC
phospholipase C
- PKC
protein kinase C
- PPADS
pyridoxal-5′-phosphate-6-azophenyl-2,4-disulfonate
- RB-2
Reactive blue 2
- U73122
(1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)-hexyl)-1H-pyrrole-2,5-dione)
- UDP
uridine-5′-diphosphate
- UTP
uridine-5′-triphosphate
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Miki T, Miura T, Bunger R, Suzuki K, Sakamoto J, Shimamoto K. Ecto-5′-nucleotidase is not required for ischemic preconditioning in rabbit myocardium in situ. Am J Physiol. 1998;275:H1329–37. doi: 10.1152/ajpheart.1998.275.4.H1329. [DOI] [PubMed] [Google Scholar]
- 3.Miura T, Ishimoto R, Sakamoto J, Tsuchida A, Suzuki K, Ogawa T, et al. Suppression of reperfusion arrhythmia by ischemic preconditioning in the rat: is it mediated by the adenosine receptor, prostaglandin, or bradykinin receptor. Basic Res Cardiol. 1995;90:240–6. doi: 10.1007/BF00805667. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Kloner RA. The cardioprotective effects of ischemic preconditioning are not mediated by adenosine receptors in rat hearts. Circulation. 1993;87:1642–8. doi: 10.1161/01.cir.87.5.1642. [DOI] [PubMed] [Google Scholar]
- 5.Hu K, Nattel S. Mechanisms of ischemic preconditioning in rat hearts. Involvement of alpha1B-adrenoceptors, pertussis toxin-sensitive G proteins, and protein kinase C. Circulation. 1995;92:2259–65. doi: 10.1161/01.cir.92.8.2259. [DOI] [PubMed] [Google Scholar]
- 6.Goto M, Liu Y, Yang XM, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995;77:611–21. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- 7.Miki T, Cohen MV, Downey JM. Opioid receptor contributes to ischemic preconditioning through protein kinase C activation in rabbits. Mol Cell Biochem. 1998;186:3–12. [PubMed] [Google Scholar]
- 8.Ninomiya H, Otani H, Lu K, Uchiyama T, Kido M, Imamura H. Enhanced IPC by activation of pertussis toxin-sensitive and -insensitive G protein-coupled purinoceptors. Am J Physiol Heart Circ Physiol. 2002;282:H1933–H1943. doi: 10.1152/ajpheart.00771.2001. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Bolis L, Straub RW, editors. Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach. New York: Raven Press; 1978. pp. 107–18. [Google Scholar]
- 10.Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM. Advances in signalling by extracellular nucleotides. The role and transduction mechanisms of P2Y receptors. Cell Signal. 2000;12:351–60. doi: 10.1016/s0898-6568(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–93. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- 12.Eliseev VV, Rodionova OM, Sapronov NS, Selizarova NO. The effect of uridine and uridine nucleotides on isolated rat heart performance in regional myocardial ischemia. Patol Fiziol Eksp Ter. 2002;2:13–5. [PubMed] [Google Scholar]
- 13.Oldenburg O, Cohen MV, Yellon DM, Downey JM. Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res. 2002;55:429–37. doi: 10.1016/s0008-6363(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 14.Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–45. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- 15.Shneyvays V, Nawrath H, Jacobson KA, Shainberg A. Induction of apoptosis in cardiac myocytes by an A3 adenosine receptor agonist. Exp Cell Res. 1998;243:383–97. doi: 10.1006/excr.1998.4134. [DOI] [PubMed] [Google Scholar]
- 16.Safran N, Shneyvays V, Balas N, Jacobson KA, Shainberg A. Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol Cell Biochem. 2001;217:143–52. doi: 10.1023/a:1007209321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shneyvays V, Mamedova L, Zinman T, Jacobson K, Shainberg A. Activation of A3 adenosine receptor protects against doxorubicin-induced cardiotoxicity. J Mol Cell Cardiol. 2001;33:1249–61. doi: 10.1006/jmcc.2001.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fixler D, Tirosh R, Zinman T, Shainberg A, Deutsch M. Differential aspects in ratio measurements of [Ca2+]i relaxation in cardiomyocyte contraction following various drug treatments. Cell Calcium. 2002;31:279–87. doi: 10.1016/s0143-4160(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 19.Shneyvays V, Safran N, Halili-Rutman I, Shainberg A. Insights into adenosine A1 and A3 receptors function: cardiotoxicity and cardioprotection. Drug Dev Res. 2000;50:324–37. [Google Scholar]
- 20.Stern MD, Silverman HS, Houser SR, Josephson RA, Capogrossi MC, Nichols CG, et al. Anoxic contractile failure in rat heart myocytes is caused by failure of intracellular calcium release due to alteration of the action potential. Proc Natl Acad Sci USA. 1988;85:6954–8. doi: 10.1073/pnas.85.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Ani D, Jacobson KA, Shainberg A. Characterization of adenosine receptors in intact cultured heart cells. Biochem Pharmacol. 1994;48:727–35. doi: 10.1016/0006-2952(94)90050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieminen AL, Gores GJ, Bond JM, Imberti R, Herman B, Lemasters JJ. A novel cytotoxicity screening assay using a multiwell fluorescence scanner. Toxicol Appl Pharmacol. 1992;115:147–55. doi: 10.1016/0041-008x(92)90317-l. [DOI] [PubMed] [Google Scholar]
- 23.Puceat M, Vassort G. Purinergic stimulation of rat cardiomyocytes induces tyrosine phosphorylation and membrane association of phospholipase C gamma: a major mechanism for InsP3 generation. Biochem J. 1996;318:723–8. doi: 10.1042/bj3180723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pham TM, Morris JB, Arthur JF, Post GR, Brown JH, Woodcock EA. UTP but not ATP causes hypertrophic growth in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2003;35:287–92. doi: 10.1016/s0022-2828(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 25.Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br J Pharmacol. 1998;124:428–30. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippov AK, Simon J, Barnard EA, Brown DA. Coupling of the nucleotide P2Y4 receptor to neuronal ion channels. Br J Pharmacol. 2003;138:400–6. doi: 10.1038/sj.bjp.0705043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogdanov Y, Rubino A, Burnstock G. Characterisation of subtypes of the P2X and P2Y families of ATP receptors in the foetal human heart. Life Sci. 1998;62:697–703. doi: 10.1016/s0024-3205(97)01168-5. [DOI] [PubMed] [Google Scholar]
- 28.Hou M, Malmsjo M, Moller S, Pantev E, Bergdahl A, Zhao XH, et al. Increase in cardiac P2X1-and P2Y2-receptor mRNA levels in congestive heart failure. Life Sci. 1999;65:1195–206. doi: 10.1016/s0024-3205(99)00353-7. [DOI] [PubMed] [Google Scholar]
- 29.Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 1995;35:541–79. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- 30.von Kügelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–23. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 31.Arthur JF, Matkovich SJ, Mitchell CJ, Biden TJ, Woodcock EA. Evidence for selective coupling of alpha 1-adrenergic receptors to phospholipase C-beta 1 in rat neonatal cardiomyocytes. J Biol Chem. 2001;276:37341–6. doi: 10.1074/jbc.M106572200. [DOI] [PubMed] [Google Scholar]
- 32.Zheng JS, Boluyt MO, Long X, O’Neill L, Lakatta EG, Crow MT. Extracellular ATP inhibits adrenergic agonist-induced hypertrophy of neonatal cardiac myocytes. Circ Res. 1996;78:525–35. doi: 10.1161/01.res.78.4.525. [DOI] [PubMed] [Google Scholar]
- 33.Podrasky E, Xu D, Liang BT. A noval phospholipase C- and cAMP-independent positive inotropic mechanism via a P2 purinoceptor. Am J Physiol. 1997;273:2380–7. doi: 10.1152/ajpheart.1997.273.5.H2380. [DOI] [PubMed] [Google Scholar]
- 34.Jaconi M, Bony C, Richards SM, Terzic A, Arnaudeau S, Vassort G, et al. Inositol 1,4,5-trisphosphate directs Ca2+ flow between mitochondria and the endoplasmic/sarcoplasmic reticulum: a role in regulating cardiac autonomic Ca2+ spiking. Mol Biol Cell. 2000;11:1845–58. doi: 10.1091/mbc.11.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bony C, Roche S, Shuichi U, Sasaki T, Crackower MA, Penninger J, et al. A specific role of phosphatidylinositol 3-kinase gamma. A regulation of autonomic Ca2+ oscillations in cardiac cells. J Cell Biol. 2001;152:717–28. doi: 10.1083/jcb.152.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belous A, Wakata A, Knox CD, Nicoud IB, Pierce J, Anderson CD, et al. Mitochondrial P2Y-like receptors link cytosolic adenosine nucleotides to mitochondrial calcium uptake. J Cell Biochem. 2004;92:1062–73. doi: 10.1002/jcb.20144. [DOI] [PubMed] [Google Scholar]
- 37.Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- 38.Williams M, Jarvis MF. Purinergic and pyrimidinergic receptors as potential drug targets. Biochem Pharmacol. 2000;59:1173–85. doi: 10.1016/s0006-2952(99)00341-x. [DOI] [PubMed] [Google Scholar]


