Abstract
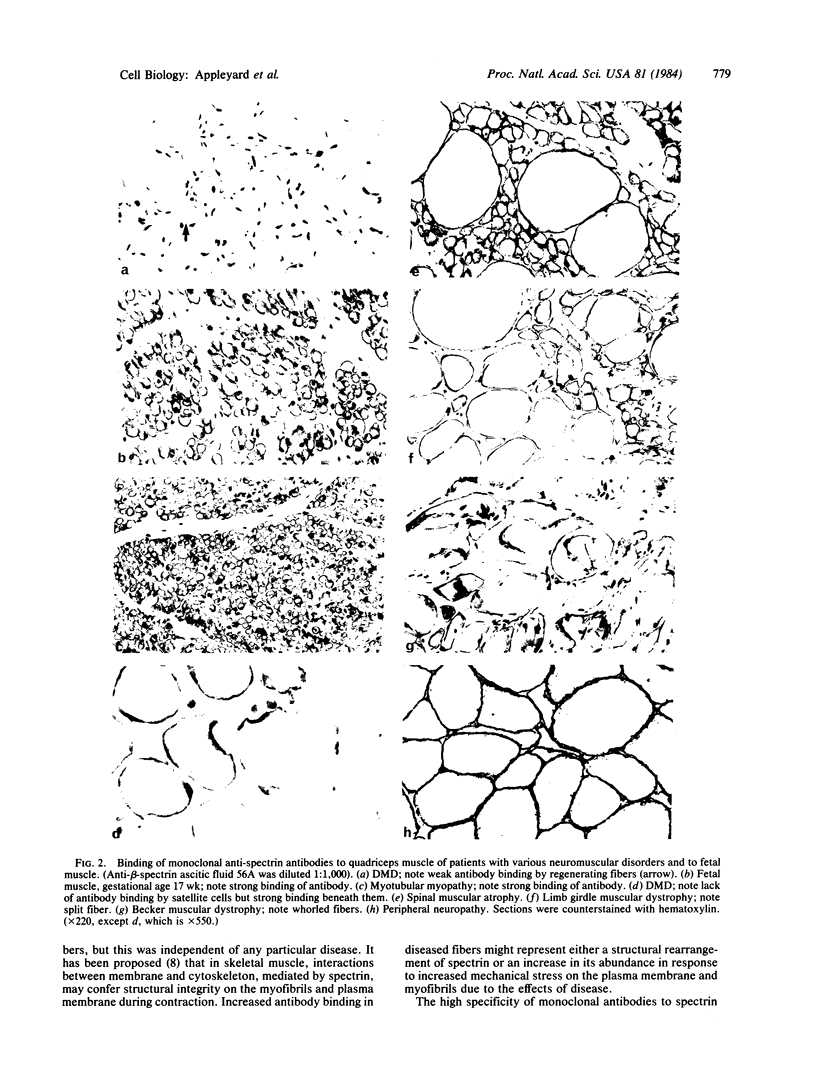
Spectrin is the major protein of the erythrocyte membrane skeleton, which is bound to the cytoplasmic surface of the membrane's lipid bilayer and is responsible for cell shape and membrane elasticity. Inability to identify spectrin in other cell types led to the assumption that this protein was unique to erythrocytes. However, spectrin-like proteins have been demonstrated recently in a variety of cell types, including skeletal and cardiac muscle, in several species. We used monoclonal antibodies against human erythrocyte spectrin subunits in an immunocytochemical study to detect related proteins in normal and diseased human skeletal muscle. Six of seven monoclonal antibodies against beta-spectrin determinants were bound at the cytoplasmic surface of muscle fiber plasma membranes, whereas none of six monoclonal antibodies against alpha-spectrin determinants was bound. Muscle fibers of patients with neuromuscular diseases showed similar distribution and specificity of antibody binding to those of normal subjects, but the intensity of binding was increased. In contrast, probable regenerating fibers in muscle of patients with muscular dystrophies showed reduced binding of antibodies, but reduced binding was not seen in fetal muscle fibers nor in those of a patient with a myotubular myopathy. We conclude that human skeletal muscle fibers possess a spectrin-related protein associated with their plasma membrane that shows extensive beta-chain similarities to erythrocyte spectrin but differs significantly with respect to the alpha-subunit. Its function may be associated with the maintenance of membrane and myofibril integrity during contraction, and the increased antibody binding in diseased muscle may reflect a structural rearrangement of spectrin or a compensatory increase in spectrin abundance in response to increased stress on these systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines A. J. The spread of spectrin. Nature. 1983 Feb 3;301(5899):377–378. doi: 10.1038/301377b0. [DOI] [PubMed] [Google Scholar]
- Bennett V., Davis J., Fowler W. E. Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature. 1982 Sep 9;299(5879):126–131. doi: 10.1038/299126a0. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979 Apr 10;254(7):2533–2541. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Burke B. E., Shotton D. M. Dissection of the human erythrocyte spectrin molecule using monoclonal antibodies. EMBO J. 1982;1(4):505–508. doi: 10.1002/j.1460-2075.1982.tb01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Kelly T., Mangeat P. Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J Cell Biol. 1982 Nov;95(2 Pt 1):478–486. doi: 10.1083/jcb.95.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. J., Mastaglia F. L. Morphological changes in dystrophic muscle. Br Med Bull. 1980 May;36(2):145–122. doi: 10.1093/oxfordjournals.bmb.a071630. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Osborn M., Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982 Apr;28(4):843–854. doi: 10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. Erythroid spectrin, brain fodrin, and intestinal brush border proteins (TW-260/240) are related molecules containing a common calmodulin-binding subunit bound to a variant cell type-specific subunit. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4002–4005. doi: 10.1073/pnas.79.13.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. F-actin-binding and cross-linking properties of porcine brain fodrin, a spectrin-related molecule. J Biol Chem. 1982 Aug 25;257(16):9781–9787. [PubMed] [Google Scholar]
- Goodman S. R., Zagon I. S., Kulikowski R. R. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7570–7574. doi: 10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G., Weber K. Spectrin is absent in various tissue culture cells. Nature. 1977 Mar 10;266(5598):181–183. doi: 10.1038/266181a0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Cheney R. E., Willard M. Location of a protein of the fodrin-spectrin-TW260/240 family in the mouse intestinal brush border. Cell. 1983 Mar;32(3):953–965. doi: 10.1016/0092-8674(83)90080-6. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Witkowski J. A. Membrane abnormalities in Duchenne muscular dystrophy. J Neurol Sci. 1983 Feb;58(2):159–174. doi: 10.1016/0022-510x(83)90214-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Expression of spectrin in nonerythroid cells. Cell. 1982 Dec;31(3 Pt 2):505–508. doi: 10.1016/0092-8674(82)90306-3. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I. Immunolocalization of a novel, cytoskeleton-associated polypeptide of Mr 230,000 daltons (p230). J Cell Biol. 1983 Mar;96(3):703–716. doi: 10.1083/jcb.96.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Expression of the beta subunit of spectrin in nonerythroid cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):363–367. doi: 10.1073/pnas.80.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Sheetz M., Singer S. J. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1359–1363. doi: 10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repasky E. A., Granger B. L., Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982 Jul;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Biochemistry of muscle membranes in Duchenne muscular dystrophy. Muscle Nerve. 1980 Jan-Feb;3(1):3–20. doi: 10.1002/mus.880030103. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Sobue K., Kanda K., Inui M., Morimoto K., Kakiuchi S. Actin polymerization induced by calspectin, a calmodulin-binding spectrin-like protein. FEBS Lett. 1982 Nov 8;148(2):221–225. doi: 10.1016/0014-5793(82)80811-9. [DOI] [PubMed] [Google Scholar]
- Stephens H. R., Duance V. C., Dunn M. J., Bailey A. J., Dubowitz V. Collagen types in neuromuscular diseases. J Neurol Sci. 1982 Jan;53(1):45–62. doi: 10.1016/0022-510x(82)90079-x. [DOI] [PubMed] [Google Scholar]