Abstract
Agonists of adenosine A1 receptors have been frequently proposed as candidates for clinical development in treatment of cerebral ischemia and stroke. Numerous experimental studies have shown that pre- and postischemic administration of these drugs results in a very significant reduction of postischemic brain damage. However, only a few studies determined the impact of cerebral ischemia and drug treatment on postischemic recovery of spatial memory. The present paper demonstrates that preischemic i.p. administration of adenosine amine congener (ADAC) at 100 μg/kg in gerbils results in a significant (P < 0.05) reduction of postischemic mortality and hippocampal, cortical and striatal morbidity. Postischemic Morris’ water maze tests show that preischemic treatment with ADAC also leads to a very significant (P < 0.001) reduction of postischemic spatial memory loss. Our results indicate feasibility of further consideration of adenosine A1 receptor agonists as a clinically applicable acute treatment of brain ischemia. Recent development of neuroprotective adenosine A1 receptor agonists that are free of cardiovascular side effects supports such development.
Keywords: Cerebral ischemia, Adenosine receptor, Spatial memory, Water maze, (Gerbil)
1. Introduction
Despite intense research aimed at the development of effective therapeutic interventions, the means of preventing ischemic brain damage are as elusive as ever. Among numerous therapies under current testing, approaches based on the stimulation of adenosine A1 receptors offer a substantial promise in reducing ischemia-related structural and functional damage (for recent reviews, see Rudolphi et al., 1992; Miller and Hsu, 1992; Von Lubitz et al., 1996).
Multiple studies have shown that acute stimulation of adenosine A1 receptors results in decreased calcium influx, enhanced membrane hyperpolarization, suppressed release of neurotransmitters (including neurotoxic glutamate), and depression of NMDA receptor excitability (reviewed by Schubert and Kreutzberg, 1990; Rudolphi et al., 1992; Von Lubitz and Marangos, 1992; Von Lubitz et al., 1996). As a result of these effects, neuronal excitability and firing rate are reduced causing, in turn, a substantial reduction of brain metabolic demands (Schubert and Kreutzberg, 1990).
The events elicited by stimulation of adenosine A1 receptor stimulation counteract several processes recognized as key participants in the generation of ischemic brain damage (reviewed by Von Lubitz et al., 1996; Bengtsson and Siesjö, 1990; Choi, 1992). Hence, it is not surprising that several successful attempts have been made to demonstrate the neuroprotective value of treatment with agonists acting at this adenosine receptor subtype (reviewed by Von Lubitz et al., 1996). Although most of these studies demonstrated unequivocally that both pre- and postischemically administered adenosine A1 receptor agonists reduce postischemic neuronal loss (reviews by Miller and Hsu, 1992; Rudolphi et al., 1992; Von Lubitz et al., 1996), only a few described the effect of these drugs on postischemic neurological recovery (e.g., Phillis and O’Regan, 1988; Von Lubitz and Marangos, 1990; Héron et al., 1994).
It is well known that both focal and global ischemia may result in very significant disturbances of cognitive functions in both man (Mohr et al., 1986; Mohr, 1986) and in animals subjected to an experimental cerebrovasculatory arrest (Bothe et al., 1986; Auer et al., 1989; Le Peillet et al., 1989; Jaspers et al., 1990; Katoh et al., 1992). Short term and spatial memory appear to be particularly affected (Mohr et al., 1986). In view of the current interest in adenosine A1 receptor agonists as therapeutics in stroke and cerebral ischemia, it is surprising that, while only one paper presents the effect of adenosine A1 receptor agonist on postischemic learning (Héron et al., 1994), there are no reports describing the direct effect of such treatment on spatial memory. We have, therefore, investigated the impact of preischemic administration of a selective adenosine A1 receptor agonist ADAC (Maillard et al., 1993) on the mortality, brain damage, and spatial memory following moderate forebrain ischemia in gerbils.
2. Materials and methods
2.1. Animals
Female gerbils (Tumblebrook Farms, MA, USA) weighing 70 g were used in the study. Prior to inclusion in the experiments, animals were tested for susceptibility to spontaneous seizures using the method described by Lee et al. (1984). Seizure-prone gerbils were rejected.
2.2. Drug and its administration
Adenosine amine congener [ADAC, (N6-[4-[[[(2-aminoethyl)amino]carbonyl]methyl]phenyl]adenosine)] was purchased from RBI (Natick, MA, USA). The drug was dissolved in a 20 : 80 (v/v) mixture of Alkamuls EL-620 (Rhône-Poulenc, Cranbury, NJ, USA) and phosphate buffered saline and administered at 0.1 mg/kg in a 0.15 ml volume. The drug was given 15 min prior to ischemia. Control animals received an equivalent injection of the vehicle.
2.3. Water maze
The details of the water maze have been described in our previous paper (Von Lubitz et al., 1993). The system consisted of a tank (1 m diameter) which was filled with opaque water to a depth of 30 cm. The tank contained a colorless target platform (15 cm diameter) submerged to the depth of 1.5 cm and placed in the center of the north-west quadrant. Two highly contrasting spatial markers were suspended from the north and east walls of the tank facing toward its centre. The starting point for each trial was the south-east wall. During trials, animal movements were tracked using the Watermaze data acquisition program (Infallible Software, Research Triangle Park, NC, USA).
2.4. Water maze training
Prior to water maze trials, gerbils were acclimatized to swimming by daily immersion in water of incrementing depth (i.e., 3 cm, 10 cm, 15 cm, and 30 cm). During acclimatization, all spatial clues and the targets were removed from the tank and its vicinity. Since exposure to water induced spontaneous seizures in some animals, seizing gerbils were excluded from further experiments. After the final exclusions, 30 animals entered water maze training.
2.4.1. Preischemic training
Preischemic training consisted of three phases: the invisible target acquisition phase, the probe test, and the visible target test. Global learning in the water maze paradigm was tested during the acquisition phase. The probe test, during which the target was removed, served to isolate and test the spatial component of the learned task. Finally, the visible target was used to investigate both visual and motor ability of animals to reach the target, and to test their motivation to perform the water maze task.
Each acquisition trial lasted 120 s. The acquisition trials were continued until the animals reached a stable performance plateau. Following 3 days of stable target acquisition times, animals were subjected to the probe trial lasting 60 s. The visible target trial was administered on the same day as ischemia. In order to preclude the influence of swimming stress on the postischemic outcome, the visible test was performed 8 h prior to the occlusion. Additional details of the water maze testing procedure have been described elsewhere (Sei et al., 1992; Von Lubitz et al., 1993).
2.4.2. Postischemic training
Postischemic training was commenced after an 8 day recovery period. The training schedule followed the same pattern as that prior to the insult. Acquisition training was discontinued after control animals reached preischemic target latency. Probe trial and visible target trial were administered during the 2 days immediately following the postischemic acquisition phase.
2.5. Ischemia
Prior to 10 min ischemia, gerbils were randomly divided into the control and drug testing groups. Since our previous studies indicated 50–60% postischemic mortality among control animals (Von Lubitz and Marangos, 1992; Von Lubitz et al., 1994) and maximally 20% among drug treated gerbils, in order to assure an adequate number of survivors available for postischemic water maze testing, 20 animals were assigned to the control group, and 10 to the ADAC group. Bilateral carotid occlusion was performed under Halothane anesthesia as described previously (Von Lubitz et al., 1994). Body/brain temperature was maintained at the preischemic level by means of an infrared heating lamp and a heating blanket (Harvard Instruments, South Natick, MA, USA). During postischemic recovery, animals were monitored each day for signs of functional damage (e.g., pareses) that might affect their subsequent water maze performance. Postischemic mortality was also monitored every day.
2.6. Postischemic locomotor activity testing
Several studies have demonstrated that cerebral ischemia induces persistent postischemic locomotor hyperactivation (e.g., Phillis and O’Regan, 1988; Katoh et al., 1992; Gao and Phillis, 1994; see also Mileson and Schwartz, 1991). Since locomotor hyperactivation may result in a spurious improvement of performance in the water maze (Von Lubitz et al., 1993), open field activity of all gerbils was assessed 7 days after the occlusion (i.e., one day prior to the water maze experiments). During locomotor testing, horizontal displacement of each gerbil was monitored for 10 min in 2 min epochs by means of a Digiscan activity monitor (Omnitech Electronics, Columbus, OH, USA).
2.7. Histology
One day after the last postischemic water maze trial, all animals were heavily anesthetized with Nembutal (60 mg/kg) and perfused through the aorta with buffered (pH 7.4) formaldehyde. Frozen sections of the brains were made as described previously (Lin et al., 1990). The sections were stained using the method of Nissl, and the extent of the neuronal loss was determined in the cortex, hippocampal CA1 sector, and striatum following a published protocol (Von Lubitz and Marangos, 1992, Von Lubitz et al., 1994).
2.8. Statistical analysis
Dunnett’s test was used to analyze histological data, while Fisher’s exact test was used to evaluate the end-point mortality differences. Genstat 3.0 (Infallible Software, Research Triangle Park, NC) was used in these analyses with P < 0.05 indicating significance.
Single factor repeated measures analysis of variance (ANOVA) was used to analyze locomotor activity and water maze data. Individual group differences were assessed by means of contrast analyses using univariate F-tests. P < 0.01 was considered significant in behavioural studies. All statistical errors are expressed as ± S.E.M. Statistical parametres were computed using Systat 5.03 program (Evanston, IL, USA).
3. Results
3.1. Postischemic mortality
Seven control animals died during the initial 12 h postischemia (35%). Three additional control gerbils died between day 1 and day 3. Finally, two animals were found dead on day 14. The two latter gerbils died 5–8 h following the last water maze trial. Whether their deaths can be ascribed to the consequences of ischemia alone or combination of ischemia and swimming stress is uncertain.
In the ADAC group, only two animals died during the entire monitoring period. One animal died on day 3, and one on day 6 after ischemia.
End point mortality was 60% and 20% in the control and ADAC groups, respectively.
3.2. Locomotor activity prior to postischemic water maze trials
Locomotor activity tests performed 7 days after ischemia revealed a significant main effect of drug treatment [F(1,8) = 220.824, P < 0.001] with all ADAC treated animals consistently showing lower open field activity than the controls when measured over a 10 min period (i.e., 135 ± 6 cm vs. 235 ± 3 cm).
3.3. Water maze trials
3.3.1. Preischemia
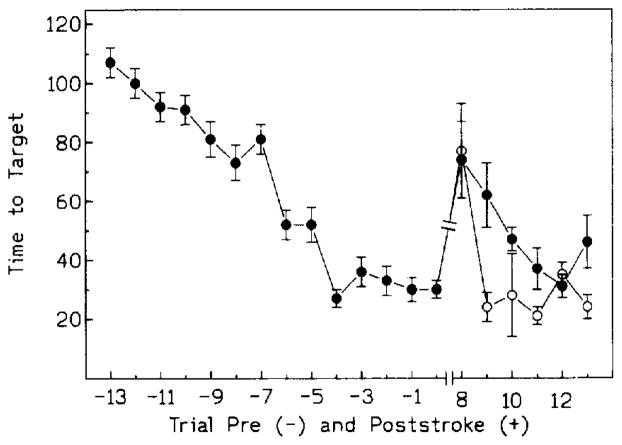
All gerbils swam without any difficulties although most experienced problems in climbing the target on day 1 of the trials. Thereafter, all animals mounted the target readily and remained on it until lifted 15 s later. The overall pattern of search was similar to that described previously for mice and rats (Sei et al., 1992; Morris et al., 1982; Von Lubitz et al., 1993). Thus, during the first 3 trials, a wall-hugging (thigmotactic) pattern was evident. Thigmotaxis was subsequently modified into a center-cross pattern, in which gerbils freely crossed the entire width of the water surface. From the 8th day onwards, a direct, target-oriented pattern dominated. There was a significant main effect of trials [F(13,507) = 37.283, P < 0.001], with significantly different target latencies from trial 5 (i.e. day 9 preischemia, Fig. 1) onwards.
Fig. 1.
Pre-(−) and postischemic ( + ) target acquisition trials. Ischemia induced on day 0. Postischemic trials were resumed on day 8 after ischemia. Open circles: ADAC treated group; black circles: controls. Significance markers have been omitted for the sake of clarity. For details of statistical analysis and significance, see section 3.2.1 and 3.2.2.
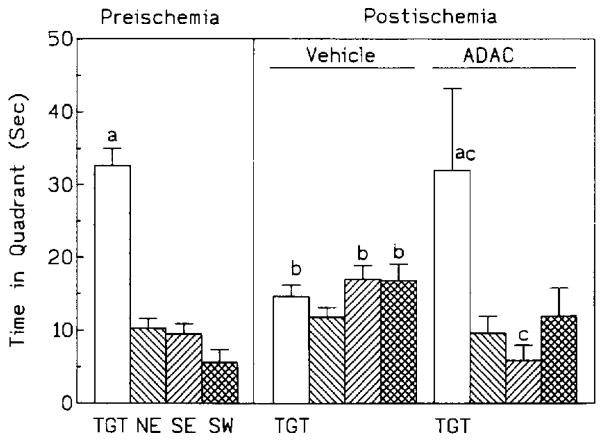
The probe trial revealed a significant main effect of quadrants [F(3,27) = 35.351, P < 0.001), with the time spent in the target quadrant (37.7 ± 2.3 s) being significantly different from that spent in the other three quadrants (Fig. 2, P < 0.001).
Fig. 2.
Comparison of time spent in each quadrant during pre- and postischemic probe trials. TGT: target quadrant; NE: north-east quadrant; SE: south-east quadrant; SW: south-west quadrant. Statistical significance: a P < 0.01 within the group; b P < 0.01 between pre- and postischemia; c P < 0.01 between postischemic control and ADAC groups. Further details of statistical analysis described in sections 3.2.1. and 3.2.2.
Target latency during the preischemic visible target trial was significantly shorter than during the last preischemic acquisition trial (12.0 ± 1.2 s vs. 27.9 ± 2.9 s, respectively, P < 0.01).
3.3.2. Postischemia
Postischemic water maze training revealed a significant main effect of trials ([F(5,125) = 14.906, P < 0.001) on the latency to target. Although there were no main effects of drug treatment on target latencies, between group comparisons revealed significantly shorter latencies in the ADAC group on the 2nd and 6th postischemic trial (Fig. 1). Moreover, in the ADAC group there were no statistically significant differences between pre- and postischemic target latencies beginning with the second postischemic trial (i.e., day 9 postischemia, Fig. 1). In the control group, on the other hand, statistically significant differences between pre- and postischemic trials prevailed during the 2nd, 3rd and 6th trial (i.e., days 9, 10 and 14 postischemia).
The postischemic probe trial showed a significant main effect of quadrants [F(3,48) = 8.190, P < 0.001)] and a significant interaction between treatment groups and quadrants [F(3,48) = 9.208, P < 0.001], with the control animals spending significantly less lime in the target quadrant [14.6 ± 1.6 s control vs. 32.0 ± 3.9 s ADAC, P < 0.001, (Fig. 2)]. Moreover, while in the ADAC group the time spent in the target quadrant was significantly (P < 0.001) longer than in any other quadrant, time distribution was approximately equal for all quadrants in the control group (Fig. 2). Finally, when pre- and postischemic data were compared, there was a significant postischemic decrease in time spent in the target quadrant in the control but not in the ADAC group (Fig. 2).
Postischemic latencies to the visible target did not differ between the control and ADAC groups (11.1 ± 2.5 s vs. 13.2 ± 2.5 s) and comparison of pre- and postischemic data failed to show statistically significant differences.
3.4. Neuronal survival
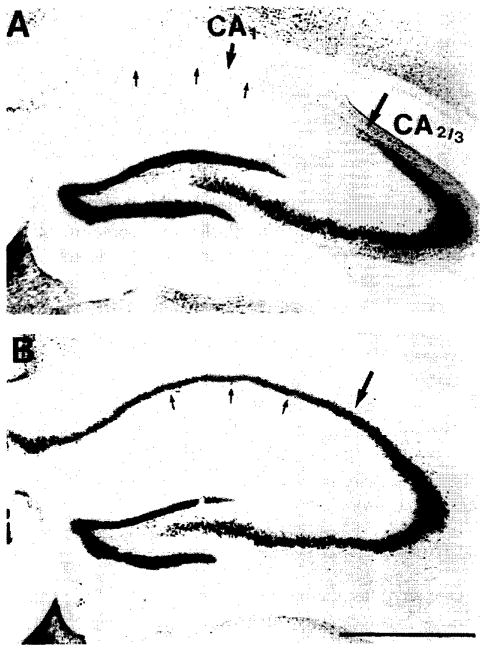
Two weeks after ischemia, very severe damage was present in the hippocampal CA1 region of the control animals (23% neurons surviving, Fig. 3). The damage was also present in the cortex and in the dorsolateral striatum (83% and 25%, respectively). Preischemic treatment with ADAC resulted in a very significant reduction of the hippocampal damage (81% surviving neurons, P < 0.001, Fig. 3) and a complete elimination of neuronal loss from both cortex and striatum.
Fig. 3.
Neuronal damage in the CA1 sector of the control gerbil (A) and its amelioration in an animal treated 15 min prior to ischemia with a single dose of 100 μg/kg ADAC (B).
4. Discussion
Cardiac arrest and cerebrovascular arrest involving either middle or posterior cerebral artery frequently result in retro- and anterograde amnesia in humans (Mohr, 1986; Mohr et al., 1986). Typically, all of these insults involve severe damage to the hippocampus, i.e., the brain region postulated to be intimately involved in learning processes related to spatial coding (O’Keefe and Nadel, 1978). Amnesia, determined in a wide variety of learning paradigms, has been also frequently reported in several animal species exposed to experimentally induced loss of the hippocampal formation (Mishkin, 1978; Morris et al., 1982; Bothe et al., 1986; Hagan and Beughard, 1990; Jaspers et al., 1990).
The Morris water maze (Morris et al., 1982) is now considered the most direct means of assessing postischemic memory and learning of spatially oriented tasks (Corbett et al., 1992). To our knowledge, ours is the first study of the neuroprotective effects of a drug in which the gerbil model of cerebral ischemia was combined with the water maze test in order to address the problem of postischemic recovery of spatial memory. The study shows that preischemic treatment with adenosine A1 receptor agonist ADAC results in neuronal survival and mortality reduction that are fully comparable to previous reports of experiments in which other adenosine A1 receptor agonists have been used (reviewed by Rudolphi et al., 1992; Von Lubitz et al., 1996). Interestingly, however, while most of the former studies used drug doses in the milligram range (reviews by Rudolphi et al., 1992; Miller and Hsu, 1992), we now show that 100 μg/kg ADAC is equally efficacious. Moreover, at this dose, ADAC appears to be free of the disturbing cardiovascular side effects (e.g., bradycardia and hypotension; Von Lubitz et al., unpublished observations) that accompany higher doses of adenosine A1 receptor agonists. Such side effects are considered the most serious obstacle in clinical development of agents stimulating adenosine A1 receptors (Williams, 1993). Recently, however, a series of other adenosine A1 receptor agonists characterized by a side effect-free profile has been reported (Knutsen et al., 1995). The efficacy of these new CNS-selective compounds as anticonvulsants and neuroprotectants appears to be fully comparable (Knutsen et al., 1995) to the drugs used in the earlier studies (Von Lubitz et al., 1989, 1994) and to ADAC (present paper). Importantly, the present study shows that the therapeutic dose of ADAC is significantly lower than that of previously studied agonists of adenosine A1 receptors.
Our study shows that preischemic administration of ADAC significantly attenuates ischemia-induced disruption of spatial memory. Yet, although significant differences between ADAC and control groups were observed on the 2nd day of the postischemic acquisition trials, overall ADAC does not appear to improve postischemic acquisition in the water maze. It has to be remembered, however, that when tested in the open field paradigm, locomotor activity of control gerbils was significantly higher than that of ADAC treated animals. Moreover, comparison of our historical open field data obtained during studies of naive, non-ischemic gerbils (Beenhakker, unpublished) with the postischemic activity of ADAC treated gerbils (present study) revealed absence of statistical differences. It is, therefore, likely that the lack of a clear-cut difference between ADAC and control gerbils during acquisition trials is not caused by a limited efficacy of ADAC per se, but by a faster movement of control animals resulting in an increased likelihood of a chance encounter with the target. This conclusion is supported by the results of probe trials which showed a significant postischemic target quadrant preference in the ADAC group. Moreover, postischemic target quadrant preference in the ADAC group was indistinguishable from that observed during the preischemic probe trial. In the control group, on the other hand, postischemic quadrant preference was absent, and the comparison of pre- and postischemic probe trial data showed a significant postischemic reduction in the time spent by control animals in the target quadrant. The convincing nature of the postischemic probe trial is emphasized by the results of the visible target test, in which target latencies of both ADAC and control groups did not differ statistically from each other or from the latencies observed preischemia. The latter data indicate that once the target is made visible and the element of ‘uncertainty’ is removed, all animals perform equally well, and no other additional factors (e.g., surgical trauma) influence the outcome.
The results of our study contrast those of Héron et al. (1994) who showed that administration of another A1 receptor agonist, R-phenylisopropyladenosine (R-PIA), while resulting in a significant diminution of glutamate release and improvement of postischemic neurological scores in the ‘4-vessel’ model of rat global ischemia, failed to have any beneficial effect on either hippocampal morphology or postischemic memory. Very similar histological results were obtained by Roussel et al. (1991) who used the model of permanent middle cerebral occlusion in rats. However, both groups employed low microgram doses of the drug and we have recently shown (Von Lubitz et al., 1994) that a comparably low dose of another adenosine A1 receptor agonist (N6-cyclopentyladenosine, CPA) decreases postischemic mortality, but has no effect on the postischemic survival of hippocampal neurons. Higher doses of both R-PIA and CPA (i.e., 1 mg/kg), on the other hand, have been shown to be protective in all studied measures (reviewed by Rudolphi et al., 1992; Miller and Hsu, 1992; Von Lubitz et al., 1996). The same argument may apply to the negative memory data presented by Héron et al. (1994). However, since the latter authors used active avoidance tests, it can not be excluded that the failure of R-PIA in this paradigm may be due to the different impact of ischemia on different forms of memory. The subject merits further investigations.
The results of our present study reaffirm conclusions of several previous authors (see reviews by Miller and Hsu, 1992; Rudolphi et al., 1992; Von Lubitz et al., 1996) who showed that adenosine A1 receptor agonists have a very significant potential as clinical agents in treatment of cerebral ischemia and stroke. The advent of new selective agonists active at adenosine A1 receptors and characterized by minimal or non-existent cardiovascular side effects (Knutsen et al., 1995) emphasizes the potential of adenosine receptor based therapies even more strongly.
References
- Auer RN, Jensen ML, Winshaw IQ. Neurobehavioural deficit due to ischemic brain damage limited to half of the CA1 sector of the hippocampus. J Neurosci. 1989;9:1641. doi: 10.1523/JNEUROSCI.09-05-01641.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson F, Siesjö BK. Cell damage in cerebral ischemia: physiological, biochemical, and structural aspects. In: Schurr A, Rigor BM, editors. Cerebral Ischemia and Resuscitation. CRC Press; Boca Raton: 1990. p. 215. [Google Scholar]
- Bothe H-W, Bosma H-J, Hofer H, Hossman K-A, Angermeier WF. Selective vulnerability of hippocampus and disturbances of memory storage after mild unilateral ischemia of gerbil brain. Stroke. 1986;17:1160. doi: 10.1161/01.str.17.6.1160. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;9:1261. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Corbett D, Evans SJ, Nurse SM. Impaired acquisition of the Morris water maze following global ischemic damage in the gerbil. Neuroreport. 1992;3:204. doi: 10.1097/00001756-199202000-00021. [DOI] [PubMed] [Google Scholar]
- Gao Y, Phillis JW. CGS 15943. an adenosine A2 receptor antagonist, reduces cerebral ischemic injury in the Mongolian gerbil. Life Sci. 1994;55:3. doi: 10.1016/0024-3205(94)00889-2. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Beughard M. The effects of forebrain ischemia on spatial learning. Behav Brain Res. 1990;41:151. doi: 10.1016/0166-4328(90)90150-d. [DOI] [PubMed] [Google Scholar]
- Héron A, Lekieffre D, Le Peillet E, Lasbennes F, Seylaz J, Plotkine M, Boulu RG. Effects of an adenosine A1 agonist on neurochemical, behavioral and histological consequences of ischemia. Brain Res. 1994;641:217. doi: 10.1016/0006-8993(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Jaspers RMA, Block F, Heim C, Sontag K-H. Spatial learning is affected by transient occlusion of common carotid arteries (2VO): comparison of behavioural and histopathological changes after ‘2VO’ and ‘four-vessel-occlusion’ in rats. Neurosci Lett. 1990;117:149. doi: 10.1016/0304-3940(90)90135-v. [DOI] [PubMed] [Google Scholar]
- Katoh A, Ishibashi Ch, Shiomi T, Takahara Y, Eigyo M. Ischemia-induced irreversible deficit of memory function in gerbils. Brain Res. 1992;577:57. doi: 10.1016/0006-8993(92)90537-j. [DOI] [PubMed] [Google Scholar]
- Knutsen LJS, Lau J, Sheardown MJ, Eskesen K, Thomsen C, Weis JU, Judge ME, Klitgaard H. Anticonvulsant actions of novel and reference adenosine agonists. In: Belardinelli L, Pelleg A, editors. Adenosine and Adenine Nucleotides: from Molecular Biology to Integrative Physiology. Kluwer Academic Publishers; Boston: 1995. p. 479. [Google Scholar]
- Le Peillet E, Lekieffre D, Plotkine M, Boulu RG. Modified open-field activity test to study the protective activity of drugs in cerebral ischemia in rats (4 vessel model) In: Krieglstein J, editor. Pharmacology of Cerebral Ischemia 1988. CRC Press; Boca Raton: 1989. p. 369. [Google Scholar]
- Lee RJ, Bajorek JG, Lomax P. Similar anticonvulsive, but unique behavioural effects of opioid agonists in the seizure-sensitive Mongolian gerbil. Neuropharmacology. 1984;5:517. doi: 10.1016/0028-3908(84)90024-8. [DOI] [PubMed] [Google Scholar]
- Lin CS, Polsky K, Nadler JV, Crain B. Selective neocortical and thalamic cell death in the gerbil after transient ischemia. Neuroscience. 1990;2:289. doi: 10.1016/0306-4522(90)90083-g. [DOI] [PubMed] [Google Scholar]
- Maillard MC, Nikodijević O, LaNoue KF, Berkich D, Ji X-D, Bartus R, Jacobson KA. Adenosine receptor prodrugs: synthesis and biological activity of derivatives of potent A1 selective agonists. J Pharm Sci. 1993;1:46. doi: 10.1002/jps.2600830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileson B, Schwartz RD. The use of locomotor activity as a behavioral screen for neuronal damage following transient forebrain ischemia in gerbils. Neurosci Lett. 1991;128:71. doi: 10.1016/0304-3940(91)90762-i. [DOI] [PubMed] [Google Scholar]
- Miller LP, Hsu C. Therapeutic potential for adenosine receptor activation in ischemic brain injury. J Neurotrauma. 1992;9(Suppl 2):S563. [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Mohr JP. Posterior cerebral artery. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Stroke. Churchill Livingstone; New York: 1986. p. 451. [Google Scholar]
- Mohr JP, Gautier JC, Hier DB, Stein RW. Middle cerebral artery. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Stroke. Churchill Livingstone; New York: 1986. p. 377. [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation in rats with hippocampal lesions. Nature. 1982;297:681. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Clarendon; Oxford: 1978. [Google Scholar]
- Phillis JW, O’Regan MH. Deoxycoformycin prevents ischemia-induced locomotor hyperactivity in the unanesthetized gerbil. Med Sci Res. 1988;16:897. [Google Scholar]
- Roussel S, Pinard E, Seylaz J. Focal cerebral ischemia in chronic hypertension: no protection by R-phenylisopropylaenosine. Brain Res. 1991;545:171. doi: 10.1016/0006-8993(91)91283-7. [DOI] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Adenosine and brain ischemia. Cerebrovasc Brain Metab Rev. 1992;4:346. [PubMed] [Google Scholar]
- Schubert P, Kreutzberg GW. Neuroprotective mechanisms of endogenous adenosine action and pharmacological implications. In: Krieglstein J, Oberpichler H, editors. Pharmacology of Cerebral Ischemia. Wissenschaftliche Verlagsgesselschaft; Stuttgart: 1990. p. 417. [Google Scholar]
- Sei Y, Arora PK, Skolnick P, Paul IA. Spatial learning impairment in a murine model of AIDS. FASEB J. 1992;6:3008. doi: 10.1096/fasebj.6.11.1644264. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Marangos PJ. Cerebral ischemia in gerbils: postischemic administration of cyclohexyladenosine and 8-sulphophenyl-theophylline. J Mol Neurosci. 1990;2:53. doi: 10.1007/BF02896926. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Marangos PJ. Self-defense of the brain: adenosinergic strategies in neurodegeneration. In: Marangos PJ, Lai H, editors. Emerging Strategies in Neuroprotection. Birkhauser; Boston: 1992. p. 151. [Google Scholar]
- Von Lubitz DKJE, Dambrosia JM, Redmond DJ. Protective effect of cyclohexyl adenosine in treatment of cerebral ischemia in gerbils. Neuroscience. 1989;2:451. doi: 10.1016/0306-4522(89)90265-0. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DKJE, I, Paul A, Bartus RT, Jacobson KA. Effects of chronic administration of adenosine A1 receptor agonist and antagonist on spatial learning and memory. Eur J Pharmacol. 1993;249:271. doi: 10.1016/0014-2999(93)90522-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Lin RC-S, Melman N, Ji X-d, Carter MF, Jacobson KA. Chronic administration of adenosine A1 receptor agonist or antagonist in cerebral ischemia. Eur J Pharmacol. 1994;256:161. doi: 10.1016/0014-2999(94)90241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Carter MF, Beenhakker M, Lin RC-S, Jacobson KA. Adenosine: a prototherapeutic concept in neurodegeneration. In: Trembly B, Slikker B Jr, editors. Ann New York Acad Sci; Neuroprotective Agents, Proceedings of II International Conference; 1996. p. 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. Purinergic drugs: opportunities for the 1990s. Drug Dev Res. 1993;28:438. [Google Scholar]