Abstract
Objective To examine the association of family organization with metabolic control in adolescents with type 1 diabetes through the mechanisms of family self-efficacy for diabetes and disease management. Method Data from the baseline assessment of a longitudinal RCT were used, wherein 257 adolescent–parent dyads (adolescents aged 11–14) each completed the family organization subscale of the Family Environment Scale, the self-efficacy for Diabetes Self-Management Scale, the Diabetes Behavior Rating Scale, and 2 24-hr diabetes interviews. Results Structural equation modeling showed greater family organization was associated indirectly with better disease management behaviors via greater family self-efficacy (β = .38, p < .001). Greater self-efficacy was indirectly associated with better metabolic control via better disease management both concurrently (β = −.37, p < .001) and prospectively (β = −.26, p < .001). The full model indicates more family organization is indirectly associated with better metabolic control concurrently and prospectively through greater self-efficacy and better disease management (β = −.13, p < .001). Conclusions Understanding the mechanisms by which family organization is associated with metabolic control provides insight into possible avenues of prevention/intervention for better diabetes management.
Keywords: disease management, family organization, self-efficacy, type 1 diabetes
Introduction
Type 1 diabetes is a chronic childhood condition characterized by the inability to produce insulin and subsequent blood glucose (BG) instability (NIH, 2006). In order to manage this condition, children must perform a series of behavioral tasks, including frequent BG monitoring, insulin administration, and careful monitoring of diet and exercise (Greening, Stoppelbein, Konishi, Jordan & Moll, 2007; NIH, 2006). Though the regimen is complex and time-consuming, failure to adhere to it may result in serious short- and long-term medical problems [American Diabetes Association, 2011; Diabetes Control and Complications Trial Research Group (DCCT), 1993; Greening et al., 2007].
As a result of the involved management process and the serious consequences of poorer management, youth with diabetes may feel overwhelmed (Greening et al., 2007; Lemanek, Kamps & Chung, 2001). This situation is complicated further for adolescents since diabetes self-care behaviors may interfere with perceived independence and social acceptance and be associated with poorer metabolic control (La Greca & Bearman, 2002; Silverstein et al., 2005). However, family support and involvement in the diabetes care regimen can help reduce the challenges for youth and is associated with better metabolic control (Ellis et al., 2007; La Greca & Bearman, 2002).
Family Organization
Strong evidence supports the importance of parental involvement in treatment (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997; Ellis et al., 2007; Wysocki et al., 2009). Positive aspects of family functioning and the home environment are associated with improved adolescent adherence to diabetes care. Families with more organization and routine have children who are more likely to evidence better treatment adherence, better metabolic control, and successful adaptation to the diabetes regimen, suggesting that family organization is associated with better diabetes self-care behaviors (Greening et al., 2007; Grey, Boland, Yu, Sullivan-Bolyai, & Tamborlane, 1998; Hauser et al., 1990; Safyer et al., 1993; Seiffge-Krenke, 1998). Likewise, youth who have higher levels of regimen adherence, BG monitoring and better nutrition were more likely to have parents who reported higher levels of family organization, and youths themselves were more likely to perceive better family organization (Hauser et al., 1990).
Examination of families with alternative family structures reveals congruent findings. Parents in nontraditional family structures, such as single-parent families report less family organization, regardless of whether or not their children have diabetes (Overstreet et al., 1995). Further, parents of children with diabetes from nontraditional family structures report more child behavior problems than two-parent families (Overstreet et al., 1995) along with poorer metabolic control, lending credence to the idea that higher levels of family organization may relate to better self-care behaviors and subsequently, more favorable metabolic control (Overstreet et al., 1995; Silverstein et al., 2005). Greater familial organization is hypothesized to help allay youths’ fears associated with self-management (Hauser et al., 1989; Safyer et al., 1993) and to increase their self-efficacy, in turn leading to improved adherence behaviors and better metabolic control, although this model has not been empirically tested.
Therefore, to better understand how general family organization, defined as the relative importance of familial organization in family activities and responsibilities (Moos & Moos, 2002), may be associated with better metabolic control potential mediators of this relation need to be examined. Greater family organization may provide more routine structure that supports diabetes tasks. For example, a predictable schedule may make it easier for youth to remember to check their BG levels or to administer insulin. Disease management that is easier to integrate into routine daily life may be associated with youth and parental perceptions of greater diabetes self-efficacy to manage diabetes tasks. A sense of greater self-efficacy, as well as better disease management may be two mechanisms through which family organization is associated with better metabolic control.
Potential Mediators
Self-Efficacy for Diabetes
Family organization may relate to adherence through self-efficacy since greater self-efficacy for diabetes management relates to better disease care. For example, in a review of existing literature, Sigurardóttir (2005) noted that greater self-efficacy for diabetes is associated with better self-care behaviors and better metabolic control. Further, Ott et al. (2000) determined that lower self-efficacy mediated the relation between less supportive parental behaviors and less BG monitoring. Recently, Berg et al. (2011) found that self-efficacy mediates the relation between child–parent relationship quality and metabolic control. However, no studies yet have examined the relations among family organization, youth and parent self-efficacy, and disease management.
Disease Management
Poorer diabetes adherence is not only detrimental but can also be dangerous to the health of youth with type 1 diabetes (Morris et al., 1997; Sokol, McGuigan, Verbrugge, & Epstein, 2005). Poorer diabetes adherence contributes to poorer glycemic control, hospitalization, and instances of diabetic ketoacidosis in youth (Morris et al., 1997; Sokol et al., 2005). A better understanding of factors that relate to youth disease management behaviors, including family organization and self-efficacy for diabetes and their interrelations may yield multiple points of treatment intervention to facilitate disease care.
Current Study
Although family organization has been shown to positively relate to adherence behaviors and to metabolic control in youth with diabetes, the mechanisms of their interrelations are unclear. Higher levels of family organization reasonably could be associated with greater youth and parent self-efficacy, since the family environment is presumptively more supportive of effective disease management, which in turn is associated with better metabolic control. The current study explores these relations through examination of individual and family factors in adolescents’ diabetes disease management with multimethod, multi-informant data from the baseline assessment of a longitudinal randomized clinical trial. Specifically, family organization is hypothesized to be associated with better metabolic control, via greater youth/parent self-efficacy for diabetes management and better disease management behaviors.
Materials and Methods
Participants
Eligibility requirements for participants included (a) youth age between 11 and 14 years at time of recruitment, (b) illness duration of at least 1 year, (c) absence of severe diabetes-related complications or other medical diagnoses, and (d) fluency in English. Questionnaire and interview data were collected during baseline assessments with 257 family dyads composed of an adolescent with type 1 diabetes (49% female) and one parent (92% mothers) enrolled in an ongoing randomized clinical trial; adolescent HbA1c values were collected at a youth’s baseline and follow-up clinic appointment ∼3 months later before the initiation of psychological treatment. Mean age at baseline was 12.80 (SD = 1.21). Most of the participants were Caucasian (69%; 19%, African-American; 6%, Latino; 2%, Asian-American; 4%, other) and lived in a home with two or more adults (77%) of primarily upper-middle or middle-class socioeconomic status based on the Hollingshead Four-Factor Scale (80% class II or III; M = 2.45, SD = 0.87; Hollingshead, 1975). Adolescent participants had a mean illness duration of 5.16 years (SD = 3.11) and a mean HbA1C of 8.81% (SD = 1.64%) at baseline and 8.94% (SD = 1.56%) 3 months later. The majority of youth (65%) was on an intensive insulin regimen of multiple daily basal bolus injections or an insulin pump.
Procedure
Psychological data were from baseline assessments of an ongoing multisite randomized clinical trial (RCT). HbA1c data were collected at baseline and again approximately 3 months later. The RCT began after the collection of all baseline psychological and HbA1c data as well as the 3 month follow-up HbA1c data; the intervention/control sessions began following the youth’s clinic visit, after the HbA1c data were already obtained. RCT participation involved four brief sessions of either behavioral intervention or diabetes information with one parent and a youth conducted at the time of regular endocrinology appointments. Participants were recruited from diabetes clinics at two mid-Atlantic Children’s hospitals. Potentially eligible families were identified based on clinic lists and contacted by informational letter and follow-up telephone call by trained research assistants. Written consent and assent were obtained. Data for the trial were collected by self-report questionnaires and interviews conducted by research assistants at regularly scheduled clinic visits and via a follow-up telephone call within 2 weeks of baseline, as well as medical record reviews. The baseline protocol required ∼60 min to complete. Families were given a $25 gift card in appreciation of their time. Of the 404 eligible families successfully contacted, 285 provided consent to participate (71%) in the larger trial. Those declining consent primarily cited lack of interest and of time. Baseline data were provided by 257 dyads (90%). The appropriate institutional review boards approved the study.
Measures
Background Information
Demographic and medical information were obtained through a 33-item questionnaire developed by the research team. Socioeconomic status was assessed via the Hollingshead scale (Hollingshead, 1975), with categorical scores ranging from 1 to 5, for which lower scores indicate higher socioeconomic class. Parent marital status also was assessed via this measure. The glycosylated hemoglobin A1C, or HbA1c assay, is routinely performed at regular clinic visits, and results were extracted from medical records. Medical data were verified by record review by research assistants.
Family Organization
The 9-item organization subscale from the Family Environment Scale (FES; Moos & Moos, 2002) assesses general, not diabetes-specific, family organization. Both parents and youth answered true/false items to indicate their perception of their family’s organization, for example, “activities in our family are pretty carefully planned”. The FES generally demonstrates satisfactory levels of internal consistency and test–retest reliability (Moos & Moos, 2002), however, variability exists (Loveland-Cherry, Youngblut, & Leidy, 1989). In this sample, the internal consistency for separate parent and youth report was marginal (parent report α = .55, youth report α = .58). However, when parent and youth report were considered together in the creation of a family organization, latent variable reliability was adequate (combined report α = .70). As such, family organization was treated as a latent construct in this study. A family organization latent construct also allows for the identification of the construct’s true score (Kline, 2011; Llabre, 2010).
Family Self-Efficacy
Parents and youth completed the self-efficacy for Diabetes Self-Management Scale (SEDSM; Iannotti, Schneider, et al., 2006), a 10-item self-report scale that assesses each person’s perceived self-efficacy to complete or perform diabetes care behaviors, such as BG checks and insulin injections. Participants rate each item on a 1–10 scale to indicate different levels of youth/parent self-efficacy (1 = not sure at all that I could do “X” behavior, 10 = completely sure that I could do “X behavior”). Higher scores on this measure indicate higher levels of self-efficacy for diabetes self-care behaviors. Youth and parent diabetes-related self-efficacy both show high internal consistency (α = .90) and test–retest reliability (α = .89; Ianotti, Schneider, et al., 2006). Internal consistencies in the present sample also were high (parent report α = .85, youth report α = .85). Similar to the family organization variable, youth and parent self-efficacy scores were combined into a latent variable to provide a measure of family self-efficacy in the current study. Creation of a latent construct partials out error variance due to reporter bias and can identify the true score of a family self-efficacy construct (Kline, 2011; Llabre, 2010).
Disease Management
The Diabetes Behavior Rating Scale (DBRS; Iannotti, Nansel, et al., 2006) was completed by youth and parents separately to assess management of diabetes care. The DBRS is a youth- and parent-proxy report of diabetes management behaviors over the previous week, with slight variations in item content based on insulin regimen for injections or pump. For these analyses, the total scale score was used, which consists of 36 items for insulin injection regimens and 37 items for pump regimens. Respondents rated the approximate frequency with which routine diabetes care behaviors occurred such as BG monitoring, insulin administration, and behavioral adjustments to specific situations on a 5-point Likert scale ranging from “never” to “always”. Scores were transformed into proportions of tasks completed and averaged across insulin regimen groups. A score of 1 is the maximum and higher scores reflect greater adherence. The DBRS has good internal consistency and test–retest reliability. The questionnaire has a significant association with the interview form of the measure and with HbA1c (Iannotti, Nansel, et al., 2006). In this sample, the reliability coefficient was adequate for both versions (pump: parent report α = .69, youth report α = .81; nonpump: parent report α = .79, youth report α = .80).
Frequency of Blood Glucose Checks
To assess frequency of BG checks, an important indicator of self-management, youth and parents separately completed the 24-h Diabetes Interview (DI; Holmes et al., 2006, adapted from Johnson, Silverstein, Rosenbloom, Carter, & Cunningham, 1986). In separate interviews, parents and youth reported diabetes management tasks over the previous 24 h, including the total number of BG checks performed. For the current study, the DI was administered on 2 weekdays within a 2-week period and BG frequency responses were averaged across the two interviews for each parent and each adolescent within a dyad. Total number of BG checks reported over 2 days by a parent and by the youth was averaged separately. The 24-h methodology is a reliable, valid, “well-established” measure of self-care behaviors in type 1 diabetes (Freund, Johnson, Silverstein, & Thomas, 1991; Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008). Parent and youth reports on the DI were used as ecologically valid indicators of disease management since this measure was designed to minimize memory errors and reporter biases prone to questionnaires.
Data Analytic Plan
The current study evaluated a path model of the association of family organization (parent and youth report), family self-efficacy for diabetes (parent and youth self-report), disease management behaviors (parent and youth report, and parent and youth report of BG check frequency), and metabolic control (HbA1c at baseline and 3 months later). The model was analyzed using Structural Equation Modeling in Mplus 6 (Muthen & Muthen, 1998–2010). The full information maximum likelihood procedure was used to include participants who had individual data points missing, presumed to be missing at random. This procedure, which is the default in Mplus 6, estimates missing data values based on the current estimate of known parameters and then re-estimates the parameters based on known and imputed data (Collins et al., 2001). This is a preferred method for handling missing data, as it includes all available data in statistical analyses (Collins et al., 2001). Demographic data were not estimated.
The data were screened for outliers, and all indicators were found to be within normal limits for skewness (≤3) and kurtosis (≤10; Kline, 2011). Overall model fit was assessed with a chi-square analysis, root-mean-square error of approximations (RMSEA; values below .06 indicate good fit; Kline, 2011), comparative fit index (CFI; values above .90 indicate acceptable fit; Hu & Bentler, 1998, 1999), and the standardized root mean square residual (SRMR; values less than .08 are acceptable; Kline, 2011). However, due to the large sample size of the current study, the chi-square value is not considered the best indicator of model fit, as the chi-square statistic is closely related to sample size (Kline, 2011). Instead, the CFI, RMSEA, and SRMR values are considered better indicators. As such, these latter three indicators will be the focus of subsequent analyses.
Results
Descriptive information
Parents and youth in the current sample reported normative levels of family organization (parent M = 54.89, SD = 10.40; youth M = 51.93, SD = 10.62; Boyd, Gullone, Needleman & Burt, 1997). Both parents and youth reported relatively high, though normative, levels of self-efficacy for diabetes (parent M = 8.10, SD = 1.26; youth M = 7.08, SD = 1.73; Iannotti, Schneider et al., 2006). Across 2 days, parents reported a daily mean of 3.11 BG checks (SD = 1.86) and youth reported a daily mean of 3.63 checks (SD = 1.62). Mean adolescent HbA1c was higher than levels recommended by the ADA (2011) and ranged from 5.90% to 14.00% (baseline M = 8.81%, SD = 1.64; 3 months M = 8.94%, SD = 1.56). For a complete report of the means and correlations among variables, see Table 1.
Table I.
Correlations among Key Study Variables, Means, and SDs
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child age (years) | 1 | 12.80 (1.21) | |||||||||||
| 2. SESa | −.06 | 1 | 2.44 (0.87) | ||||||||||
| 3. Number of parentsb | −.06 | −.35*** | 1 | 0.83 (0.38) | |||||||||
| 4. Family org (P) | −.06 | .00 | .03 | 1 | 54.89 (10.40) | ||||||||
| 5. Family org (Y) | −.11 | −.16* | .14* | .52*** | 1 | 51.93 (10.62) | |||||||
| 6. Self-efficacy (P) | −.19** | −.05 | −.02 | .23*** | .23*** | 1 | 8.10 (1.26) | ||||||
| 7. Self-efficacy (Y) | .12 | −.21** | .20** | .16* | .28*** | .31*** | 1 | 7.08 (1.73) | |||||
| 8. Disease management (P) | −.14* | −.20** | .08 | .19** | .19** | .35*** | .33*** | 1 | 0.67 (0.11) | ||||
| 9. Disease management (Y) | −.09 | −.22*** | .12 | .05 | .19** | .18** | .45*** | .39*** | 1 | 0.63 (0.13) | |||
| 10. BG freq. (P) | −.30*** | −.04 | .19** | .21** | .21** | .23*** | .21** | .27*** | .20** | 1 | 3.11 (1.86) | ||
| 11. BG freq. (Y) | −.15* | −.19** | .25*** | .16* | .22** | .13* | .27*** | .38*** | .21** | .67*** | 1 | 3.63 (1.62) | |
| 12. HbA1c (Base.) | .13* | .32*** | −.18** | −.07 | −.15* | −.18** | −.34*** | −.30*** | −.17** | −.18** | −.26*** | 8.81 (1.64) | |
| 13. HbA1c (3 months) | .15* | .28*** | −.20** | −.16* | −.15* | −.20** | −.36*** | −.23** | −.19** | −.26*** | −.27*** | .78*** | 8.94 (1.56) |
Note. P = Parent report, Y = Youth report.
aCategorical classification was employed with lower numbers equal to higher socioeconomic status (SES).
bCoded in reference to two parent households.
*p< .05, **p< .01, ***p< .001.
Model Testing
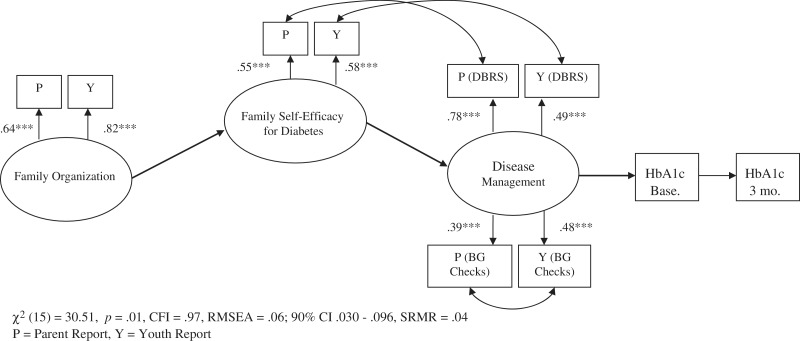
The hypothesized measurement model consisted of three latent variables. The first, family organization, included parent and youth report of family organization from the FES. Information from each reporter was used in order to derive a latent variable free of reporter bias, thereby improving reliability of the construct (Kline, 2011; Llabre, 2010). The second latent variable, family self-efficacy for diabetes, included parent and youth report of self-efficacy for diabetes from the SEDSM. The third latent variable, disease management, included parent and youth DBRS Total scale scores and parent and child report of average BG checks across two days from the 24-h DI. The measurement model was tested to ensure that the observed variables combined appropriately as indicated by the hypothesized latent variables. The proposed model did not fit the data well, as indicated by all fit indices being outside of suggested ranges [χ2 (34) = 379.59, p < .001, CFI = .51, RMSEA = .20, SRMR = .16]. However, all indicators sufficiently loaded onto the hypothesized latent variables (all factors: β > .32, p < .001). To improve model fit in accordance with MPlus suggested modification guidelines (Kline, 2011; MacCallum & Austin, 2000), correlations in error variances for parent and youth report of BG frequency, for youth-reported self-efficacy for diabetes and youth report of the DBRS Total score, and parent-reported self-efficacy and report of DBRS Total score were added. As these correlations in error variances made theoretical sense as a result of shared methodological bias, covariances were added to the model (Kline, 2011). With these correlations included, the measurement model evidenced good fit for the data [χ2 (15) = 30.51, p = .01, CFI = .97, RMSEA = .06; 90% CI .03–.10, SRMR = .04; see Figure 1].
Figure 1.
Measurement model with correlations.
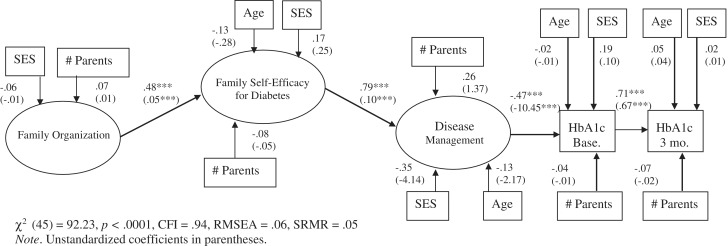
The hybrid model was evaluated, including covariates indicated by initial correlations, age, socioeconomic status, and number of parents in the household (Table 1). A significant overlap occurred between the ethnicity (Caucasian coded as 1) and the SES variables (r = −.33, p < .001) in the present sample. Due to the ethnic heterogeneity of the current sample, SES only was used in subsequent analyses. Demographic covariates of age, SES, and number of parents were regressed on the latent variables with which they demonstrated a significant correlation and all of them were regressed on HbA1c at baseline and follow-up. The resulting model demonstrated good fit [χ2 (45) = 92.23, p < .001, CFI = .94, RMSEA = .06; 90% CI = .05–.08, SRMR = .05; see Figure 2]. Family organization was significantly related to greater self-efficacy for diabetes (β = .48, p < .001). In turn, greater family self-efficacy for diabetes was related to better disease management behaviors (β = .79, p < .001). Better disease management behaviors were significantly related to concurrent lower HbA1c (β = −.47, p < .001). Finally, baseline HbA1c was significantly related to prospective HbA1c, measured 3 months later (β = .71, p < .001). The indirect paths among the variables also were significant, including the relations between family organization and HbA1c (baseline HbA1c β = −.18, p < .001; 3 month follow-up HbA1c β = −.13, p < .001), between family organization and disease management (β = .38, p < .001), and between family self-efficacy for diabetes and HbA1c (baseline HbA1c β = −.37, p < .001; 3 month follow-up HbA1c β = −.26, p < .001). Overall, this model accounted for 22% of the variance in baseline HbA1c, 60% of the variance in follow-up HbA1c, 23% of the variance in family self-efficacy for diabetes, and 62% of the variance in disease management. None of the demographic variables demonstrated a significant association with the latent variables with the exception of SES, which trended toward significance, indicating that higher SES is associated with lower concurrent HbA1c (β = .19, p = .06). Results demonstrate a chain of associations between family organization, greater family self-efficacy for diabetes, better disease management, and lower HbA1c (Figure 2).
Figure 2.
Final mediation model with demographic variables. Note. Unstandardized coefficients in parentheses.
Discussion
The current study indicates that general family organization is associated with disease management behaviors and metabolic control via greater youth and parent self-efficacy for diabetes. Specifically, higher levels of family organization relate to greater concurrent youth and parent self-efficacy for diabetes, which in turn is associated with higher concurrent levels of disease management. Better disease management, then, relates to better metabolic control. This model supports existing separate literatures on the importance of family organization (Greening et al., 2007; Grey et al., 1998; Hauser et al., 1990; Safyer et al., 1993; Seiffge-Krenke, 1998) and self-efficacy (Sigurardóttir, 2005; Ott et al., 2000) in diabetes adherence but is the first to examine the joint role these two factors play simultaneously in adolescent disease management and metabolic control.
As expected, general family organization was related to disease management in adolescents indirectly via youth and parent self-efficacy for diabetes (Figure 2). These relations indicate that families who report more household structure and organization also have children and parents who report feeling more capable of handling their diabetes. It is possible that family organization influences diabetes self-efficacy both directly and indirectly; for example, organized families may have an extant system of routines and procedures (i.e., afterschool activities and chores), into which an adolescent’s diabetes management can be more easily incorporated and handled, as has been found in prior research (Greening et al., 2007). Additionally, these families may have more precisely arranged homes, in which a child’s possessions may be found easily such that diabetes management supplies and materials may be more accessible. However, these speculations would benefit from further research. Putatively, these youth and their families may possess the necessary organizational skills to successfully manage diabetes and, in turn, experience increased opportunities for successful management.
Together with the demographic variables, family organization and family self-efficacy accounted for 62% of the variance in disease management in the current study. Having general routines and organization within a family appears to be associated with youth and parents feeling more efficacious at managing diabetes, which in turn is associated with improved disease management. As supported in previous research, it is likely that when youth and their parents feel capable to treat their disease, they report engaging in more disease management behaviors (Ott et al., 2000). Youth and parents who feel confident in their ability to actively and flexibly manage diabetes may be more likely to complete necessary disease management behaviors, particularly during stressful experiences like extremely high or low BG levels.
Further, as expected, self-efficacy for diabetes was related to concurrent and prospective HbA1c indirectly via disease management. Specifically, the more self-efficacy a family reported, the more likely they were to report good disease management, which was then associated with HbA1c concurrently and ∼3 months later. The full model accounted for 22% of the variance in baseline HbA1c and 60% of the variance in HbA1c at 3 months follow-up. This series of relations is important as it extends the model to include a biological correlate, indicating that families who report more general family organization and diabetes-specific self-efficacy also report better disease management behaviors, which in turn relates to better adolescent metabolic control.
The current model of psychological factors accounted for a large portion of the variance in explaining disease management (62%), but a substantially smaller portion of the variance in concurrent glycemic control (22%). Results implicate other factors that relate to adolescents’ disease care behaviors and metabolic control. For example, other studies have found that family conflict (Ingerski, Anderson, Dolan, & Hood, 2010) and pubertal development (Palmer et al., 2004) also contribute to metabolic control in adolescence.
The current study expands the existing literature by examining family and individual factors simultaneously via advanced statistical techniques to model the pathways that are associated with better diabetes management and ultimately, better metabolic control. This study reinforces the extant literature that has found increased familial organization, including the use of routines, and is associated with favorable metabolic control and treatment adherence and a more successful adaptation to the diabetes regimen (Fiese & Wamboldt, 2000; Greening et al., 2007; Hauser et al., 1990; Moes & Frea, 2000; Ryan & Wagner, 2003; Safyer et al., 1993; Seiffge-Krenke, 1998). More importantly, this study also offers a possible explanation regarding the mechanisms of change leading to metabolic control. By examining these factors concurrently, this study has identified a potential causal pathway, which could be targeted in prevention and intervention work to help maintain or improve diabetes care in adolescents. However, other pathways should be examined in future research, as the current study evaluated only one pathway of influence in a predominantly cross-sectional study.
Clinical Implications
The clinical implications of this research may include introducing prevention and intervention treatments for families of youth with diabetes which focus on increasing general family organization and family self-efficacy for diabetes. These programs could emphasize the long-term development, implementation, and maintenance of family organization and its relevance to diabetes management. For example, helping youth and parents to know their roles and responsibilities, and encouraging planning for family activities/schedule use. Techniques for developing adolescents’ and parents’ self-efficacy should also be targeted such as developmentally appropriate psycho-education, practicing diabetes management strategies in treatment, and tracking adolescents’ and parents’ successful management experiences. Parents, in particular, should be aware of the role they play in terms of facilitating family organization and routine development, as well as their role in supporting appropriate independent self-care behaviors in their teens. Structured family interventions aimed at developing strong problem-solving skills, such as Behavioral Family Systems Therapy, may be useful in developing both skill sets. Additional longitudinal research regarding complementary facets of family involvement such as family conflict is also necessary, however, since these relationships are likely transactional.
Strengths and Limitations for Future Directions
Strengths of the present study include the use of multimethod, multi-informant data collection procedures, including parent and adolescent report of family organization, youth, and parent self-efficacy for diabetes and diabetes management, as well as a biological measure of metabolic control collected at two time points. Additionally, through the use of advanced statistical modeling techniques, various direct and indirect relations were evaluated simultaneously among the relevant variables. Although the current study may help elucidate the relations between family organization and metabolic control, then, there are a few limitations to note. First, the data are predominantly cross-sectional and cannot determine causality; it is possible that the direction of the studied relationships could be reversed or be considered bidirectional. Longitudinal assessment will be required in future research to address these issues. Second, in this study, the FES Organization subscale evidenced lower than desirable reliability when parent and youth report were considered separately; when parent and youth report were combined, however, internal consistency was found to be adequate. Additionally, the lower reliability in the current sample seems to be in line with that found by other researchers (Loveland-Cherry et al., 1989). Furthermore, as the model was analyzed using structural equation modeling in Mplus 6 (Muthen & Muthen, 1998–2010), the error variance due to reporter bias was removed and a more stable true score, as represented by the latent variable, was used in analyses (Kline, 2011; Llabre, 2010). Nevertheless, future research should consider inclusion of alternative measures of family organization to create a latent variable of this important construct. Diabetes-specific measures of family organization could also be valuable to develop in future research as the current study assessed general organization.
Conclusions
In summation, family organization appears to play a key role in successful management of type 1 diabetes in adolescents. Family organization is concurrently associated with greater self-efficacy for diabetes, such that greater household order may facilitate opportunities to successfully manage diabetes. Furthermore, youth and parents who feel self-efficacious exhibit better concurrent disease management behaviors, and in turn, youth exhibit better metabolic control. In short, family organization is a contributor to better disease management and metabolic control which may have possible implications for prevention and intervention programs.
Funding
The National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (5R01DK070917-04 to C.H.).
Conflicts of interest: None declared.
References
- American Diabetes Association. 2011. Living with diabetes: Complications. Retrieved from http://www.diabetes.org/living-with-diabetes/complications/?loc=DropDownLWD-complications. [Google Scholar]
- Anderson B J, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. The Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Berg C A, King P S, Butler J M, Pham P, Palmer D, Wiebe D J. Parental involvement and adolescents’ diabetes management: The mediating role of self-efficacy and externalizing and internalizing behaviors. Journal of Pediatric Psychology. 2011;36:329–339. doi: 10.1093/jpepsy/jsq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C P, Gullone E, Needleman G L, Burt T. The Family Environment Scale: Reliability and normative data for an adolescent sample. Family Process. 1997;36:369–373. doi: 10.1111/j.1545-5300.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- Collins L M, Schafer J L, Kam C M. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods. 2001;6(4):330–351. [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Ellis D A, Podolski C, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: Impact on regimen adherence in youth with Type 1 diabetes. Journal of Pediatric Psychology. 2007;32(8):907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- Fiese B H, Wamboldt F S. Family routines, rituals, and asthma management: A proposal for family-based strategies to increase treatment adherence. Families, Systems, & Health. 2000;18(4):405–418. [Google Scholar]
- Freund A, Johnson S B, Silverstein J, Thomas J. Assessing daily management of childhood diabetes using 24-hour recall interviews: Reliability and stability. Health Psychology. 1991;10:200–208. doi: 10.1037//0278-6133.10.3.200. [DOI] [PubMed] [Google Scholar]
- Greening L, Stoppelbein L, Konishi C, Jordan S S, Moll G. Child routines and youths’ adherence to treatment for type 1 diabetes. Journal of Pediatric Psychology. 2007;32:437–447. doi: 10.1093/jpepsy/jsl029. [DOI] [PubMed] [Google Scholar]
- Grey M, Boland E A, Yu C, Sullivan-Bolyai S, Tamborlane W V. Personal and family factors associated with quality of life in adolescents with diabetes. Diabetes Care. 1998;21(6):909–914. doi: 10.2337/diacare.21.6.909. [DOI] [PubMed] [Google Scholar]
- Hauser S T, Jacobson A M, Lavori P, Wolfsdorf J I, Herskowitz R D, Milley J E, Bliss R, Wertlieb D, Stein J. Adherence among children and adolescents with insulin-dependent diabetes mellitus over a four-year longitudinal follow-up: II. Immediate and long-term linkages with the family milieu. Journal of Pediatric Psychology. 1990;15(4):527–542. doi: 10.1093/jpepsy/15.4.527. [DOI] [PubMed] [Google Scholar]
- Hauser S T, Jacobson A M, Wertlieb D, Wolfsdorf J, Herskowitz R, Vieyra M, Orleans J. Family contexts of self-esteem and illness adjustment in diabetic and acutely-ill children. In: Ramsey C, editor. The science of family medicine. New York: Guilford; 1989. [Google Scholar]
- Hollingshead AB. Four-factor Index of Social Status. New Haven, CT: Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Holmes CS, Chen R, Streisand R, Marschall DE, Souter S, Swift EE, Peterson CC. Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. Journal of Pediatric Psychology. 2006;31:770–784. doi: 10.1093/jpepsy/jsj083. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler P. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Iannotti R J, Nansel T R, Schneider S, Haynie D L, Simons-Morton B, Sobel D O, Clark L. Assessing regimen adherence of adolescents with type 1 diabetes. Diabetes Care. 2006;29:2263–2267. doi: 10.2337/dc06-0685. [DOI] [PubMed] [Google Scholar]
- Iannotti R J, Schneider S, Nansel T R, Haynie D L, Plotnick L P, Clark L M, Sobel DO, Simons-Morton B. Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. Journal of Developmental and Behavioral Pediatrics. 2006;27(2):98–105. doi: 10.1097/00004703-200604000-00003. [DOI] [PubMed] [Google Scholar]
- Ingerski L M, Anderson B J, Dolan L M, Hood K K. Blood glucose monitoring and glycemic control in adolescence: Contribution of diabetes-specific responsibility and family conflict. Journal of Adolescent Health. 2010;47(2):191–197. doi: 10.1016/j.jadohealth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S B, Silverstein J, Rosenbloom A, Carter R, Cunningham W. Assessing daily management in childhood diabetes. Health Psychology. 1986;5:545–564. doi: 10.1037/0278-6133.5.6.545. [DOI] [PubMed] [Google Scholar]
- Kline R B. Principles and practice of structural equation modeling. 3rd ed. New York, NY: Guilford; 2011. [Google Scholar]
- La Greca A M, Bearman K J. The Diabetes Social Support Questionnaire-Family Version: Evaluating adolescents’ diabetes-specific support from family members. Journal of Pediatric Psychology. 2002;27(8):665–676. doi: 10.1093/jpepsy/27.8.665. [DOI] [PubMed] [Google Scholar]
- Llabre M M. Structural equation modeling in behavioral medicine research. In: Steptoe A, editor. Handbook of behavioral medicine: methods and applications. New York: Springer; 2010. pp. 895–908. [Google Scholar]
- Lemanek K L, Kamps J, Chung N B. Empirically supported treatments in pediatric psychology: Regimen adherence. Journal of Pediatric Psychology. 2001;26(5):253–275. doi: 10.1093/jpepsy/26.5.253. [DOI] [PubMed] [Google Scholar]
- Loveland-Cherry C J, Youngblut J M, Leidy N K. A psychometric analysis of the Family Environment Scale. Nursing Research. 1989;38(5):262–266. [PubMed] [Google Scholar]
- MacCallum R C, Austin J T. Applications of structural equation modeling in psychological research. Annual Review of Psychology. 2000;51:201–226. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- Moes D R, Frea W D. Using family context to inform intervention planning for the treatment of a child with autism. Journal of Positive Behavior Interventions. 2000;2(1):40–46. [Google Scholar]
- Moos R H, Moos B S. Family environment scale manual: Development, applications, research. 3rd ed. Stanford, CA: Stanford University Medical Centers; 2002. [Google Scholar]
- Morris A D, Boyle D I, McMahon A D, Greene S A, MacDonald T M, Newton N R. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus. Lancet. 1997;350(9090):1505–1510. doi: 10.1016/s0140-6736(97)06234-x. [DOI] [PubMed] [Google Scholar]
- Muthen L K, Muthen B O. Mplus User’s Guide. 6th ed. Los Angeles, CA: Muthen & Muthen; 1998-2010. [Google Scholar]
- NIH. (2006, April). Your Guide to Diabetes: Type 1 and Type 2. National Diabetes Information Clearinghouse (NDIC): A Service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH. Retrieved from http://www.diabetes.niddk.nih.gov/dm/pubs/type1and2/index.htm. [Google Scholar]
- Ott J, Greening L, Palardy N, Holderby A, DeBell W K. Self-efficacy as a mediator variable for adolescents’ adherence to treatment for insulin-dependent diabetes mellitus. Children’s Health Care. 2000;29(1):47–63. [Google Scholar]
- Overstreet S, Goins J, Chen R S, Holmes C S, Greer T, Dunlap W P, Frentz J. Family environment and the interrelation of family structure, child behavior, and metabolic control for children with diabetes. Journal of Pediatric Psychology. 1995;20(4):435–447. doi: 10.1093/jpepsy/20.4.435. [DOI] [PubMed] [Google Scholar]
- Palmer D L, Berg C A, Wiebe D J, Beveridge R M, Korbel C D, Upchurch R, Swinyard MT, Lindsay R, Donaldson D L. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. Journal of Pediatric Psychology. 2004;29(1):35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- Quittner A L, Modi A C, Lemanek K L, Ievers-Landis C E, Rapoff M A. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology. 2008;33:16–36. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G W, Wagner G J. Pill taking ‘routinization’: A critical factor to understanding episodic medication adherence. AIDS Care. 2003;15(6):795–806. doi: 10.1080/09540120310001618649. [DOI] [PubMed] [Google Scholar]
- Safyer A W, Hauser S T, Jacobson A M, Bliss R, Herskowitz R D, Wolfsdorf J I, Wertlieb D. The impact of the family on diabetes adjustment: A developmental perspective. Child & Adolescent Social Work Journal. 1993;10(2):123–140. [Google Scholar]
- Seiffge-Krenke I. The highly structured climate in families of adolescents with diabetes: Functional or dysfunctional for metabolic control? Journal of Pediatric Psychology. 1998;23(5):313–322. doi: 10.1093/jpepsy/23.5.313. [DOI] [PubMed] [Google Scholar]
- Sigurardóttir A K. Self-care in diabetes: Model of factors affecting self-care. Journal of Clinical Nursing. 2005;14(3):301–314. doi: 10.1111/j.1365-2702.2004.01043.x. [DOI] [PubMed] [Google Scholar]
- Silverstein J H, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Deeb L, Grey M, Anderson B, Holzmeister L A, Clark N. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Sokol M C, McGuigan K A, Verbrugge R R, Epstein R S. Impact of medication adherence on hospitalization risk and healthcare cost. Medical Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Nansel T R, Holmbeck G N, Chen R, Laffel L, Anderson B J, Weissberg- Benchell J. Collaborative involvement of primary and secondary caregivers: Associations with youths’ diabetes outcomes. Journal of Pediatric Psychology. 2009;34:869–881. doi: 10.1093/jpepsy/jsn136. [DOI] [PMC free article] [PubMed] [Google Scholar]