Abstract
In the present study, rapid resolution liquid chromatography was coupled with quadrupole time-of-flight tandem mass spectrometry (RRLC-Q-TOF-MS) to identify the absorbed components and metabolites in rat urine after oral administration of Buyang Huanwu decoction (BYHWD). After oral administration of BYHWD, urine samples were collected and pretreated by solid phase extraction. The mass measurements were accurate within 5 ppm of error for all the protonated molecules, and subsequent fragment ions offered higher quality structural information for interpretation of the fragmentation pathways of various compounds. A total of 50 compounds were detected in rat urine samples within 20 min, including 12 parent compounds and 38 metabolites. Except for three prototype components (Hydroxysafflor yellow A, Paeoniflorin, and Amygdalin), the metabolites identified mainly came from Radix Astragali, Radix Angelicae Sinensis, and Rhizoma Chuanxiong. The results indicated that glucuronidation and sulfation were the major metabolic pathways of isoflavonoids, while glutathione conjugation, glucuronidation and sulfation were the main metabolic pathways of phthalides. No saponin-related metabolites were detected. The present study provided important structural information on the metabolism of BYHWD. Furthermore, the results of this work have demonstrated the feasibility of the RRLC/ESI-QTOF-MS approach for rapid and reliable characterization of metabolites from herbal medicines.
Keywords: Buyang Huanwu decoction, quadrupole time-of-flight tandem mass spectrometry, metabolite profiling, urine
1. Introduction
Recently, Traditional Chinese Medicine (TCMs) has attracted considerable attention worldwide due to its high effectiveness against many diseases coupled with its low toxicity [1–3]. In Chinese herbal therapy, Chinese herbal prescriptions, comprising several crude drugs at an intrinsic mass ratio according to TCM formulation concepts, exert an important role in clinical applications. Buyang Huanwu Decoction (BYHWD) is one famous traditional Chinese prescription consisting of seven medicinal crude drugs, including Radix Astragali, Radix Angelicae Sinensis, Radix paeoniae Rubra, Rhizoma Ligustici Chuanxiong, Flos Carthami, Semen Persicae and Lumbricus in a ratio of 120:6:4.5:3:3:3:3. This prescription has been extensively used to improve the syndromes of Qi deficiency and blood stasis for many years in China, owing to its significant therapeutic performance [4]. In clinical applications, BYHWD has proved effective in treating cerebrovascular and cardiovascular diseases [5–7]. Previous pharmacological studies have demonstrated that BYHWD and its major components may prevent VSMC proliferation by interfering with the ERK transduction pathway[8], the formation of arterial thrombosis, and aortic vascular intimal hyperplasia after balloon catheter injury [9], as well as regulate endothelium-derived vasoactive factors in rats [4]. It is also reported that BYHWD can promote the growth and differentiation of neural cells and inhibit apoptosis of nerve cells [10, 11].
Although the pharmacokinetic parameters of BYHWD have been investigated [12], up to now the literature data on the metabolism of BYHWD is still limited. In order to get much metabolism information of BYHWD from the complex biological matrices, it is necessary to develop effective and reliable analytical methods for detection. Recently, liquid chromatography/quadrupole time-of-flight mass spectrometry (LC-Q-TOF/MS), a high-speed and sensitive technique with shorter analysis times and greater m/z value accuracy, has attracted increasing attention [13]. The LC-Q-TOF/MS has been successfully applied to identify active ingredients and metabolites in TCM [14].
In our previous work [15,16], the LC/MS analytical method was developed for quantitative and qualitative analysis of the major constituents in BYHWD, in which a rapid resolution liquid chromatography was coupled with quadrupole time-of-flight tandem mass spectrometry (RRLC-Q-TOF-MS) for elucidation of the chemical profile of BYHWD. Fifty-four major constituents were identified or tentatively characterized, enabling further in vivo elucidation of the metabolites in the prescription. In this investigation, we expanded the scope of our earlier study, by using an RRLC-Q-TOF/MS system to identify known and tentative metabolites in rat urine after oral administration of BYHWD. To our knowledge, it is the first time that the urinary metabolic profile of BYHWD has been established, and it will provide helpful information for further pharmacological and pharmacokinetics research on BYHWD.
2. Experimental
2.1 Herbal materials and chemicals
Radix Astragali (root of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao), and Radix Angelicae Sinensis (root of Angelica sinensis (Oliv.) Diels) were collected from authenticated locations in Min County, Shanxi Province, and Hunyuan County, Gansu Province, in China, respectively. Rhizoma Chuanxiong (root of Ligusticum chuanxiong Hort.), Radix paeoniae Rubra (root of Paeonia lactiflora Pall.) and Flos Carthami (flower of Carthamus tinctorius L.) samples were collected from Sichuan Province in China. Semen Persicae (seed of Prunus persica (L.) Batsch) and Pheretima (Pheretima aspergillum (E. Perrier)) samples were bought from a local pharmacy.
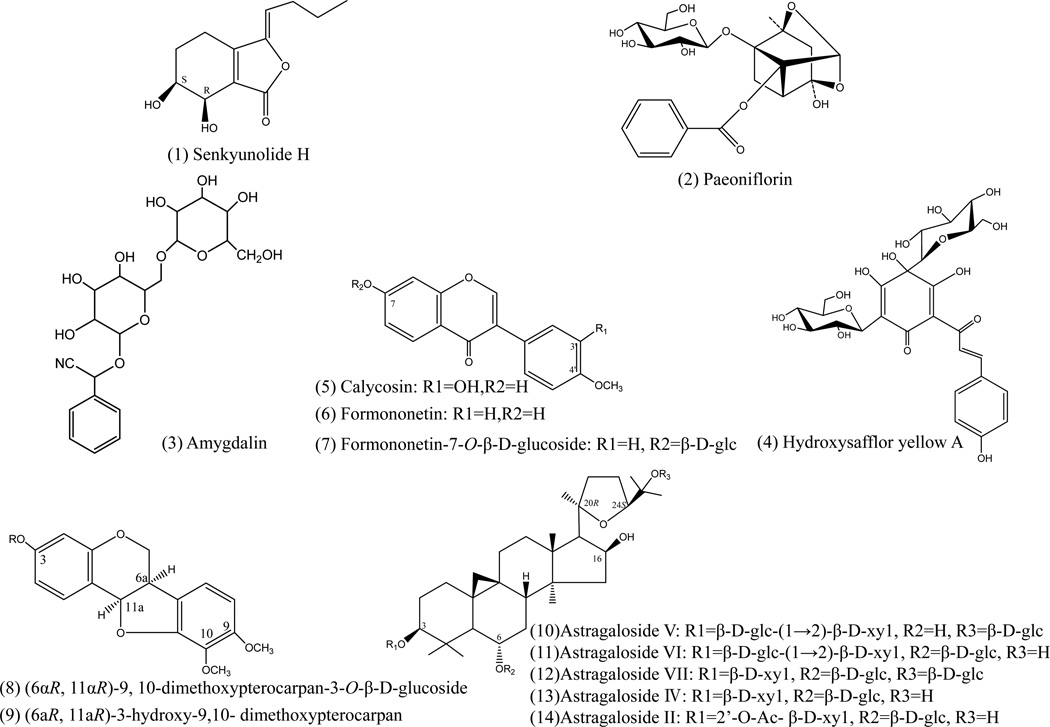
The standards, hydroxysafflor yellow A, amygdalin, and paeoniflorinand ferulic acid were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Senkyunolide H and Z-ligustilide were isolated in our laboratory from Angelica sinensis (Oliv.) Diels. Isoflavonoid and saponin standards were isolated from the dried roots of Astragalus. membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao in our laboratory [17, 18]. Their structures (as shown in Figure 1) were elucidated and unequivocally identified by spectroscopic methods (UV, IR, MS, 1H-NMR and 13C NMR). The purity of each standard compound was determined to be higher than 95% by normalization of the peak area detected by RRLC-DAD-TOFMS.
Figure 1.
Chemical structures of 12 parent compounds of DBT identified in the drug-containing urine.
HPLC-grade acetonitrile (ACN) was supplied by Merck (Darmstadt, Germany). Formic acid (FA) (purity: 96%) was purchased from Tedia (Fairfield, OH, USA). Ethanol was purchased from the First Chemical Company of Nanjing (Jiangsu, China). Distilled water (18 MΩ) was further purified by a Milli-Q system (Millipore, Bedford, MA, USA). Other reagents and chemicals were of analytical grade.
2.2 Preparation of BYHWD
The powdered sample of BYHWD (142.5 g), consisting of Radix Astragali, Radix Angelicae Sinensis, Radix paeoniae Rubra, Rhizoma Ligustici Chuanxiong, Flos Carthami, Semen Persicae and Pheretima in a ratio of 120:6:4.5:3:3:3:3, was immersed in 2000 mL 70% ethanol for 1 h and extracted in a reflux water bath for 1 h. The mixture was refluxed for 2 h at 75°C and filtered, and the residue was refluxed again with another 2000mL 70% ethanol under the same conditions. The extracts were merged and evaporated by rotary evaporation under vacuum at 45°C. The residue was then freeze-dried. Finally, the dried residue was dissolved in water to obtain BYHWD oral solution with a concentration of 3g/mL of crude drug.
2.3 Animal experiments
Animal studies were conducted according to protocols approved by the Review Committee of Animal Care and Use. Six Sprague-Dawley rats (260±15 g) were purchased from Slac Laboratory Animal Co., Ltd. (Shanghai, China). The animals were acclimatized to the facilities for 5 days and then fasted (on plastic bottom cages) with free access to water for a 12-h period prior to the experiment. Then, the blank urine sample from each rat was collected using a metabolic cage. Prior to drug administration, the experimental animals were deprived of food for 12 h once more, and then were exposed orally to the BYHWD preparation at a dose of 60 g/kg (i.e., 2 mL per 100 g body weight). The drug-containing urine samples were collected during the 0–24 h period after administration. All urine samples were centrifuged and then stored at −70°C until additional extraction and analysis.
2.4 Urine sample preparation
A solid-phase extraction (SPE) method was used to extract metabolites from the urine samples. 1.0 mL urine sample was added and flowed through LC-18 SPE columns (1 mL/100mg volume, Supelco, USA) using gravity. The SPE columns were washed with 3 mL deionized water, and then the compounds were eluted with 2 mL methanol. The eluant was then centrifuged at 12000 g at 4°C for 10 min, and 2 mL aliquots of the supernatants were injected into the LC/ESI-TOFMS system for analysis.
2.5 Analytical System
Chromatographic analysis was performed on an Agilent 1200 Series LC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a binary pump, an online degasser, an autoplate-sampler and a thermostatically controlled column compartment. Chromatographic separation was carried out at 25°C on an Agilent ZorBax SB-C18 column (4.6×250 mm, 1.8mm).
The chromatographic conditions were as follows: flow rate of 0.6 mL/min, sample injection volume of 2 µL, column temperature of 25 °C, mobile phase A (0.1% formic acid) and mobile phase B (100% acetonitrile). The gradient profile was optimized as the following: 0–2.0 min, 5–10% B; 2.0–4.0 min, 10–15% B; 4.0–6.0 min, 15% B; 6.0–10.0 min, 15–28% B; 10.0–16.0 min, 28–40% B; 16.0–18.0 min, 40–80% B; 18.0–20.0min, 80–100% B.
Detections were performed using a 6520 Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with an electrospray (ESI) interface. The ESI source of the MS was operated in both positive and negative modes, and the operating parameters were: drying gas (N2) flow rate, 10.0 L/min; drying gas temperature, 325 °C; nebulizer, 35 psig; capillary, 3000 V; Oct RFV, 750 V; and fragmentor voltage, 100 V, and skimmer voltage 65 V. All operations, acquisition, and analysis of data were monitored by Agilent LC–Q-TOF-MS MassHunter Acquisition Software Version A.01.00 (Agilent Technologies) and operated under MassHunter Acquisition Software Version B.02.00 (Agilent Technologies). Mass spectra were recorded across the range m/z 120–2000 with accurate mass measurement of all mass peaks. To optimize signals and obtain maximal structural information, the collision energy was adjusted from 5 V to 40 V for MS/MS experiments.
3. Results and Discussion
3.1 Detection and identification of components in rat urine
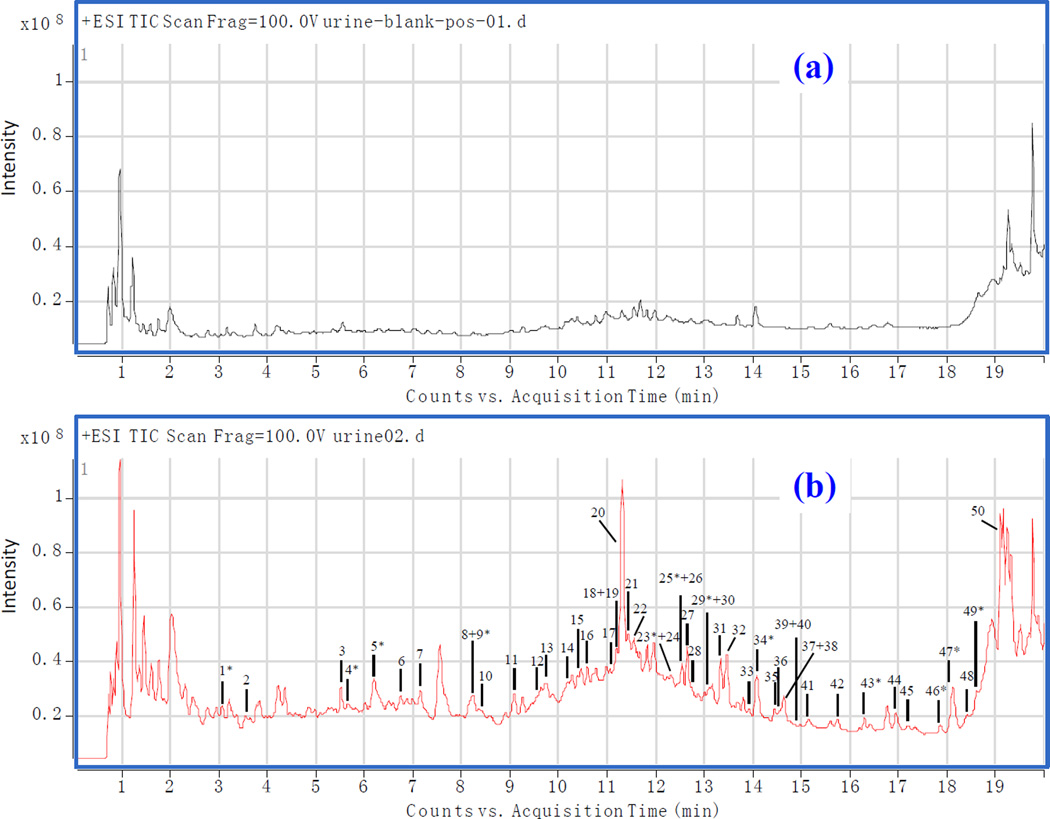
The screening, identification and further characterization of the metabolites of BYHWD were firstly performed by UHPLC/TOFMS in both the positive and negative ion mode. The typical total ion chromatograms (TICs) of blank urine and drug-containing urine samples in positive ion mode are shown in Figure 2(a) and (b), respectively.
Figure 2.
TICs of urine samples of rat by RRLC-QTOF/MS (a): The blank urine in positive mode; (b): The drug-containing urine in positive mode (*The parent constituents in Buyang Huanwu Decoction).
Endogenous interferences from complex biological matrices usually affect the metabolite identification. In order to increase sensitivity and to facilitate data processing, the extracted ion chromatograms (EICs) of TOF/MS were used by comparison of the EICs of the drug-containing urine samples (n=6) with the EICs of blank urine samples, a total of 50 compounds, including 12 prototype components and 38 potential metabolites, were detected in the drug-containing urine (Table 1). The retention time of each compound was ascertained by employing its mass to generate the extracted ion chromatogram (EIC) of the drug-containing urine sample. The fragment ions of the metabolites were then correlated based on their co-elution with their corresponding protonated ions, as well as the possible metabolic or fragmentation pathways of constituents in the BYHWD preparation. Then, the most probable molecular formulas of the metabolites were determined using different criteria, including mass accuracy <5 ppm, the nitrogen rule, the double-bond equivalent index, the isotopic pattern, and modifications based on the common metabolic pathways. Furthermore, structures of the metabolites were elucidated based on product ion spectra generated via adjustment of the fragmentor voltage (5 V, 30 V, and 40 V). Ultimately, 22 of the 38 potential metabolites were tentatively identified.
Table 1.
Metabolites identified from BYHWD in rat urine samples by RRLC-ESI-QTOF/MS
| No. |
tR (min) |
m/z [M+H]+ |
m/z [M+Na]+ |
Formula [M+H]+ |
Fragment ions | ppm error | Identification |
|---|---|---|---|---|---|---|---|
| 1 (P1) | 3.07 | 225.1108 | C12 H16O4 | −1.37 | Senkyunolide H | ||
| 2 (M1) | 3.53 | 383.1319 | C17H19O8 S | 1.68 | |||
| 3 (M2) | 5.65 | 146.0607 | C9H8NO | −4.32 | 4(1H)-quinolinone or 4-hydroxyquinoline | ||
| 4 (P2) | 5.74 | 613.1764 | C27H32O16 | 2.13 | Hydroxysafflor yellow A | ||
| 5 (P3) | 6.2 | 458.1605 | C27H22NO6 | 1.37 | Amygdalin | ||
| 6 (M3) | 6.80 | 641.1653 | C29H39O16 | 479, 465, 303,167,123 | 2.48 | 7,2’-dihydroxy-3’,4’-trimethoxyisoflavan-7-β-O-D-glucuronide-2´-O-β-glucuronide | |
| 7 (M4) | 7.15 | 623.1616 | 645.1432 | C21H19O10 | 461, 447, 285, 270, 253, 225, 197,137. | 3.79 | Calycosin-7-O-β-D-glucoside-3´-O-β-glucuronide |
| 8 (M5) | 8.25 | 247.1545 | 269.1364 | C12H23O5 | −1.93 | ||
| 9 (P4) | 8.32 | 525.1613 ([M+HCOO]−) | C24H28O13 | 0.94 | Paeoniflorin | ||
| 10 (M6) | 8.42 | 431.0970 | 453.0788 | C22H22O9 | 253*, 255, 224, 223, 195, 133 | 0.62 | Daidzein-7-O-β-D-glucuronide |
| 11 (M7) | 9.09 | 453.2078 | 467.1884 | C18H33N2O9 | 0.08 | ||
| 12 (M8) | 9.64 | 477.1028 | 499.0847 | C22H21O12 | 301, 285, 213, 187, 137, | −0.01 | glucuronide conjugate of hydroxylated calycosin |
| 13 (M9) | 9.74 | 311.0699 | 333.0517 | C13H14N2O5 S | −0.86 | ||
| 14 (M10) | 10.18 | 386.2743 | C17H24NO7 S | 2.81 | butylidenephthalide-related metabolites(glutathione-derived metabolites) | ||
| 15 (M11) | 10.46 | 383.1336 ([M−H]−) | 405.1148 | C18H23O9 | 189, 171, 143, 128, 115 | 0.25 | 3-hydroxybutylphthalide-3-O-β-D-glucuronide |
| 16 (M12) | 10.61 | 447.0925 | 469.0738 | C21H19O11 | 271,253, 225, 213, 197,137 | −0.29 | 4'-demethycalycosin-7-O-β-D-glucuronide |
| 17 (M13) | 11.07 | 479.1909 | 501.1727 | C24H31O10 | 0.42 | ||
| 18 (M14) | 11.16 | 335.0217 | C15H11O7S | 253*, 255, 224, 223, 195, 167, 133, | 1.02 | Daidzein-7-O-sulfate or Daidzein-4'-O-sulfate | |
| 19 (M15) | 11.19 | 383.1354 ([M−H]−) | C18H23O9 | 0.25 | isomer of 3-hydroxybutylphthalide-3-O-β-D-glucuronide | ||
| 20 (M16) | 11.32 | 461.1079 | 483.0903 | C22H21O11 | 285, 270, 253, 225, 197, 137, | 1.27 | Calycosin-7-O-β-D-glucuronide |
| 21 (M17) | 11.44 | 597.1756 | 619.1574 | C34H29O10 | −0.08 | ||
| 22 (M18) | 11.53 | 365.0321 | C16H13O8S | 1.15 | calycosin-7-O-sulfate or calycosin-3′-O-sulfate | ||
| 23 (P5) | 12.23 | 463.1587 | C23H26O10 | 2.47 | (6αR, 11αR)-9, 10-dimethoxypterocarpan-3-O-β-D-glucoside | ||
| 24 (M19) | 12.33 | 383.1348 ([M−H]−) | C18H23O9 | 0.26 | isomer of 3-hydroxybutylphthalide-3-O-β-D-glucuronide | ||
| 25 (P6) | 12.50 | 431.1327 | C22H23O9 | 2.22 | Formononetin-7-O-β-D-glucoside | ||
| 26 (M20) | 12.54 | 477.1018 | 499.0844 | C22H21O12 | 301, 285, 213, 187, 137, | 2.00 | glucuronide conjugates of hydroxylated calycosin |
| 27 (M21) | 12.66 | 445.1138 | C22H21O10 | 269, 254, 226, 197, 181, 169, 141, 137 | −1.08 | Formononetin-7-O-β-D-glucuronide | |
| 28 (M22) | 12.88 | 465.1379 | 487.1202 | C22H25O11 | 2.72 | ||
| 29 (P7) | 13.09 | 285.0760 | C16H13O5 | −0.22 | Calycosin | ||
| 30 (M23) | 13.09 | 509.1633 | 531.1642 | C24H29O12 | 333 | −2.08 | 7,2’-dihydroxy-3’,4’,5’-trimethoxyisoflavan-7-β-O-D-glucuronide |
| 31 (M24) | 13.34 | 479.1541 | 501.1361 | C23H27O11 | 303, 167,123. | 1.57 | (3R)-7,2’-dihydroxy-3’,4’-dimethoxyisoflavan-7-O-β-D-glucuronide |
| 32 (M25) | 13.48 | 477.1370 | 499.1202 | C23H25O11 | 301,167,152. | 2.68 | (6αR,11αR)-3-hydroxy-9,10-dimethoxypterocarpan-3-O-β-D-glucuronide |
| 33 (M26) | 13.94 | 287.0904 | C21H30NO4S | −4.17 | |||
| 34 (P 8) | 14.15 | 947.5184 | C47H78O19 | −0.2 | Astragaloside V/VI/VII | ||
| 35 (M27) | 14.45 | 381.1175 | 403.0988 | C18H21O9 | 1.45 | ||
| 36 (M28) | 14.55 | 188.0709 | 210.0526 | C11H19NO2 | 0.95 | ||
| 37 (M29) | 14.64 | 370.1321 | 392.1134 | C17H24NO6S | 354, 207, 161, 189, 179 | −0.13 | acetylcyteine conjugate of senkyunolide |
| 38 (M30) | 14.76 | 354.1376 | 376.1178 | C17H24NO5S | 0.19 | acetylcysteine conjugate of dehydroxylated senkyunolide | |
| 39 (M31) | 14.89 | 352.1204 | 374.1022 | C17H22NO5S | 189, 171, 143, 129 | 2.56 | 3-hydroxybutylphthalide-3-S-acetylcysteine |
| 40 (M32) | 14.99 | 259.1643 | 281.1471 | C12H23N2O4 | −2.54 | ||
| 41 (M33) | 15.14 | 216.1959 | 238.1777 | C12H25NO2 | −0.20 | ||
| 42 (M34) | 15.75 | 257.2223 | 279.2043 | C14H28N2O2 | −0.18 | ||
| 43 (P9) | 16.30 | 807.4509 | C41H68O14 | 1.3 | Astragaloside IV | ||
| 44 (M35) | 16.94 | 373.2737 | 391.2839 | C24H37O3 | −0.03 | ||
| 45 (M36) | 17.21 | 352.1213 | 374.1024 | C17H22NO5S | 0.1900 | isomer of 3-hydroxybutylphthalide-3-S-acetylcysteine | |
| 46 (P10) | 17.87 | 849.4613 | C43H70O15 | −4.4 | Astragaloside II | ||
| 47 (P11) | 18.11 | 269.0823 | C16H12O4 | 2.6 | Formononetin | ||
| 48 (M37) | 18.41 | 271.0971 | C16H15O4 | 253, 225, 213, 197, 137 | −2.29 | 4'-demethylcalycosin | |
| 49 (P12) | 18.61 | 301.1079 | C17H16O5 | −4.4 | (6aR, 11aR)-3-hydroxy-9,10dimethoxypterocarpan | ||
| 50 (M38) | 19.18 | 287.1497 | 309.1321 | C14H23O6 | −3.67 |
P: The parent constituents in Buyang Huanwu Decoction; M: metabolites;
Fragmentations were obtained in the negative ion mode.
3.2 Identification of parent components in drug-containing urine
By comparing the accurate masses of peaks appearing in the chromatograms of drug-containing urine with those previously identified in BYHWD [15], 12 peaks were detected as prototype components of BYHWD. As shown in Table 1, most of the prototype compounds were identified by comparison of their retention times and TOFMS data with those of the standards, and their structures are shown in Figure 1. Among these 12 compounds, there are five isoflavonoids ((6αR, 11αR)-9, 10-dimethoxypterocarpan-3-O-β-D-glucoside, Formononetin-7-O-β-D-glucoside, calycosin, formononetin, and (6αR,11αR)-3- hydroxy-9,10-dimeth-oxypterocarpan), and three saponins (Astragaloside V/VI/VII, Astragaloside IV and Astragalo-sideII) from Radix Astragali, and four other types of compounds (Senkyunolide H, Hydroxysafflor yellow A, Paeoniflorin and Amygdalin) from Radix Angelicae Sinensis, Flos Carthami, Radix paeoniae Rubra and Semen Persicae.
3.3 Identification of metabolites in drug-containing urine
Beyond the 12 prototype compounds, more than 38 peaks were tentatively predicted to be metabolites of BYHWD, and could be generally divided into two groups: isoflavonoid-related and phthalide-related metabolites.
3.3.1 Identification of isoflavonoid-related metabolites
A total of 14 compounds detected in rat urine were tentatively assigned as metabolites originating from isoflavonoids. According the aglycone type, they are generally divided into four groups named G1, G2, G3 and G4, possessing calycosin, formononetin, (6αR,11αR)-3-hydroxy-9,10-dimethoxypterocarpan, and (3R)-7, 2′- hydroxy-3′,4′-dimethoxy- isoflavan as the aglycone, respectively (Table 1). Ions of [M+H]+, [M+Na]+, or [M+NH4]+ in the positive mode and [M−H]− in the negative mode can be easily observed for most components. Moreover, diagnostic losses corresponding to conjugates were observed in the mass spectra of these metabolites, including C6H8O6 (176Da) for glucuronide conjugates and SO3 (80Da) for sulfate conjugates.
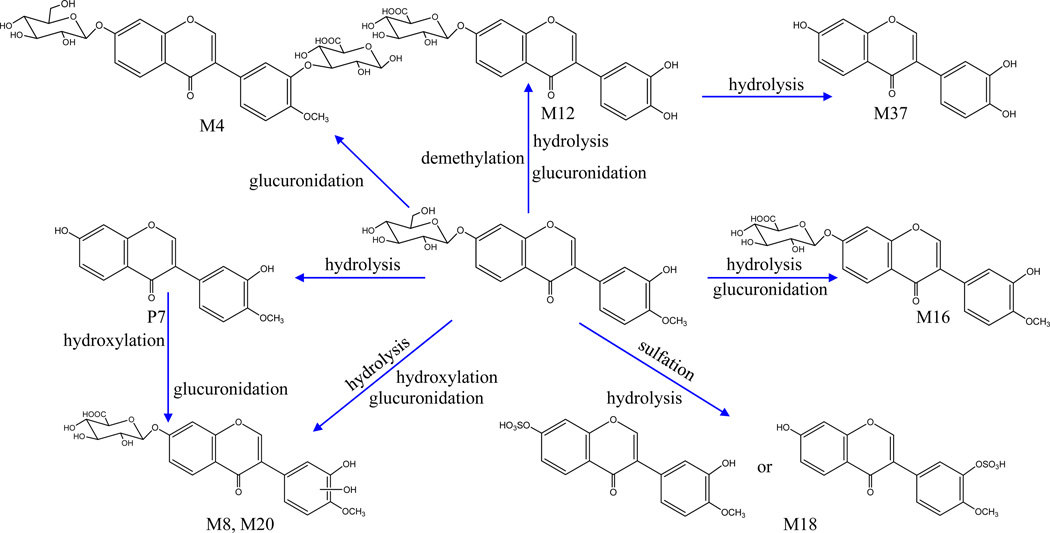
Metabolites of G1
Five glucuronide conjugates (M4, M8, M12, M16 and M20), one sulfate conjugate (M18), and one demethylated product (M37) were assigned as metabolites of G1 (Figure 3). Among them, M4, M16 and M18 comprised the same aglycone, calycosin, and yielded a series of characteristic ions of calycosin at m/z 285, 270, 253, 225, 213, 197, and 137. The aglycone of M12 and M37 was assigned as 4’-demethylcalycosin, due to the presence of the aglycone ion at m/z 271.0615, 14Da (CH2) less than the protonated ion of calycosin, together with the observation of the other fragment ions shown in Table 1. Meanwhile, the aglycone ion of M8 and M20 ([M+H–C6H8O6]+), which appeared at m/z 301.07 (C16H13O6+), could have been derived from calycosin (C16H13O5+) via hydroxylation, suggesting that their aglycone was hydroxylated calycosin. Since the glucuronide conjugates of isoflavonoids mainly exist as 7-O-β-D-glucuronides in vivo [19,20], M12 and M16 were tentatively identified as 4'-demethyl-calycosin-7-O-β-D-glucuronide and calycosin-7-O-β- D-glucuronide. In the MS spectrum of M4, the presence of the characteristic ions of calycosin indicated that the fragment ion at m/z 447 corresponded to calycosin-7-O-β-D-glucoside, and, accordingly, M4 was identified as calycosin-7-O-β-D- glucoside-3′-O-β-D-glucuronide.
Figure 3.
Proposed major metabolic pathways of G1(calycosin) -related metabolites.
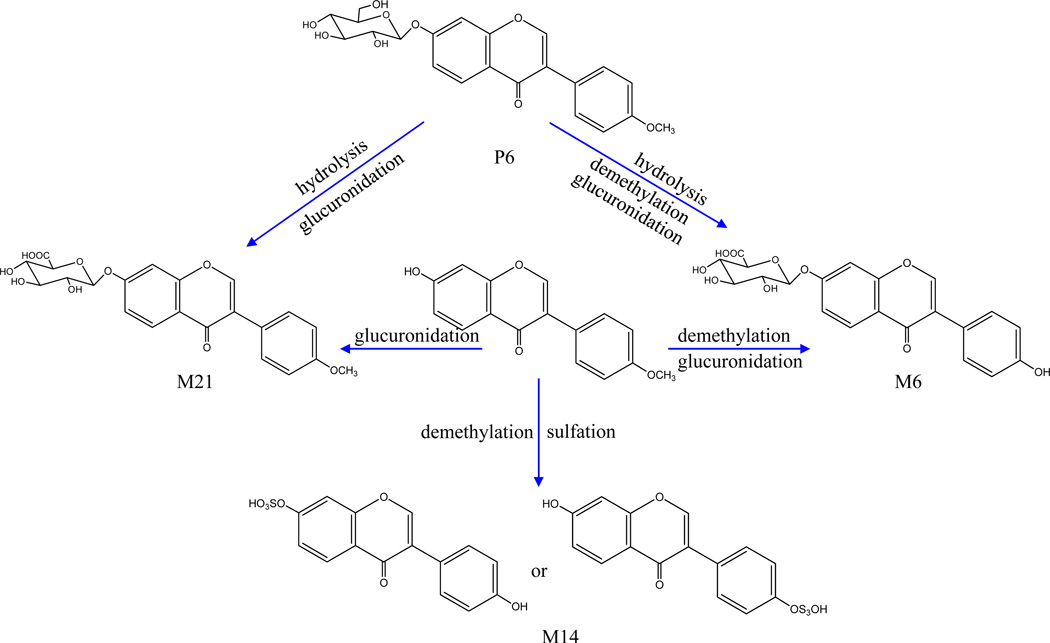
Metabolites of G2
Two glucuronide conjugates (M6 and M21) and one sulfate conjugate (M14) were assigned as metabolites of G2 (Figure 4). M21 yielded a peak at m/z 445.1138 in positive mode and showed product ions at m/z 269, 254, 226, 197, 181, 169, 141 and 137 in the MS/MS detection, suggesting that it possesses a formononetin aglycone. Both M6 and M14 exhibited the same product ion at m/z 255 (C15H11O4), which was tentatively identified as daidzein, since it was 14 Da (CH2) less than the protonated ion of formononetin; the other fragment ions shown in Table 1 were also observed. In addition, it has been well documented that formononetin can be metabolized to daidzein [21], and then to equol (C15H14O3) [22–25]. Eventually, M6 and M21 were identified as daidzein-7-O-β-D-glucuronide and formononetin-7-O-β-D- glucuronide, respectively, due to only one hydroxyl group at the C-7 position of their skeleton.
Figure 4.
Proposed major metabolic pathways of G2 (formononetin) -related metabolites.
Metabolites of G3
M25 was considered as a metabolite of G3, owing to the production of the characteristic ions of (6αR,11αR)-3-hydroxy-9,10-dimethoxypterocarpan at m/z 167, 301 and 152 in positive mode. Since there is only one hydroxyl group available at the C-3 position of the skeleton of G3, M25 was consequently identified as (6αR,11αR)-3-hydroxy-9,10- dimethoxypterocarpan 3- O-β-D-glucuronide.
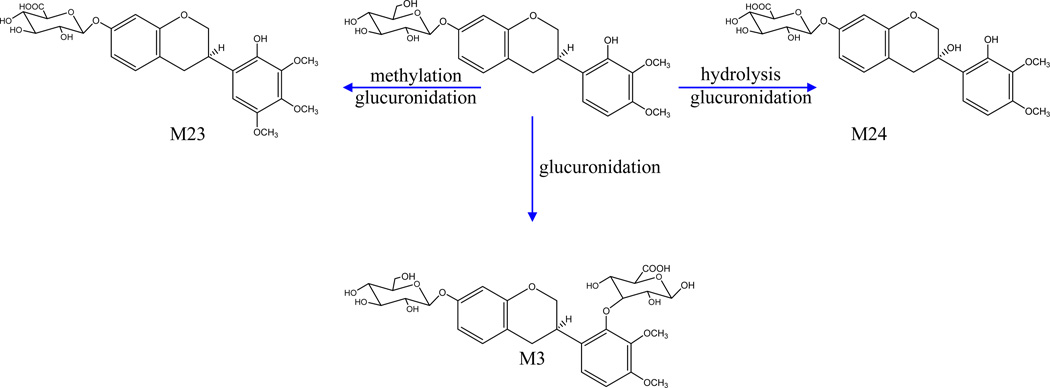
Metabolites of G4
Three glucuronide conjugates (M3, M23, and M24) were assigned as metabolites of G4 (Figure 5). M3 and M24 produced a [M+H–C6H8O6]+ ion at m/z 303.1222, and further exhibited [2,3B+H]+ and [2,3A+H]+ ions at m/z 167 and 123, respectively, indicating that they possessed the same aglycone G4. Furthermore, M3 displayed the [M+H]+ ion at m/z 641.1563 (C29H37O16), and also yielded product ions at m/z 479 and 465, corresponding to the losses of a glucose (162 Da) and a glucuronide (176 Da). Thus, M3 was tentatively assigned as (3R)-7,2’-hydroxy-3’,4’-dimethoxy- isoflavan-7- O-β-D-glucoside-2′-glucuronide. The aglycone ion of M23 at m/z 333.13 (C18H21O6) was 32Da (CH3O) higher than that of G3, implying that there is one more methoxyl in this aglycone compared with G3. According to a previous study [26], M23 was tentatively identified as 7,2′-dihydroxy-3′,4′,5′-trimethoxy- isoflavan- 7-O-β-D-glucuronide.
Figure 5.
Proposed major metabolic pathways of G4 ((3R)-7,2′-dihydroxy-3′,4′-dimethoxyiso-flavan) -related metabolites.
3.3.2 Identification of phthalide-related metabolites
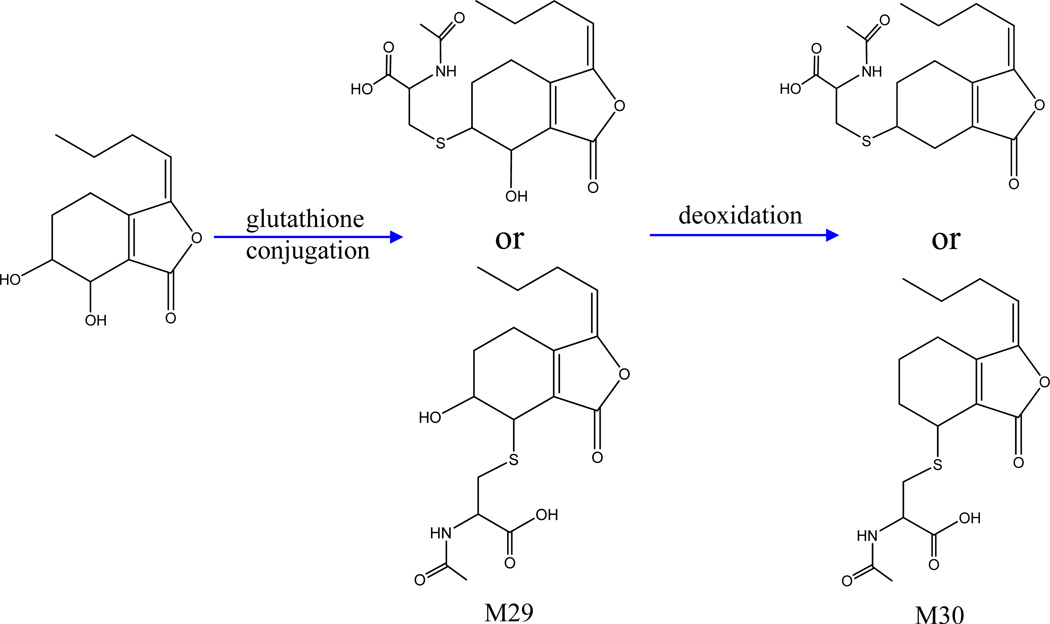
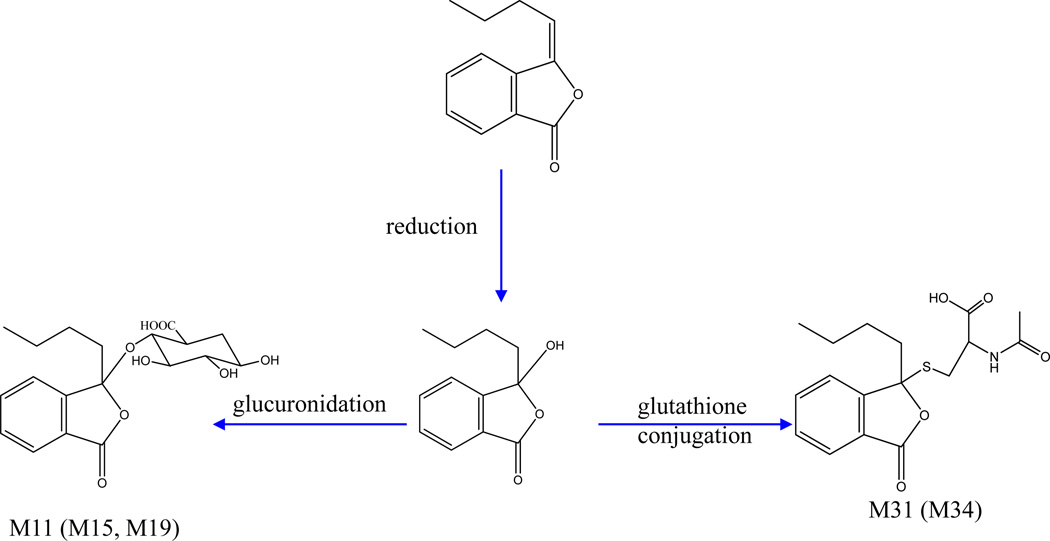
Phthalides are important bioactive compounds in Radix Angelicae Sinensis and Rhizoma Chuanxiong, two components of BYHWD. A total of 7 metabolites (M29, M30, M11, M15, M19, M31, M34) were tentatively identified as phthalide-related metabolites according to their MS/MS data listed in Table 1. Figure 6 and Figure 7 displays the possible structures of these components, including two senkyunolide-related metabolites and five butylidenephthalide-related metabolites. In addition to the glucuronide conjugates and sulfate conjugate, some of these metabolites were glutathione-derived compounds, whose fragmentation patterns show a neutral loss of C5H7NO2S (145 Da) originating from the acetylcysteine residue.
Figure 6.
Proposed major metabolic pathways of senkyunolide-related metabolites.
Figure 7.
Proposed major metabolic pathways of butylidenephthalide-related metabolites.
Senkyunolide-related metabolites
M29 displayed the [M+H]+ and [M+Na]+ ions at m/z 370.1323 and 392.1134, respectively, indicating that the molecular formula of M29 is C17H23NO6S. Further MS analysis of M29 yielded a product ion at m/z 207, which was formed by the loss of C5H9NO3S (163 Da) from the [M+H]+ ion, suggesting the presence of one acetylcysteine residue (C5H7NO2S) in M29. In addition, M29 exhibited product ions at m/z 161.0950, 179.1054, 189.0905 and 207.1014, the characteristic ions of senkyunolide in positive mode, indicating that it is a senkyunolide-related metabolite. According to previous studies [27], M29 was identified as the acetylcysteine conjugate of senkyunolide.
As for M30, the protonated ion at m/z 354.1373 (C17H24NO5S) was 16Da (O) less than the protonated ion of M29, suggesting that it was a dehydroxylation product of M29.
Butylidenephthalide-related metabolites
M11 and M31 were considered as butylidenephthalide related metabolites owing to the production of the diagnostic ions of butylidenephthalide at m/z 189, 171, 143, 128 and 115. Furthermore, M11 yielded the aglycone ion by the loss of C6H8O6 (176 Da), while M31 yielded the aglycone ion by the loss of C5H7NO2S (145 Da). According to a previous study [27], M11 and M31 were tentatively identified as 3-hydroxybutylphthalide-3-O-β-D-glucuronide and 3-hydroxybutylphthalide-3-S-acetylcysteine, respectively. Meanwhile, M15 and M19 have the same chemical formula as M11. Since no other fragment ions of M15 and M19 were found, they were tentatively assigned as the isomers of M11. In the same way, M34 was tentatively identified as the isomer of M31 due to having the same chemical formula.
3.4 Analysis of the urinary metabolite profile of BYHWD
BYHWD is a well-known traditional Chinese prescription with many good therapeutic effects. However, the lack of information on the metabolites of BYHWD has made it difficult to obtain a consistent picture of its efficacy and pharmacology. In the present study, 50 metabolites were detected in rat urine samples within 20 min. 22 of them, including isoflavonoids, phthalides and saponins, were tentatively identified based on MS data. Except for the three prototype components (Hydroxysafflor yellow A, Paeoniflorin, and Amygdalin), the metabolites identified mainly came from the crude drugs Radix Astragali, Radix Angelicae Sinensis and Rhizoma Chuanxiong.
Isoflavonoids were the most abundant metabolites in the drug-containing urine. A total of 19 isoflavonoids, including 5 parent compounds and 14 metabolites, were identified in the drug-containing urine. The detection of two isoflavonoid glycosides (P5 and P6) demonstrated that isoflavonoid glycosides could be absorbed directly, which was supported by a previous study [26]. Based on these metabolites, the possible metabolic pathways of isoflavonoids in BYHWD were proposed and summarized in Figure 3–5. The results revealed that glucuronidation and sulfation were the main metabolic pathways of isoflavonoids in BYHWD. In addition, it showed that some Phase I reactions, such as hydrolysis, demethylation, methylation, hydroxylation and reduction also occurred in the metabolism of isoflavonoids in BYHWD.
Saponins also play an important role in BYHWD. However, only very low amounts of astragaloside II, astragaloside IV (P9) and astragaloside V/VI/VII (P8) were detected in the drug-containing urine. This can be possibly attributed to the fact that saponins were poorly absorbed directly, and were mainly excreted through feces or bile.
As for the phthalides from Radix Angelicae Sinensis and Rhizoma Ligustici Chuanxiong, both prototype components and metabolites were detected in the drug-containing urine. According to metabolism information, in addition to glucuronidation and sulfation, glutathione conjugation also occurs in the metabolism of phthalides.
Conclusion
In this study, a rapid and reliable UPLC-Q-TOF/MS method was successfully established to identify the metabolites of BYHWD in rat urine samples collected 24 h after oral administration of BYHWD. In total, 50 metabolites were detected, and 22 of them were tentatively identified. To our knowledge, this is the first study to establish the urinary metabolic profile of BYHWD by UPLC-Q-TOF/MS. The urinary metabolites and their metabolic pathways proposed in this study are very helpful in revealing the bioactive chemical basis of BYHWD. Furthermore, it was demonstrated that the high-speed and sensitive UPLC-Q-TOF/MS analytical system was a useful tool to investigate the active ingredients and metabolites of TCM.
Acknowledgements
This work was supported in part by the Program for Changjiang Scholars and Innovative Research Teams in Universities (No. IRT0868), the National Science Foundation of China (No. 30973884) and the NIH/NCCAM P01 AT004418 and K01 AT005362.
References
- 1.Jiang WY. Therapeutic wisdom in traditional Chinese medicine: A perspective from modern science. Trends Pharmacol. Sci. 2005;26:558–563. doi: 10.1016/j.tips.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Normile D. The new face of traditional Chinese Medicine. Science. 2003;299:188–190. doi: 10.1126/science.299.5604.188. [DOI] [PubMed] [Google Scholar]
- 3.Wang ZG, Ren J. Current status and future direction of Chinese herbal medicine. Trends Pharmacol. Sci. 2002;23:347–348. doi: 10.1016/s0165-6147(02)02051-5. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Wang WR, Lin R, Zhang JY, Ji QL, Lin QQ, Yang LN. Buyang Huanwu decoction ameliorates coronary heart disease with Qi deficiency and blood stasis syndrome by reducing CRP and CD40 in rats. J. Ethnopharmacol. 2010;130:98–102. doi: 10.1016/j.jep.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Wang WR, Lin R, Zhang H, Lin QQ, Li N, Yang, Zhang KF, Ren F. The effects of Buyang Huanwu Decoction on hemorheological disorders and energy metabolism in rats with coronary heart disease. J. Ethnopharmacol. 2011;137:214–220. doi: 10.1016/j.jep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Zeng SM. Clinical research of Buyang Huanwu Decoction for residuals of cerebral infarction. Hainan Medical Journal (in Chinese) 2005;16:136–137. [Google Scholar]
- 7.Gai FC. Application of Buyang Huanwu Decoction in cardio-cerebralvessels diseases. Journal of Emergency in Traditional Chinese Medicine (in Chinese) 2005;14:376–377. [Google Scholar]
- 8.Chen G, Wu L, Deng CQ. The effects of BuYang HuanWu Decoction and its effective components on proliferation-related factors and ERK1/2 signal transduction pathway in cultured vascular smooth muscle cells. J Ethnopharmacol. 2011;135:7–14. doi: 10.1016/j.jep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Zhang W, Li H, Chen BY, Zhang GM, Tang YH, Hee FY, Deng CQ. Inhibition of aortic intimal hyperplasia and cell cycle protein and extracellular matrix protein expressions by BuYang HuanWu Decoction. J. Ethnopharmacol. 2009;125:423–435. doi: 10.1016/j.jep.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Jiang DM. Neuroprotective effect of Buyang Huanwu Decoction on spinal ischemia/reperfusion injury in rats. J. Ethnopharmacol. 2009;124:219–223. doi: 10.1016/j.jep.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Chen A, Wang H, Zhang JW, Wu XQ, Liao J, Li H, Cai WJ, Luo XG, Ju G. BYHWD rescues axotomized neurons and promotes functional recovery after spinal cord injury in rats. J. Ethnopharmacol. 2008;117:451–456. doi: 10.1016/j.jep.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Yang G, Lin R, Hu Z. Determination of paeoniflorin, calycosin7-O-β-D-glucoside, ononin, calycosin and formononetin in rat plasma after oral administration of Buyang Huanwu decoction for their pharmacokinetic study by liquid chromatography–mass spectrometry. Biomed. Chromatogr. 2011;25:450–457. doi: 10.1002/bmc.1466. [DOI] [PubMed] [Google Scholar]
- 13.Lv Y, Zhang X, Liang X, Liu XR, Dai WX, Yan SK, Zhang WD. Characterization of the constituents in rat biological fluids after oral administration of Fufang Danshen tablets by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2010;52:155–159. doi: 10.1016/j.jpba.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhu JB, Guo XJ, Fu SP, Zhang XL, Liang XM. Characterization of steroidal saponins in crude extracts from Dioscorea zingiberensis C. H. Wright by ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010;53:462–474. doi: 10.1016/j.jpba.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Liu EH, Qi LW, Peng YB, Cheng XL, Wu Q, Li P, Li CY. Rapid separation and identification of fifty-four major constituents in Buyang Huanwu Decoction by ultra-fast HPLC system coupled with DAD-TOF/MS. Biomed. Chromatogr. 2009;23:828–842. doi: 10.1002/bmc.1193. [DOI] [PubMed] [Google Scholar]
- 16.Liu EH, Qi LW, Cheng XL, Peng YB, Li P. Simultaneous determination of twelve bioactive constituents in Buyang Huanwu decoction by HPLC-DAD-ELSD and HPLC-TOF/MS. Biomedical Chromatography. 2010;24:125–131. doi: 10.1002/bmc.1269. [DOI] [PubMed] [Google Scholar]
- 17.Qi LW, Wen XD, Cao J, Li CY, Li P, Yi L. Rapid and sensitive screening and characterization of phenolic acids, phthalides, saponins and isoflavonoids in Danggui Buxue Tang by rapid resolution liquid chromatography/diode-array detection coupled with time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:2493–2509. doi: 10.1002/rcm.3638. [DOI] [PubMed] [Google Scholar]
- 18.Qi LW, Cao J, Li P, Yu QT, Wen XD, Wang YX, Li CY, Bao KD, Ge XX, Cheng XL. Qualitative and quantitative analysis of Radix Astragali products by fast high-performance liquid chromatography-diode array detection coupled with time-of-flight mass spectrometry through dynamic adjustment of fragmentor voltage. J. Chromatogr. A. 2008;1203:27–35. doi: 10.1016/j.chroma.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Clarke DB, Lloyd AS, Botting NP, Oldfield MF, Needs PW, Wiseman H. Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with (13C(3)) isoflavone internal standards. Anal. Biochem. 2002;309:158–172. doi: 10.1016/s0003-2697(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 20.Bursztyka J, Perdu E, Tulliez J, Debrauwer L, Delous G, Canlet C, De Sousa G, Rahmani R, Benfenati E, Cravedi JP. Comparison of genistein metabolism in rats and humans using liver microsomes and hepatocytes. Food Chem. Toxicol. 2008;46:939–938. doi: 10.1016/j.fct.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Tolleson WH, Doerge DR, Churchwell MI, Marques MM, Roberts DW. Metabolism of Biochanin A and Formononetin by human Liver Microsomes in Vitro. J. Agr. Food Chem. 2002;50:4783–4790. doi: 10.1021/jf025549r. [DOI] [PubMed] [Google Scholar]
- 22.Chang YC, Nair MG. Metabolism of daidzein and genistein by intestinal bacteria. J. Nat. Prod. 1995;58:1892–1896. doi: 10.1021/np50126a014. [DOI] [PubMed] [Google Scholar]
- 23.Joannou GE, Kelly GE, Reeder AY, Waring M, Nelson C. A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J. Steroid Biochem. Mol. Biol. 1995;54:167–184. doi: 10.1016/0960-0760(95)00131-i. [DOI] [PubMed] [Google Scholar]
- 24.Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, Hur HG, Han KO. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006;101:246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Heinonen SM, Hoikkala A, Wähälä K, Adlercreutz H. Identification of new metabolites having an intact isoflavonoid skeleton. J. Steroid Biochem. Mol. Biol. 2003;87:285–299. doi: 10.1016/j.jsbmb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Xu F, Zhang Y, Xiao SY, Lu XW, Yang DH, Yang XD, Li CL, Shang MY, Tu PF, Cai SQ. Absorption and metabolism of Astragali radix decoction: in silico, in vitro, and a case study in vivo. Drug Metab. Dispos. 2006;34:913–924. doi: 10.1124/dmd.105.008300. [DOI] [PubMed] [Google Scholar]
- 27.Yan R, Ko NL, Li SL, Tam YK, Lin G. Pharmacokinetics and metabolism of ligustilide, a major bioactive component in Rhizoma Chuanxiong, in the rat. Drug Metab. Dispos. 2008;36:400–408. doi: 10.1124/dmd.107.017707. [DOI] [PubMed] [Google Scholar]