Abstract
CD1d-restricted invariant natural killer T (iNKT) cells bear characteristics of innate and adaptive lymphocytes, which allow them to bridge the two halves of the immune response and play roles in many disease settings. Recent work has characterized precisely how their activation is initiated and regulated. Novel antigens from important pathogens have been identified, as has an abundant self-antigen, β-glucopyranosylcaramide, capable of mediating an iNKT-cell response. Studies of the iNKT T-cell receptor (TCR)–antigen–CD1d complex show how docking between CD1d–antigen and iNKT TCR is highly conserved, and how small sequence differences in the TCR establish intrinsic variation in iNKT TCR affinity. The sequence of the TCR CDR3β loop determines iNKT TCR affinity for ligand–CD1d, independent of ligand identity. CD1d ligands can promote T helper type 1 (Th1) or Th2 biased cytokine responses, depending on the composition of their lipid tails. Ligands loaded into CD1d on the cell surface promote Th2 responses, whereas ligands with long hydrophobic tails are loaded endosomally and promote Th1 responses. This information is informing the design of synthetic iNKT-cell antigens. The iNKT cells may be activated by exogenous antigen, or by a combination of dendritic cell-derived interleukin-12 and iNKT TCR–self-antigen–CD1d engagement. The iNKT-cell activation is further modulated by recent foreign or self-antigen encounter. Activation of dendritic cells through pattern recognition receptors alters their antigen presentation and cytokine production, strongly influencing iNKT-cell activation. In a range of bacterial infections, dendritic cell-dependent innate activation of iNKT cells through interleukin-12 is the dominant influence on their activity.
Keywords: immunity, innate, natural killer T cells, non-classical MHC, T-cell receptor, T cells
Introduction
Invariant natural killer T (iNKT) cells recognize antigen (foreign or endogenous glycolipid) presented by the non-classical MHC class I-like molecule CD1d. In common with conventional T cells, they are selected in the thymus on the basis of their T-cell receptor (TCR) affinity for ligand. The term ‘invariant’ derives from the very restricted TCR used by these cells; the iNKT TCR comprises Vα24Jα18 in humans and Vα14Jα18 in mice, paired with Vβ11 in humans and Vβ2, Vβ7 or Vβ8.2 in mice. Phenotypically, iNKT cells are characterized by expression of NK markers and memory effector T-cell markers.1 Other NKT-cell types exist (collectively termed ‘type 2’ NKT cells) but will not be considered in this review.
The CD1d structure, containing two deep hydrophobic pockets,2 suggested that it could present lipid antigen, and in 1997 the prototype iNKT-cell ligand α-galactosylceramide (αGalCer) was identified in marine sponge extract.3 Fluorescently labelled tetramers of CD1d loaded with αGalCer have enabled the development and activation of iNKT cells to be characterized in great detail.4
In response to antigen, iNKT cells mount a rapid response, releasing substantial amounts of cytokine within hours of activation. They are among the first lymphocytes to produce interferon-γ (IFN-γ) in response to bacterial infection,5 and contain pre-formed cytokine mRNA to enable their reaction speed.6 Fast release of cytokines by activated iNKT cells is sufficient to transactivate other lymphocytes and shape the course of a subsequent adaptive response. The iNKT-cell response to αGalCer includes secretion of the T helper type 1 (Th1) cytokine IFN-γ and Th2 cytokine interleukin-4 (IL-4).7 However, other iNKT-cell antigens may elicit a response polarized towards Th2 or Th1 cytokine release. Synthetic Th1-biasing or Th2-biasing iNKT-cell ligands have been developed to exploit this for therapeutic effect.8,9 A range of pathogen-derived iNKT-cell antigens have been characterized,10 and accumulation of self-antigen can also activate iNKT cells.11
Their power to modulate adaptive immunity implicates iNKT cells in the control of a range of disease states and makes them an attractive target for intervention.12 In patients with autoimmune conditions, iNKT-cell numbers are lowered, and increasing their numbers can ameliorate disease.13 However, iNKT-cell frequencies vary even in healthy individuals, and there are questions over the relevance of iNKT-cell frequency in circulation compared with at sites of inflammation, over the mechanism of protection conferred by iNKT cells, and over whether they are protective in all cases.14 Similarly, iNKT cells can participate in anti-tumour responses,15 and iNKT-cell frequency is decreased in tumours.16 Their anti-tumour effects may be via direct cytotoxicity, an ability to activate NK cells, or through suppressing angiogenic activity of tumour-associated macrophages.17 Invariant NKT cells are not always protective against disease. They promote the development of allergic asthma through their ability to secrete Th2-type cytokines,18 colonizing mucosa in the absence of adequate early childhood exposure to microbes.19
Are all iNKT cells identical? On two counts, no; first, there are multiple iNKT-cell populations, differing in their function, location and phenotype.20 Second, the ‘invariant’ iNKT TCR does vary, influencing its affinity for ligand-CD1d.
Antigens for iNKT cells
In addition to recognizing αGalCer,3 iNKT cells are activated by myriad microbial antigens.21 The first to be identified were α-hexose-containing glycolipids derived from Borrelia burgdorferi and Sphingomonas spp.22–24 Structurally diverse foreign antigens have since been characterized, including phosphatidylinositol mannoside from Mycobacterium bovis BCG,25 and cholesteryl α-glucoside from Helicobacter pylori.26 Although each of these antigens is important in context, none of the agents from which they are derived is a sufficiently large threat to exert pressure to maintain a specialized lineage of T cells. More recently, iNKT antigens have been isolated from Streptococcus pneumoniae and group B streptococcus, both clinically important bacteria.27 As yet uncharacterized iNKT antigens are present in house dust extract, suggesting that iNKT antigens are more ubiquitous than previously thought.28
Invariant NKT cells also become activated in the absence of foreign antigen,29,30 and must be selected in the thymus by self-antigen.31 The identity of these self-antigens has been contentious. Isoglobotrihexosylceramide (iGB3) was proposed to mediate selection and activation of iNKT cells,32 but iGB3-synthase-deficient mice have a normal iNKT compartment33 and iGB3 is present in trace amounts in mice34 and absent in humans.35 β-Glucopyranosylceramide (β-GlcCer) was initially excluded as an iNKT self-antigen,36 but new work has shown how it activates iNKT cells in a CD1d-dependent manner.11 β-GlcCer is abundant in the thymus and peripheral lymphoid tissues, accumulates in response to danger signals, and its absence impairs an iNKT-cell response. This evidence places it as a major self-antigen for iNKT cells. Many other endogenous glycosphingolipids (GSL) have been extracted from CD1d, with fluorescent labelling of glycan headgroups and HPLC used to profile the eluted GSL.37 Although GSL are important for iNKT-cell activation, as shown by work with a GSL synthesis inhibitor,30 iNKT-cell antigens are not exclusively GSL.
CD1d has been found associated with glycosylphosphatidylinositol,38 and engineered forms of CD1d (protease-cleavable or tail-less, secreted CD1d) have been used to extract endogenous CD1d-associated non-GSL species.39,40 Secreted CD1d presents over 150 species, though only lysophosphatidylcholine was subsequently shown to be stimulatory.41 It remains possible that these molecules activate type 2 NKT cells.
By transfecting GSL-deficient cell lines with CD1d and characterizing the iNKT stimulatory properties of cell extracts, and confirming their results with sphingolipid-specific hydrolases, which left the antigenic activity of their extracts unaffected, Pei et al.42 confirmed that endogenous iNKT-cell antigens need not be GSL. Lipids isolated from thymocytes include ether-bonded mono-alkyl glycerophosphates, which are able to activate iNKT thymocytes in a CD1d-dependent manner. Mice deficient in ether-bonded lipids are partially deficient in their ability to select iNKT cells, so these molecules form an essential part of the endogenous iNKT-cell antigen repertoire.43 CD1d is also capable of binding long hydrophobic peptides.44,45
Despite its potency as an iNKT antigen, αGalCer-based therapy has not become established in any disease indication. There is now strong interest in developing agonist ligands to bias iNKT-cell responses towards a Th1 or Th2 cytokine profile,9 or to create a reduced response,46,47 allowing fine control of immune activation.
Structural basis of CD1d–ligand recognition
The iNKT-cell TCR functions as a pattern-recognition receptor for both pathogens and altered levels of self-antigen. Structures of the iNKT TCR in complex with ligand-CD1d illuminate how it recognizes diverse antigens.
The footprint of the iNKT TCR on CD1d runs parallel to its binding cleft, unlike the diagonal footprint on MHC characterized for many peptide–MHC-specific TCR, and covers a small surface area.48 Just as conventional TCRs have a germline-encoded predisposition to recognize peptide–MHC,49 so the iNKT TCR uses conserved sequence to recognize antigen–CD1d.50 CD1d–ligand recognition is largely mediated by complementarity-determining regions (CDR) 3α, 1α and 2β, and structures of various human and mouse iNKT TCR alone51,52 and in TCR–antigen–CD1d ternary complexes53–56 show how CD1d–ligand recognition by the iNKT TCR is highly conserved. CDR2β forms polar interactions with CD1d, CDR1α interacts exclusively with ligand, and CDR3α contacts both.48,53 Mouse Vβ8.2-containing TCR–αGalCer–CD1d complexes closely resemble those resolved for the human iNKT TCR; if the Vβ7 chain is substituted, additional contacts are made between CDR3β and CD1d.57 A Vβ2-containing ternary complex includes even more CDR3β–CD1d contacts.56
How can an invariant receptor such as the iNKT TCR show promiscuity in antigen recognition? There is limited polymorphism at position 93 of the Vα24-Jα18 chain,58 but the major variable region of the iNKT TCR is the CDR3β loop. Evidence suggests that contact between CDR3β and CD1d mitigates the energetic penalty of binding a lower affinity CD1d–ligand complex. Structures of an iNKT TCR with varied ligands clearly show that weaker ligands require more contribution from CDR3β at the TCR–CD1d interface.54 Mutagenesis studies also support this conclusion.50,59
Naturally occurring CDR3β sequence variants confer a range of CD1d–ligand affinities on the iNKT TCR. All iNKT TCRs recognize high-affinity ligands such as αGalCer, yet reduced numbers interact with weaker agonists.60,61 Invariant NKT-cell clones show bright, homogeneous staining with αGalCer–CD1d tetramers but when tetramers loaded with the weaker agonist OCH are used, stain as OCH–CD1d tetramer bright, intermediate or dim.60 The staining pattern observed for OCH–CD1d tetramers matches that for β-glycosylceramide–CD1d tetramers, and the hierarchy was confirmed by surface plasmon resonance analysis of the interaction between cloned TCRs and ligand–CD1d. The CDR3β affinity hierarchy, applicable to diverse GSL antigens, is therefore not indicative of antigen preference by different iNKT TCRs, but is a function of CDR3β sequence. Interestingly, the iNKT-cell repertoire may be selected to exclude cells with high autoreactivity.62 Mallevaey et al.62 modified the CDR3β of a naturally occurring iNKT TCR to create an extra-sticky variant that made additional hydrophobic contacts with αGalCer–CD1d from the CDR3β loop. Only appropriate iNKT cells engage in an NKT response: exposure of mouse iNKT cells to weak antigen leads to enrichment for Vβ7-expressing clones (which use more CDR3β–CD1d contacts) with each cell division cycle, whereas αGalCer, able to engage all iNKT cells, induces no bias.63 Together, these studies suggest that the iNKT repertoire is selected to fall within a delimited window of affinity for ligand–CD1d, yielding a gamut of iNKT cells of fixed reactivity. Hence, like T cells, not all iNKT cells respond to all antigens; clonal expansion of a specific population ensures an appropriate response.
Unlike TCR–pMHC complexes,64 iNKT TCR–antigen–CD1d ternary complex formation depends upon induced fit of CD1d and antigen to a rigid TCR.52,65 Consistently, the antigen–CD1d surface is moulded to resemble the topology of αGalCer–CD1d in the iNKT TCR–αGalCer–CD1d complex. Analysis of αGalCer variants demonstrates the importance of conserved contacts between the galactosyl headgroup and the iNKT TCR.63,66 Borrelia burgdorferi αGalDAG has its headgroup repositioned upon binding iNKT TCR,67 as does S. pneumoniae-derived Glc-DAG-s2.27,68
β-linked self-GSL follow the same binding stereotype as foreign α-linked antigens. The structures of CD1d-β-linked self-antigen–iNKT TCR complexes show how the headgroup is flattened so that the complex resembles that formed with αGalCer.55 The energetic penalty incurred in this squashing explains the lower affinity of the iNKT TCR for endogenous ligands. The bulky headgroup of iGb3, rather than hindering binding, contributes TCR contacts from its flattened position to compensate.69
Antigen-specific responses
The iNKT TCR affinity for an antigen in complex with CD1d is not always sufficient to predict the nature of the cytokine response (Th1 or Th2 biased) it elicits. Evidence now suggests that the strength of interaction between antigen and CD1d, the longevity of this complex on the cell surface, and antigen-presenting cell (APC) type determines the cytokine polarization seen in an iNKT-cell response (Fig. 1).
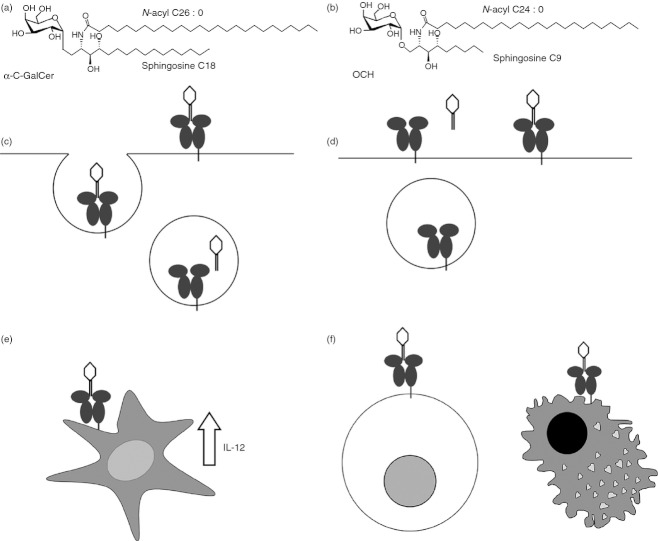
Figure 1.
Distinctions between T helper type 1 (Th1) -biasing and Th2-biasing invariable natural killer T (iNKT) -cell antigens. (a) Th1-biasing CD1d ligands, such as α-C-GalCer (shown) have long hydrophobic tails. (b) Th2-biasing CD1d ligands have unsaturated and/or shortened tails, such as OCH (shown). (c) Th1-biasing antigens are loaded in the endosome, whereas Th2-biasing antigens (d) are loaded at the cell surface. A Th1-biased iNKT response requires antigen presentation by dendritic cells, which also secrete interleukin-12 to sustain iNKT-cell activation (e), while Th2 responses are generated by antigen presentation by more diverse antigen-presenting cells including macrophages and B cells (f).
Invariant NKT antigens with Th2 cytokine-biasing effects are characterized by shortened unsaturated tails, increased overall polarity and reduced hydrophobicity. Shortening of either acyl or sphingosine chains can polarize responses towards Th2.70 For example, OCH, an αGalCer analogue with a shortened sphingosine chain, elicits a Th2 response,8,71,72 as does an acyl truncated and di-unsaturated αGalCer (C20 : 2).73
Intracellular staining for cytokines produced by iNKT cells after a short (2 hr) exposure to agonists reported as Th1 or Th2 polarizing fails to reveal a Th1 or Th2 bias.73 Cytokine measurements from culture supernatants include IFN-γ from trans-activated NK cells as well as from iNKT cells. For a Th1 bias to be measured, the activation of iNKT cells must be sustained enough to activate NK cells, requiring a strong interaction between CD1d and antigen. CD1d ligands characterized as Th1-biasing include Plakoside A analogues (structurally similar to αGalCer, and also derived from sea sponge) and analogues of αGalCer with a carbon-based glycosidic linkage (α-C-GalCer and other C-glycosides). Plakoside A analogues bind deeply inside the groove of CD1d. Similarly, C-glycoside binds CD1d very tightly, facilitating a sustained (though weak) interaction with the iNKT TCR and a Th1-biased response.74 α-C-GalCer also elicits sustained iNKT TCR interaction and a Th1 response.66 Inclusion of aromatic rings on the acyl chain of αGalCer creates a Th1 bias by enhancing the stability of a TCR–antigen–CD1d complex.75,76
Sub-cellular location of antigen loading into CD1d controls persistence of antigen–CD1d complexes, influencing the Th1 versus Th2 bias of a response. Presentation of iNKT antigens was tracked using antibody specific for the complex formed between αGalCer and CD1d.77 The Th2-biasing ligands show an ability to directly load on to CD1d at the cell surface. When CD1d trafficking through the endosome was ablated by removal of its cytoplasmic tail, Th1-biasing αGalCer analogues lost much of their activity. Further evidence that lysosomal recycling influences the cytokine secretion profile comes from work with fluorescently tagged lipids;78 Th2-biasing antigens are actively displaced at acidic (lysosomal) pH. Hence, as CD1d traffics steadily through the cell, an immune synapse containing saturated-tail, hydrophobic antigen is more likely to endure, and sustain the signalling required for a Th1 response.
Using inducible knockout CD1d mice, Bai et al.79 demonstrated that Th1-type antigen presentation requires dendritic cell (DC) -expressed CD1d, whereas Th2-type antigen, loaded into CD1d at the cell surface, is presented by a range of non-IL-12-producing APC. This distinction is important as DC-derived IL-12 induces production of IFN-γ by NK cells, explaining further how a Th1 cytokine bias is achieved.
Several studies report the influence of cell-surface receptors on iNKT cells on their cytokine response. CD40, CD4, programmed death receptor PD-1 and the A2aR adenosine receptor can all influence cytokine polarization.80–83
Danger signals
The iNKT response to danger is shaped by many factors in addition to antigen. Responses are programmed by the starting activation state of iNKT cells, and by the activity of APC. Activation of APC leads to alterations in antigen presentation, including changes in CD1d expression and changes to the repertoire of self-antigens associated with CD1d. The APC-derived cytokines also mediate activation of iNKT cells, sometimes independently of the CD1d–ligand–TCR interaction. In many infectious contexts, it is APC-derived cytokine in concert with self-antigen–CD1d signalling that activates iNKT cells (summarized in Fig. 2).
Figure 2.
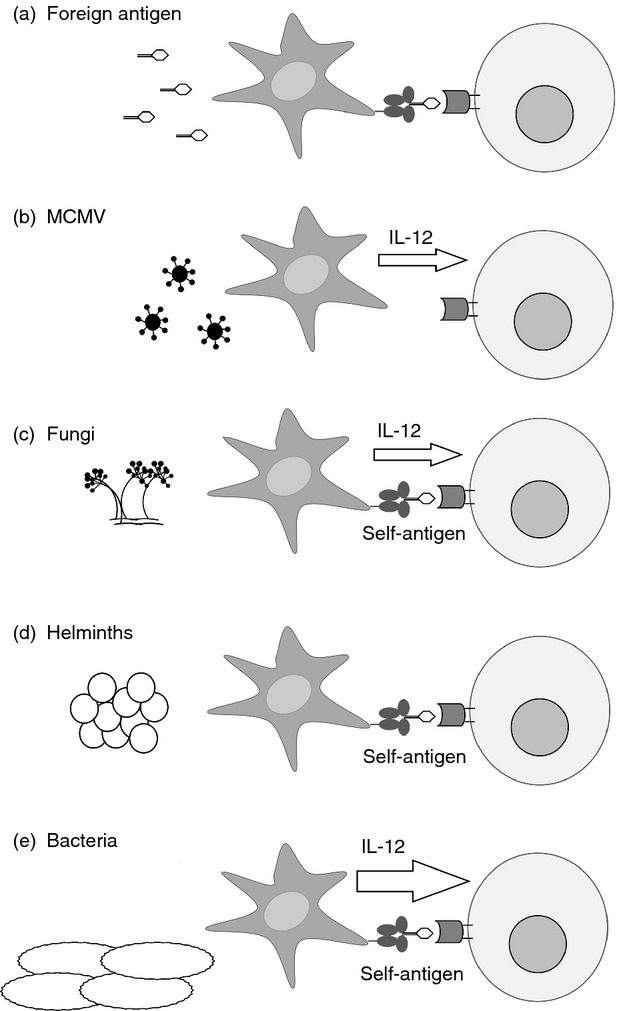
Antigen determines antigen-presenting cell (APC) signalling to invariant natural killer T (iNKT) cells. Various stimuli are detected by APC (dark grey) and cause them to activate iNKT cells (light grey) through either the iNKT cell T-cell receptor (TCR), or through cytokine release, or using a combination of both. (a) Antigens such as αGalCer activate iNKT cells through TCR recognition of antigen-CD1d. (b) In mouse cytomegalovirus infection, interleukin-12 (IL-12) from activated APC is sufficient to activate iNKT cells. (c) Fungal cell-wall glycans stimulate APC to release IL-12: recognition of self-antigen-CD1d is also required for iNKT cell activation. (d) a component of parasite eggs activates APC, which are thought to present self-antigen to activate iNKT cells. (e) Several bacterial species activate APC. This activation usually requires self-antigen–CD1d recognition by APC, but the dominant contribution to iNKT cell activation comes from APC-derived IL-12.
The recent history of an iNKT cell dictates its responsiveness. αGalCer stimulation leads to temporary anergy,84 which has impaired the development of αGalCer-based therapeutic protocols. Similarly, encounter with a range of bacteria, or the bacterial products lipopolysaccharide (LPS) and flagellin, anergizes iNKT cells.85 Neutrophils, themselves activated by iNKT cells, can also suppress iNKT-cell activity, limiting an iNKT-cell response.86 The iNKT cells that have recently encountered self-antigen have limited cytokine-secreting activity, and lowered responsiveness to foreign antigen (αGalCer).87 Such mechanisms may well restrain potentially harmful iNKT-cell activity, though recognition of CD1d-presented self-antigen also primes iNKT cells for subsequent activation by IL-12 and IL-18.87
The APC expression of CD1d is responsive to bacterial infection, which in turn affects iNKT activation. Infection of APC with Listeria monocytogenes leads to IFN-β-mediated up-regulation of CD1d (not just its redistribution to the cell surface),88 and in an M. tuberculosis infection model, IFN-γ in combination with bacterial products Pam3Cys [a Toll-like receptor 2 (TLR2) agonist] or LPS (a TLR4 agonist) was sufficient to up-regulate CD1d on macrophages.89 In vitro exposure of DC to Salmonella typhimurium or Escherichia coli-derived LPS has also been found to increase CD1d levels.90 CD1d expression is up-regulated in viral infection,91 and down-regulation of CD1d as a viral immune escape mechanism has been reported in mouse92,93 and human.94–96
The repertoire of CD1d-presented self-antigen is responsive to an APC activation state. Staining with tetramerized iNKT TCR, and comparison of the repertoire of CD1d-associated self-GSL in resting and LPS (TLR4)-stimulated myeloid DC, shows that TLR stimulation of DC causes an increase in presentation of iNKT-activating CD1d ligands.30,37 Triggering of TLR4 and TLR7 or TLR9 on DC activates iNKT cells, and this activation requires APC synthesis of charged β-linked GSL.29 In inflammation, APC levels of lysophosphatidylcholine increase, though lysophosphatidylcholine is only a weak activator of iNKT cells.41 A more important role is indicated for β-GlcCer. It is synthesized in response to TLR agonists, and inhibition of this synthesis impairs iNKT responses to DC cultured with bacteria. Further, bacterial infection of mice leads to accumulation of β-GlcCer at sites of E. coli or Streptococcus pneumoniae infection.11 In mice, TLR stimulation of DC inhibits α-galactosidase A, which normally degrades lysosomal self-antigens to prevent full iNKT activation, though this mechanism is unlikely to be important in humans.97,98
CD1d and DC-dependent but TLR-independent activation of iNKT cells has been reported in responses to fungi including Aspergillus and Candida.99 Fungal cell wall β-1,3-glucans bind pattern recognition receptors on APC to stimulate IL-12 release, which activates autoreactive iNKT cells. Invariant NKT cells also form part of the response to helminths, though the mechanism remains partly delineated. There is a requirement for CD1d, and for schistosome egg recognition by DC, though neither IL-12 nor TLR signalling is necessary.100
Activation of iNKT cells in mouse cytomegalovirus infection is antigen-independent, relying on APC-derived IL-12.101–103 In this context, iNKT cells behave as innate lymphocytes, amplifying the immune response, a capacity that widens the range of pathogen defences in which they could be involved. The APC-derived cytokines have also been demonstrated to drive antigen-independent iNKT activation in a model of E. coli infection.104
Priming of iNKT cells to be more responsive to IL-12 in the absence of foreign antigen 85 suggests that there is a hierarchy of activation stimuli for iNKT cells. For example, in response to Salmonella typhimurium, IL-12 amplifies a weak response to self-antigen,24,5 and DC from patients with advanced cancer are better able to activate iNKT cells if supplemented with IL-12.105
If exogenous antigen, self-antigen and IL-12 are all present, which is the most important in activating iNKT cells? Many studies exploring iNKT-cell activation use hybridoma cell lines, which may lack the ability to respond to both antigen and cytokine signals. To address this, Brigl et al.5 used primary mouse iNKT-cell lines and bone marrow-derived DC to compare the relative influences of ligand recognition versus cytokine stimulation on iNKT cell activation by a panel of bacteria. In response to whole bacteria, IFN-γ secretion by iNKT cells is mostly dependent on IL-12 released by DC in response to TLR stimulation, albeit with an essential role for CD1d. Interleukin-12 dependence was observed even with bacteria expressing characterized CD1d ligands such as Streptococcus pneumoniae and Sphingomonas yanoikuyae, suggesting a minimal role for CD1d presentation of foreign antigen. This relative independence of foreign antigen may be useful when the ubiquity of potential iNKT antigens is considered,28 whereas the possibility remains that iNKT-cell activation by foreign antigen is required for the establishment of pathogen-specific memory responses.
Outlook
With interest growing in designing iNKT antigens to modulate an immune response, it is important that they achieve the desired activation of iNKT cells. This in turn depends on the history of each iNKT cell and its current environment: we have seen that iNKT-cell antigens such as those in house dust are ubiquitous, that iNKT cells can exist in a primed state, and that the activation state of APC strongly influences iNKT-cell activation. Hence, responses from cultured iNKT-cell lines may not recapitulate responses achieved with the same antigen in vivo. In some contexts, antigen is dispensable for iNKT-cell activation, which also merits consideration. Exactly when does an iNKT cell act solely to amplify an innate response? Fuller understanding of the mechanisms controlling the down-regulation of an iNKT-cell response may also be relevant to understanding the activity of ‘designer’ antigens. It is also interesting to note how many inert CD1d ligands can be isolated. Are these acting as place-holders, sustaining CD1d trafficking through the cell in case more antigenic ligands are produced, or do they perform a necessary role, perhaps as ligands for type 2 NKT cells?
Regarding β-GlcCer and its role as a key self-antigen for iNKT cells, we need to understand how alterations in β-GlcCer processing and presentation (induced by disease or by the arrival of a new iNKT-cell antigen) impact on the shape of an adaptive immune response.
Disclosure
The author has no conflicts of interest to disclose.
References
- 1.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng Z, Castaño AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–45. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 3.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–54. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 6.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, Kronenberg M. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci USA. 2003;100:8395–400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–4. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 9.Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin Immunol. 2010;22:68–78. doi: 10.1016/j.smim.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–17. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 11.Brennan PJ, Tatituri RV, Brigl M, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–11. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–42. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 13.Novak J, Lehuen A. Mechanism of regulation of autoimmunity by iNKT cells. Cytokine. 2011;53:263–70. doi: 10.1016/j.cyto.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–88. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 16.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L, Asgharzadeh S, Salo J, et al. Vα24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–36. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 19.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 21.Godfrey DI, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Rossjohn J. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin Immunol. 2010;22:61–7. doi: 10.1016/j.smim.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 23.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 24.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 25.Fischer K, Scotet E, Niemeyer M, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci USA. 2004;101:10685–90. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang YJ, Kim HY, Albacker LA, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinjo Y, Illarionov P, Vela JL, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12:966–74. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingender G, Rogers P, Batzer G, et al. Invariant NKT cells are required for airway inflammation induced by environmental antigens. J Exp Med. 2011;208:1151–62. doi: 10.1084/jem.20102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paget C, Mallevaey T, Speak AO, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Salio M, Speak AO, Shepherd D, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci USA. 2007;104:20490–5. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–48. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou D, Mattner J, Cantu C, III, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 33.Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Gröne HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci USA. 2007;104:5977–82. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speak AO, Salio M, Neville DC, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci USA. 2007;104:5971–6. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christiansen D, Milland J, Mouhtouris E, et al. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 2008;6:e172. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanic AK, De Silva AD, Park JJ, et al. Defective presentation of the CD1d1-restricted natural Vα14Jα18 NKT lymphocyte antigen caused by β-d-glucosylceramide synthase deficiency. Proc Natl Acad Sci USA. 2003;100:1849–54. doi: 10.1073/pnas.0430327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muindi K, Cernadas M, Watts GF, et al. Activation state and intracellular trafficking contribute to the repertoire of endogenous glycosphingolipids presented by CD1d. Proc Natl Acad Sci USA. 2010;107:3052–7. doi: 10.1073/pnas.0915056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joyce S, Woods AS, Yewdell JW, et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541–4. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 39.Cox D, Fox L, Tian R, Bardet W, Skaley M, Mojsilovic D, Gumperz J, Hildebrand W. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol. 2009;182:4784–91. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox LM, Cox DG, Lockridge JL, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pei B, Speak AO, Shepherd D, Butters T, Cerundolo V, Platt FM, Kronenberg M. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J Immunol. 2011;186:1348–60. doi: 10.4049/jimmunol.1001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Facciotti F, Ramanjaneyulu GS, Lepore M, et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13:474–80. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- 44.Castaño AR, Tangri S, Miller JE, Holcombe HR, Jackson MR, Huse WD, Kronenberg M, Peterson PA. Peptide binding and presentation by mouse CD1. Science. 1995;269:223–6. doi: 10.1126/science.7542403. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Teige A, Mondoc E, Ibrahim S, Holmdahl R, Issazadeh-Navikas S. Endogenous collagen peptide activation of CD1d-restricted NKT cells ameliorates tissue-specific inflammation in mice. J Clin Invest. 2011;121:249–64. doi: 10.1172/JCI43964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silk JD, Salio M, Reddy BG, et al. Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J Immunol. 2008;180:6452–6. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]
- 47.Kerzerho J, Yu ED, Barra CM, et al. Structural and functional characterization of a novel nonglycosidic type I NKT agonist with immunomodulatory properties. J Immunol. 2012;188:2254–65. doi: 10.4049/jimmunol.1103049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borg NA, Wun KS, Kjer-Nielsen L, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–9. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 49.Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the αβ T-cell receptor control thymic selection. Nature. 2009;458:1043–6. doi: 10.1038/nature07812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott-Browne JP, Matsuda JL, Mallevaey T, et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–13. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 51.Gadola SD, Koch M, Marles-Wright J, et al. Structure and binding kinetics of three different human CD1d-α-galactosylceramide-specific T cell receptors. J Exp Med. 2006;203:699–710. doi: 10.1084/jem.20052369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Vα14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104–16. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wun KS, Borg NA, Kjer-Nielsen L, et al. A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR. J Exp Med. 2008;205:939–49. doi: 10.1084/jem.20072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pellicci DG, Patel O, Kjer-Nielsen L, et al. Differential recognition of CD1d-α-galactosyl ceramide by the Vβ8.2 and Vβ7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pellicci DG, Clarke AJ, Patel O, et al. Recognition of β-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat Immunol. 2011;12:827–33. doi: 10.1038/ni.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel O, Pellicci DG, Uldrich AP, et al. Vβ2 natural killer T cell antigen receptor-mediated recognition of CD1d-glycolipid antigen. Proc Natl Acad Sci USA. 2011;108:19007–12. doi: 10.1073/pnas.1109066108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mallevaey T, Scott-Browne JP, Matsuda JL, et al. T cell receptor CDR2 β and CDR3 β loops collaborate functionally to shape the iNKT cell repertoire. Immunity. 2009;31:60–71. doi: 10.1016/j.immuni.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanderson JP, Waldburger-Hauri K, Garzón D, et al. Natural variations at position 93 of the invariant Vα24-Jα18 α chain of human iNKT-cell TCRs strongly impact on CD1d binding. Eur J Immunol. 2012;42:248–55. doi: 10.1002/eji.201141956. [DOI] [PubMed] [Google Scholar]
- 59.Florence WC, Xia C, Gordy LE, et al. Adaptability of the semi-invariant natural killer T-cell receptor towards structurally diverse CD1d-restricted ligands. EMBO J. 2009;28:3579–90. doi: 10.1038/emboj.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matulis G, Sanderson JP, Lissin NM, et al. Innate-like control of human iNKT cell autoreactivity via the hypervariable CDR3 β loop. PLoS Biol. 2010;8:e1000402. doi: 10.1371/journal.pbio.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–81. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mallevaey T, Clarke AJ, Scott-Browne JP, et al. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34:315–26. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wun KS, Cameron G, Patel O, et al. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity. 2011;34:327–39. doi: 10.1016/j.immuni.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Godfrey DI, Rossjohn J, McCluskey J. The fidelity, occasional promiscuity, and versatility of T cell receptor recognition. Immunity. 2008;28:304–14. doi: 10.1016/j.immuni.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Girardi E, Wang J, Yu ED, Painter GF, Kronenberg M, Zajonc DM. The Vα14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J Exp Med. 2010;207:2383–93. doi: 10.1084/jem.20101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel O, Cameron G, Pellicci DG, et al. NKT TCR recognition of CD1d-α-C-galactosylceramide. J Immunol. 2011;187:4705–13. doi: 10.4049/jimmunol.1100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Li Y, Kinjo Y, Mac TT, Gibson D, Painter GF, Kronenberg M, Zajonc DM. Lipid binding orientation within CD1d affects recognition of Borrelia burgdorferi antigens by NKT cells. Proc Natl Acad Sci USA. 2010;107:1535–40. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Girardi E, Yu ED, Li Y, et al. Unique interplay between sugar and lipid in determining the antigenic potency of bacterial antigens for NKT cell. PLoS Biol. 2011;9:e1001189. doi: 10.1371/journal.pbio.1001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu ED, Girardi E, Wang J, Zajonc DM. Structural basis for the recognition of β-linked glycolipid antigens by invariant NKT cells. J Immunol. 2011;187:2079–83. doi: 10.4049/jimmunol.1101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goff RD, Gao Y, Mattner J, et al. Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–3. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 71.McCarthy C, Shepherd D, Fleire S, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–44. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bozna BL, Polzella P, Rankl C, et al. Binding strength and dynamics of invariant natural killer cell T cell receptor/CD1d-glycosphingolipid interaction on living cells by single molecule force spectroscopy. J Biol Chem. 2011;286:15973–9. doi: 10.1074/jbc.M110.192674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu KO, Im JS, Molano A, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc Natl Acad Sci USA. 2005;102:3383–8. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sullivan BA, Nagarajan NA, Wingender G, et al. Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J Immunol. 2010;184:141–53. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, Wong CH, Yu AL. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci USA. 2007;104:10299–304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu TN, Lin KH, Chang YJ, Huang JR, Cheng JY, Yu AL, Wong CH. Avidity of CD1d-ligand-receptor ternary complex contributes to T-helper 1 (Th1) polarization and anticancer efficacy. Proc Natl Acad Sci USA. 2011;108:17275–80. doi: 10.1073/pnas.1114255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Im JS, Arora P, Bricard G, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–98. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bai L, Sagiv Y, Liu Y, et al. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen αGalCer. Proc Natl Acad Sci USA. 2009;106:10254–9. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bai L, Constantinides MG, Thomas SY, et al. Distinct APCs explain the cytokine bias of α-galactosylceramide variants in vivo. J Immunol. 2012;188:3053–61. doi: 10.4049/jimmunol.1102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–8. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 81.Thedrez A, de Lalla C, Allain S, et al. CD4 engagement by CD1d potentiates activation of CD4+ invariant NKT cells. Blood. 2007;110:251–8. doi: 10.1182/blood-2007-01-066217. [DOI] [PubMed] [Google Scholar]
- 82.Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nowak M, Lynch L, Yue S, Ohta A, Sitkovsky M, Balk SP, Exley MA. The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur J Immunol. 2010;40:682–7. doi: 10.1002/eji.200939897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. [DOI] [PMC free article] [PubMed]
- 85.Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008;118:2301–15. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wingender G, Hiss M, Engel I, Peukert K, Ley K, Haller H, Kronenberg M, von Vietinghoff S. Neutrophilic granulocytes modulate invariant NKT cell function in mice and humans. J Immunol. 2012;188:3000–8. doi: 10.4049/jimmunol.1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Chen X, Rodenkirch L, et al. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112:4128–38. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raghuraman G, Geng Y, Wang CR. IFN-β-mediated up-regulation of CD1d in bacteria-infected APCs. J Immunol. 2006;177:7841–8. doi: 10.4049/jimmunol.177.11.7841. [DOI] [PubMed] [Google Scholar]
- 89.Sköld M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J Immunol. 2005;175:3584–93. doi: 10.4049/jimmunol.175.6.3584. [DOI] [PubMed] [Google Scholar]
- 90.Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur J Immunol. 2005;35:2100–9. doi: 10.1002/eji.200425846. [DOI] [PubMed] [Google Scholar]
- 91.Raftery MJ, Winau F, Giese T, Kaufmann SH, Schaible UE, Schönrich G. Viral danger signals control CD1d de novo synthesis and NKT cell activation. Eur J Immunol. 2008;38:668–79. doi: 10.1002/eji.200737233. [DOI] [PubMed] [Google Scholar]
- 92.Lin Y, Roberts TJ, Spence PM, Brutkiewicz RR. Reduction in CD1d expression on dendritic cells and macrophages by an acute virus infection. J Leukoc Biol. 2005;77:151–8. doi: 10.1189/jlb.0704399. [DOI] [PubMed] [Google Scholar]
- 93.Renukaradhya GJ, Webb TJ, Khan MA, Lin YL, Du W, Gervay-Hague J, Brutkiewicz RR. Virus-induced inhibition of CD1d1-mediated antigen presentation: reciprocal regulation by p38 and ERK. J Immunol. 2005;175:4301–8. doi: 10.4049/jimmunol.175.7.4301. [DOI] [PubMed] [Google Scholar]
- 94.Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115:1369–78. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rao P, Pham HT, Kulkarni A, Yang Y, Liu X, Knipe DM, Cresswell P, Yuan W. Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. J Virol. 2011;85:8093–104. doi: 10.1128/JVI.02689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moll M, Andersson SK, Smed-Sörensen A, Sandberg JK. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood. 2010;116:1876–84. doi: 10.1182/blood-2009-09-243667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal α-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–28. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen X, Wang X, Keaton JM, Reddington F, Illarionov PA, Besra GS, Gumperz JE. Distinct endosomal trafficking requirements for presentation of autoantigens and exogenous lipids by human CD1d molecules. J Immunol. 2007;178:6181–90. doi: 10.4049/jimmunol.178.10.6181. [DOI] [PubMed] [Google Scholar]
- 99.Cohen NR, Tatituri RV, Rivera A, et al. Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe. 2011;10:437–50. doi: 10.1016/j.chom.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mallevaey T, Zanetta JP, Faveeuw C, et al. Activation of invariant NKT cells by the helminth parasite Schistosoma mansoni. J Immunol. 2006;176:2476–85. doi: 10.4049/jimmunol.176.4.2476. [DOI] [PubMed] [Google Scholar]
- 101.van Dommelen SL, Tabarias HA, Smyth MJ, Degli-Esposti MA. Activation of natural killer (NK) T cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. J Virol. 2003;77:1877–84. doi: 10.1128/JVI.77.3.1877-1884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–6. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Vα14i NK T cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–13. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 105.van der Vliet HJ, Wang R, Yue SC, Koon HB, Balk SP, Exley MA. Circulating myeloid dendritic cells of advanced cancer patients result in reduced activation and a biased cytokine profile in invariant NKT cells. J Immunol. 2008;180:7287–93. doi: 10.4049/jimmunol.180.11.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]