Abstract
By using the technique of laser light-scattering spectroscopy, direct observation has been made on the intracellular accumulation of a crystallin protein within the cells of chicken embryo lens during the process of development. Appearance of delta-crystallin has been detected as early as day 4, and its concentration reaches a plateau at day 19. The measurements constitute a noninvasive determination of accumulation of protein molecules that specifically characterize the process of cell differentiation.
Full text
PDF
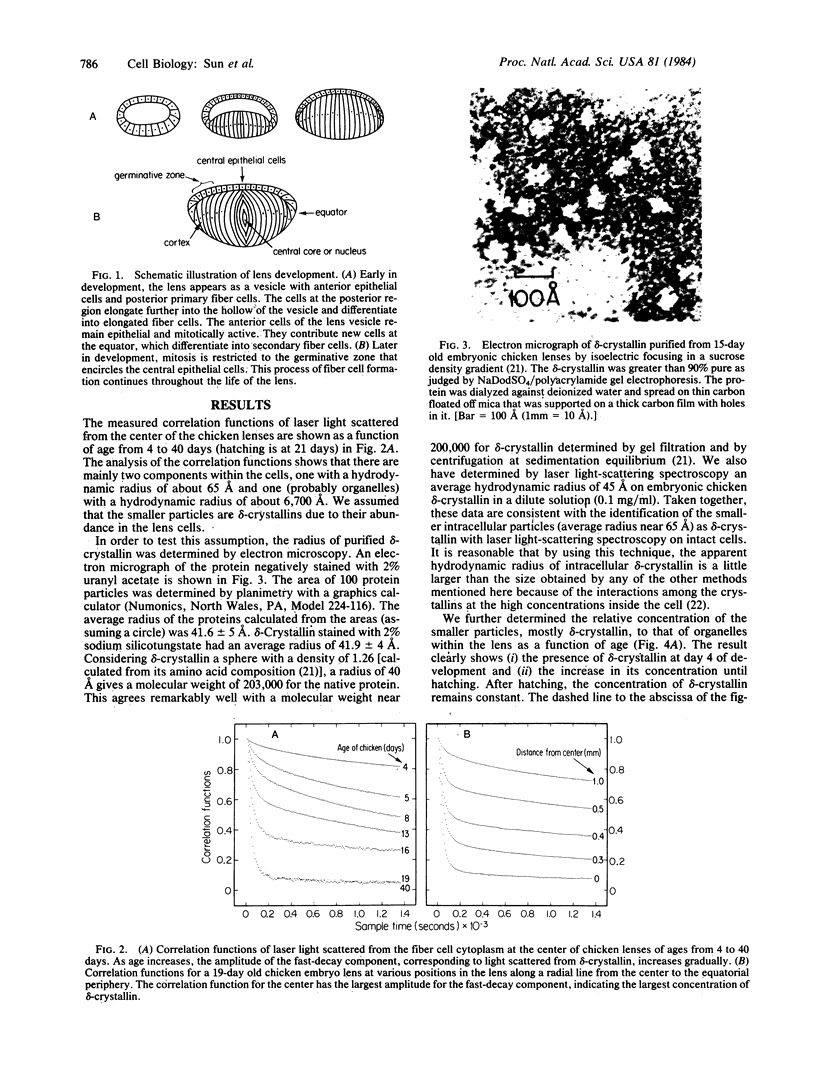
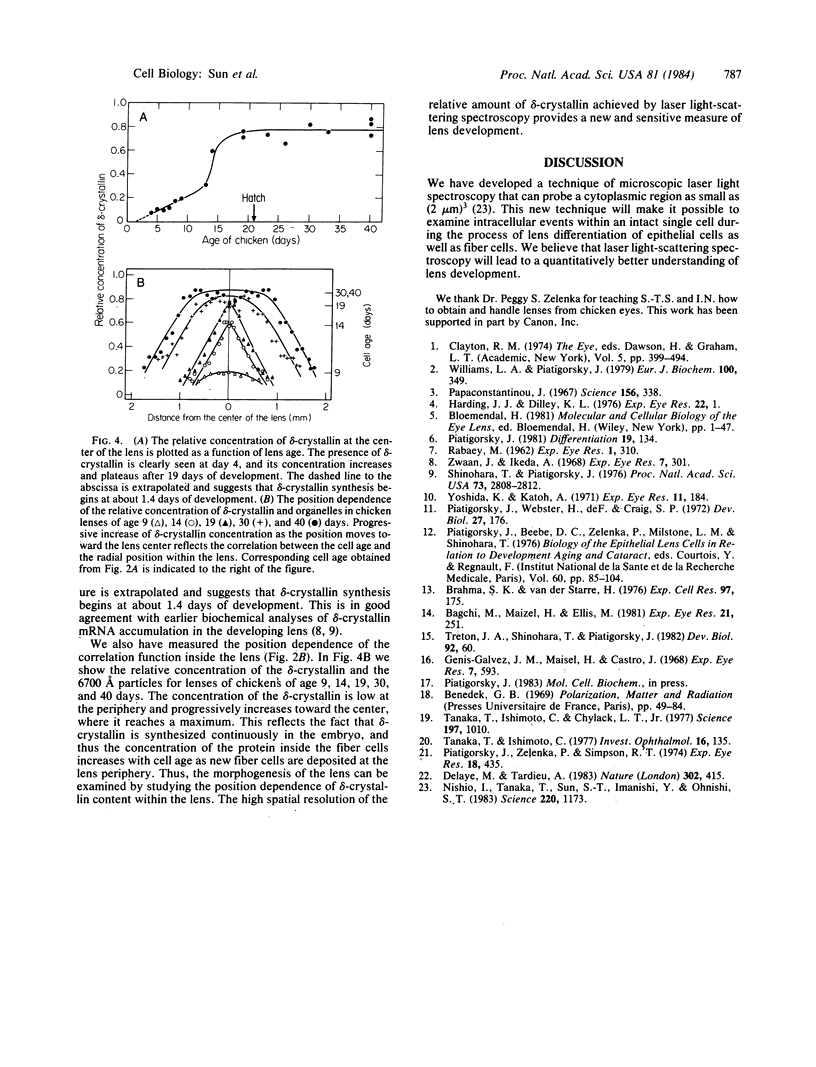
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi M., Alcala J. R., Maisel H. Delta-crystallin synthesis by the adult chicken lens. Exp Eye Res. 1981 Feb;32(2):251–254. doi: 10.1016/0014-4835(81)90013-0. [DOI] [PubMed] [Google Scholar]
- Brahma S. K., Starre H. Studies on biosynthesis of soluble lens crystallin antigens in the chick by isoelectric focusing in thin-layer polyacrylamide gel. Exp Cell Res. 1976 Jan;97:175–183. doi: 10.1016/0014-4827(76)90666-2. [DOI] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Genis-Galves J. M., Maisel H., Castro J. Changes in chick lens proteins with aging. Exp Eye Res. 1968 Oct;7(4):593–602. doi: 10.1016/s0014-4835(68)80014-4. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Dilley K. J. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976 Jan;22(1):1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Nishio I., Tanaka T., Sun S. T., Imanishi Y., Ohnishi S. T. Hemoglobin aggregation in single red blood cells of sickle cell anemia. Science. 1983 Jun 10;220(4602):1173–1175. doi: 10.1126/science.6857241. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou J. Molecular aspects of lens cell differentiation. Science. 1967 Apr 21;156(3773):338–346. doi: 10.1126/science.156.3773.338. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. D., Craig S. P. Protein synthesis and ultrastructure during the formation of embryonic chick lens fibers in vivo and in vitro. Dev Biol. 1972 Feb;27(2):176–189. doi: 10.1016/0012-1606(72)90096-6. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Zelenka P., Simpson R. T. Molecular weight and subunit structure of delta-crystallin from embryonic chick lens fibers. Exp Eye Res. 1974 May;18(5):435–446. doi: 10.1016/0014-4835(74)90080-3. [DOI] [PubMed] [Google Scholar]
- RABAEY M. Electrophoretic and immunoelectrophoretic studies on the soluble proteins in the developing lens of birds. Exp Eye Res. 1962 Jun;1:310–316. doi: 10.1016/s0014-4835(62)80017-7. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Piatigorsky J. Quantitation of delta-crystallin messenger RNA during lens induction in chick embryos. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2808–2812. doi: 10.1073/pnas.73.8.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Ishimoto C., Chylack L. T., Jr Phase separation of a protein-water mixture in cold cataract in the young rat lens. Science. 1977 Sep 2;197(4307):1010–1012. doi: 10.1126/science.887936. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ishimoto C. In vivo observation of protein diffusivity in rabbit lenses. Invest Ophthalmol Vis Sci. 1977 Feb;16(2):135–140. [PubMed] [Google Scholar]
- Tréton J. A., Shinohara T., Piatigorsky J. Degradation of delta-crystallin mRNA in the lens fiber cells of the chicken. Dev Biol. 1982 Jul;92(1):60–65. doi: 10.1016/0012-1606(82)90150-6. [DOI] [PubMed] [Google Scholar]
- Williams L. A., Piatigorsky J. Comparative and evolutionary aspects of delta-crystallin in the vertebrate lens. Eur J Biochem. 1979 Oct 15;100(2):349–357. doi: 10.1111/j.1432-1033.1979.tb04177.x. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Katoh A. Crystallin synthesis by chick lens. II. Changes in synthetic activities of epithelial and fiber cells during embryonic development. Exp Eye Res. 1971 Mar;11(2):184–194. doi: 10.1016/s0014-4835(71)80022-2. [DOI] [PubMed] [Google Scholar]
- Zwaan J., Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968 Apr;7(2):301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]



