Abstract
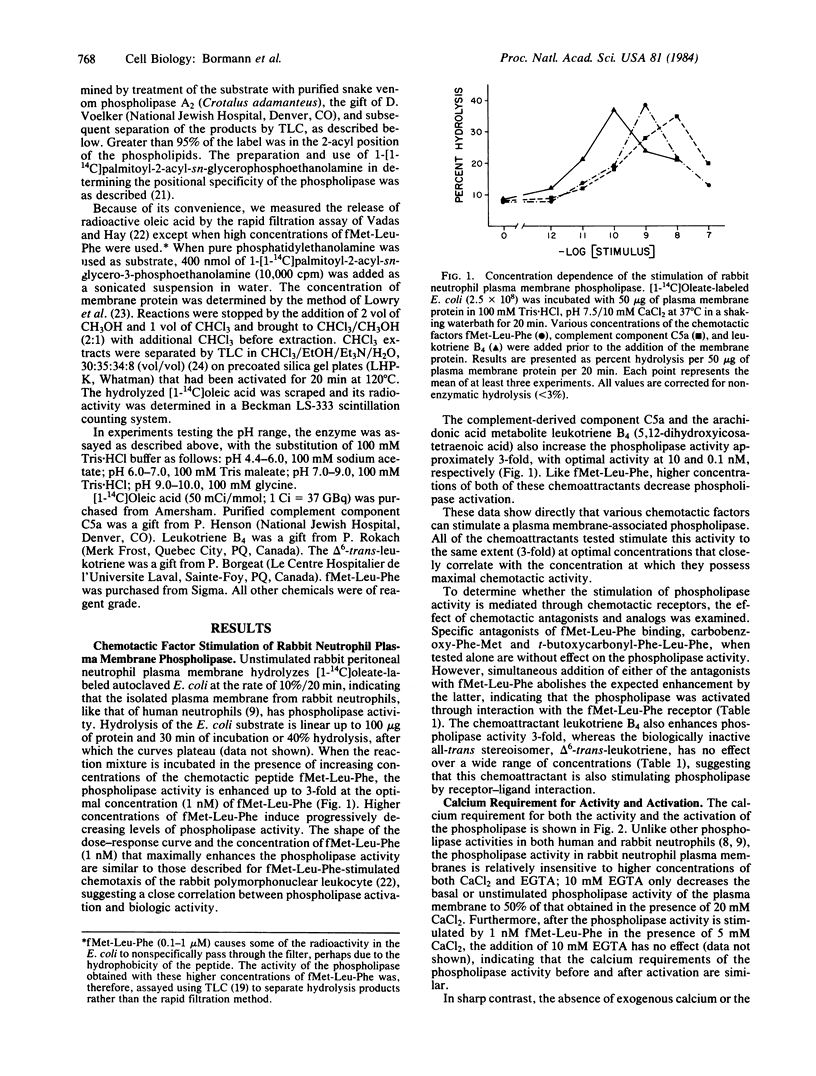
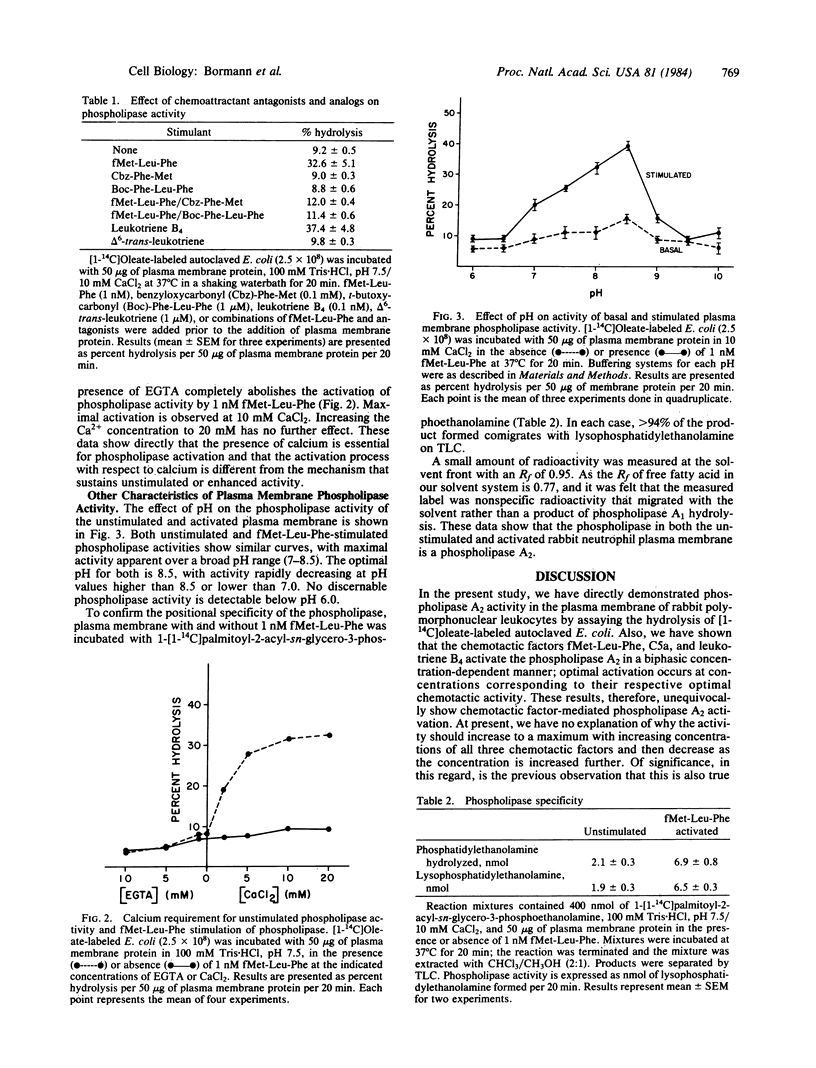
Using the exogenous substrate [1-14C]oleate-labeled autoclaved Escherichia coli, we have demonstrated that the chemotactic factors fMet-Leu-Phe, complement component C5a, and leukotriene B4 [(5S,12R)-dihydroxy-6-cis,8-trans,11-trans,14-cis-icosatetraenoic acid] stimulate a phospholipase A2 of isolated plasma membranes of rabbit peritoneal neutrophils. Each of the chemotactic factors shows a biphasic concentration dependence with the optimal concentrations occurring at 1, 10, and 0.1 nM, respectively. The specific antagonists of fMet-Leu-Phe binding, carbobenzoxy-Phe-Met and t-butoxycarbonyl-Phe-Leu-Phe, effectively block the stimulation by fMet-Leu-Phe, indicating that the activation is receptor mediated. delta 6-trans-leukotriene [(5S-12R)-dihydroxy-all-trans-6,8,10,14-icosatetraenoic acid], a biologically inactive stereoisomer of leukotriene B4, does not stimulate phospholipase activity, suggesting that the enhancement by leukotriene B4 is also receptor mediated. The unstimulated and activated phospholipase exhibit a broad range of maximal activity between pH 7.0 and pH 8.5, both with an optimal pH of 8.5. The activation of the phospholipase by fMet-Leu-Phe is completely calcium dependent; no increase in activity is demonstrable if fMet-Leu-Phe is added in the absence of exogenous calcium or in the presence of EGTA. In contrast, the unstimulated plasma membrane activity of the phospholipase, as well as the activity arising after stimulation, is relatively insensitive to the concentration of calcium, being inhibited by less than 50% in the presence of 10 mM EGTA. The phospholipase hydrolyzes 1-[1-14C]palmitoyl-2-acyl-sn-glycerophosphoethanolamine to form only radioactive lysophosphatidylethanolamine as the product, indicating that the enzyme has an A2 specificity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bareis D. L., Hirata F., Schiffmann E., Axelrod J. Phospholipid metabolism, calcium flux, and the receptor-mediated induction of chemotaxis in rabbit neutrophils. J Cell Biol. 1982 Jun;93(3):690–697. doi: 10.1083/jcb.93.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L. A multifunctional receptor on the neutrophil for synthetic chemotactic oligopeptides. J Reticuloendothel Soc. 1979 Dec;26(Suppl):701–709. [PubMed] [Google Scholar]
- Becker E. L., Talley V., Showell H. J., Naccache P. H., Sha'afi R. I. Activation of the rabbit polymorphonuclear leukocyte membrane "Na+, K+"-ATPase by chemotactic factor. J Cell Biol. 1978 May;77(2):329–333. doi: 10.1083/jcb.77.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franson R., Dobrow R., Weiss J., Elsbach P., Weglicki W. B. Isolation and characterization of a phospholipase A2 from an inflammatory exudate. J Lipid Res. 1978 Jan;19(1):18–23. [PubMed] [Google Scholar]
- Franson R., Patriarca P., Elsbach P. Phospholipid metabolism by phagocytic cells. Phospholipases A2 associated with rabbit polymorphonuclear leukocyte granules. J Lipid Res. 1974 Jul;15(4):380–388. [PubMed] [Google Scholar]
- Goldman D. W., Goetzl E. J. Specific binding of leukotriene B4 to receptors on human polymorphonuclear leukocytes. J Immunol. 1982 Oct;129(4):1600–1604. [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Sha'afi R. I. Response of adenosine cyclic 3',5'-monophosphate level in rabbit neutrophils to the chemotactic peptide formyl-methionyl-leucyl-phenylalanine. Mol Pharmacol. 1979 Sep;16(2):473–481. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rubin R. P., Sink L. E., Schrey M. P., Day A. R., Liao C. S., Freer R. J. Secretagogues for lysosomal enzyme release as stimulants of arachidonyl phosphatidylinositol turnover in rabbit neutrophils. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1364–1370. doi: 10.1016/0006-291x(79)91186-0. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Broekman M. J., Korchak H. M., Marcus A. J., Weissmann G. Endogenous phospholipid metabolism in stimulated neutrophils differential activation by FMLP and PMA. Biochem Biophys Res Commun. 1982 Aug;107(3):951–958. doi: 10.1016/0006-291x(82)90615-5. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., Williams K., Wacholtz M. C., Becker E. L. Binding of the chemotactic synthetic peptide [3H]formyl-nor-Leu-Leu-Phe to plasma membrane of rabbit neutrophils. FEBS Lett. 1978 Jul 15;91(2):305–309. doi: 10.1016/0014-5793(78)81198-3. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Phillips J., Mergenhagen S. E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and Guinea pig serum treated with immune complexes: evidence for c5a as the major chemotactic factor. Infect Immun. 1970 Jun;1(6):521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas P., Hay J. B. The release of phospholipase A2 from aggregated platelets and stimulated macrophages of sheep. Life Sci. 1980 May 19;26(20):1721–1729. doi: 10.1016/0024-3205(80)90181-2. [DOI] [PubMed] [Google Scholar]
- Victor M., Weiss J., Klempner M. S., Elsbach P. Phospholipase A2 activity in the plasma membrane of human polymorphonuclear leukocytes. FEBS Lett. 1981 Dec 28;136(2):298–300. doi: 10.1016/0014-5793(81)80639-4. [DOI] [PubMed] [Google Scholar]
- Vitkauskas G., Showell H. J., Becker E. L. Specific binding of synthetic chemotactic peptides to rabbit peritoneal neutrophils: effects on dissociability of bound peptide, receptor activity and subsequent biologic responsiveness (deactivation). Mol Immunol. 1980 Feb;17(2):171–180. doi: 10.1016/0161-5890(80)90069-3. [DOI] [PubMed] [Google Scholar]
- Waite M., van Deenen L. L. Hydrolysis of phospholipids and glycerides by rat-liver preparations. Biochim Biophys Acta. 1967 Jun 6;137(3):498–517. doi: 10.1016/0005-2760(67)90131-2. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Dechatelet L. R., Thomas M. J., O'Flaherty J. T., Waite M. Effect of phagocytosis and ionophores on release and metabolism of arachidonic acid from human neutrophils. Lipids. 1981 Feb;16(2):120–124. doi: 10.1007/BF02535685. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Waite B. M., Thomas M. J., DeChatelet L. R. Release and metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1981 Jul 25;256(14):7228–7234. [PubMed] [Google Scholar]



