Abstract
Innate and adaptive immunity are inter-related by dendritic cells (DCs), which directly recognize bacteria through the binding of pathogen-associated molecular patterns (PAMPs) to specialized receptors on their surface. After capturing and degrading bacteria, DCs present their antigens as small peptides bound to MHC molecules and prime naive bacteria-specific T cells. In response to PAMP recognition DCs undergo maturation, which is a phenotypic change that increases their immunogenicity and promotes the activation of naive T cells. As a result, a specific immune response that targets bacteria-derived antigens is initiated. Therefore, the characterization of DC–bacteria interactions is important to understand the mechanisms used by virulent bacteria to avoid adaptive immunity. Furthermore, any impairment of DC function might contribute to bacterial survival and dissemination inside the host. An example of a bacterial pathogen capable of interfering with DC function is Salmonella enterica serovar Typhimurium (S. Typhimurium). Virulent strains of this bacterium are able to differentially modulate the entrance to DCs, avoid lysosomal degradation and prevent antigen presentation on MHC molecules. These features of virulent S. Typhimurium are controlled by virulence proteins, which are encoded by pathogenicity islands. Modulation of DC functions by these gene products is supported by several studies showing that pathogenesis might depend on this attribute of virulent S. Typhimurium. Here we discuss some of the recent data reported by the literature showing that several virulence proteins from Salmonella are required to modulate DC function and the activation of host adaptive immunity.
Keywords: antigen, bacteria/bacterial immunity, Fc receptors, infection, presentation
Introduction
The initiation of adaptive immunity against bacteria requires professional antigen-presenting cells that recognize and degrade bacterial antigens and present them as peptide–MHC complexes to naive T cells.1–6 One of the most relevant antigen-presenting cells are dendritic cells (DCs), which are a link between innate and adaptive immunity. These cells have the capacity to directly recognize and uptake pathogens at the site of infection and migrate to the lymph nodes to activate bacteria-specific T cells.4,5,7–9 The DCs locate in peripheral tissues, where they are usually found in an immature state.10–13 In such a state, DCs have an elevated phagocytic capacity with reduced expression of co-stimulatory molecules, such as CD80, CD86 and CD40.8,12,14–17 After pathogen-associated molecular pattern (PAMP) detection, peripheral/immature DCs become activated and undergo functional and phenotypic changes known as maturation.18–20 This response is triggered on DCs by the signalling via specific receptors, such as Toll-like receptors, nucleotide oligomerization domain (NOD) proteins and NOD-like receptors (NLR). These receptors translate PAMP recognition into cellular responses,21–23 activating the secretion of anti-bacterial molecules and pro-inflammatory cytokines that enhance the bactericidal capacity of phagocytic cells.4,16,17,24 As the result of maturation, DCs decrease phagocytosis and increase the expression MHC-I, MHC-II and co-stimulatory molecules, which are surface molecules required for the activation of antigen-specific naive T cells.4,14,16,25,26 Maturing DCs also secrete cytokines that contribute to defining the nature and polarization of the effector function of T cells after recognition of peptide–MHC on the DC surface.27–31 Mature DCs acquire the capacity to migrate from peripheral sites of infection to the lymph nodes, where naive T cells reside.32–34 The establishment of a regulated and efficient adaptive immune response against the infecting bacteria requires DCs to prime naive T cells recognizing bacterial antigens. Consistent with this notion, the acquisition of molecular mechanisms by virulent bacteria to interfere with DC function and prevent the activation of T cells could significantly contribute to the survival and dissemination of these pathogens inside the host.7,31,35
An important intracellular bacterial pathogen is Salmonella enterica serovar Typhimurium (S. Typhimurium hereafter), which is a common cause of food poisoning and gastroenteritis in humans, as well as the aetiological agent for typhoid-like disease in mice.36,37 Infection of mice by S. Typhimurium has been widely used as a model for the typhoid fever caused by S. Typhi in humans.36,38 It is thought that orally ingested S. Typhimurium accesses the Peyer's patches after invading epithelial and M cells in the small intestine.37 Then, this pathogen spreads from the Peyer's patches to deeper organs, such as mesenteric lymph nodes, spleen and liver.39,40 In these organs, bacteria would reside in intracellular compartments,41 a feature that is probably required for the successful systemic dissemination of this bacterium.41–44
It is striking that a low dose of virulent S. Typhimurium can cause systemic infection and the death of immune-competent mice.42,45,46 It has been shown that virulent S. Typhimurium can efficiently avoid the activation of the adaptive immune response.35,44 This observation is consistent with reduced antigen presentation and T-cell activation after S. Typhimurium infection in mice.42,45,47
Because DCs are fundamental for the initiation and establishment of an anti-bacterial adaptive immunity, the interference of their function can be a pathogenicity mechanism used by S. Typhimurium to prevent recognition by the host adaptive immunity. Several S. Typhimurium virulence proteins are thought to contribute to intracellular survival and systemic dissemination of this pathogen.48–50 In agreement with this notion, recent studies have demonstrated that these virulence proteins are used by S. Typhimurium to interfere with DC function.7,42,45,47,51–53 In this review we discuss recent studies supporting the notion that virulent S. Typhimurium has the capacity to actively interfere with the function of DCs and prevent the activation of bacteria-specific T cells.
Virulence Salmonella proteins required for subversion of host cells
Several genes involved in S. Typhimurium virulence are found in pathogenicity islands 1 and 2 (SPI-1 and SPI-2).50,54–59 The expression of these proteins is regulated by the ability of S. Typhimurium to sense several molecular components of the environment.60–63 Both pathogenicity islands encode for Type Three Secretion Systems (T3SS), which inject bacterial effector proteins into host cells.64–66
Genes located in SPI-1 are preferentially expressed in the extracellular environment, such as the intestinal lumen.67,68 At this location, the virulence proteins encoded by SPI-1 are needed for bacterial entry to epithelial cells.69–71 In contrast, SPI-2 genes are expressed in the intracellular environment and S. Typhimurium uses them to survive inside eukaryotic cells.42,49,53,72 The absence of either T3SSs or effector proteins encoded by SPI-1 or SPI-2 reduces the ability of S. Typhimurium to cause a systemic illness in mice.42,73,74 It has been described that proteins encoded by genes located in other pathogenicity islands or prophages are also important for invasion and survival within host cells.56,75–79 Several reports have demonstrated that these virulence factors can also contribute to interfering with DC function during bacterial infection.80–82
Salmonella infection of the intestinal epithelium
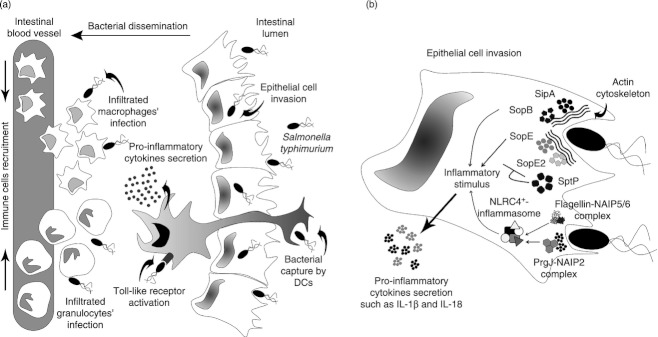
After oral infection, S. Typhimurium reaches the small intestine and invades epithelial cells in the ileum. At this location, DCs are found residing in the lamina propria (LP) and Peyer's patches.36,37 After adhering to epithelial cells,83 bacteria inject effector proteins, such as SipA, SopE, SopE2 and SopB, into the host cell cytoplasm through the T3SS encoded by the SPI-1 (T3SS-1) (Fig. 1)54,69,84–88 These proteins induce the formation and stabilization of actin filaments54,89 in epithelial and M cells, actively promoting S. Typhimurium entry to non-phagocytic cells.89,90 Together, the activity of these proteins leads to the formation of plasmatic membrane extensions, known as ruffles, which engulf extracellular bacteria (Fig. 1).91,92
Figure 1.
Lamina propria invasion and inflammatory immune response during Salmonella enterica Typhimurium infection. (a) Once Salmonella arrives at the lamina propria (LP), it can traverse cell intestinal layers and cause cytokine secretion and immune cells recruitment. Intestinal dendritic cells (DCs) can sample the pathogen, so enhancing the inflammatory environment by Toll-like receptor (TLR) activation after pathogen-associated molecular pattern (PAMP) recognition. Different types of cells, such as granulocytes and macrophages, can reach the site of infection increasing both the infiltrate and the acute gastroenteritis. (b) Salmonella pathogenicity island 1 (SPI-1) -derived proteins responsible for epithelial cell invasion during LP infection. After Salmonella recognize epithelial cell surface, SPI-1-encoded proteins are expressed and injected into host cells. Virulence effectors such as SipA, SopE, SopE2 and SopB are responsible for reorganizing actin cytoskeleton, promoting bacterial engulfment. Other SPI-1-secreted factors, such as SptP, decrease this process avoiding host cell death by excessive intracellular bacterial load. Proteins, such as flagellin and PrgJ can also be translocated into the host cell cytosol where they activate nucleotide oligomerization domain-like receptor (NLR)-containing inflammasomes to promote the secretion of inflammatory cytokines, including interleukin-1β (IL-1β) and IL-18.
The active invasion mechanism used by S. Typhimurium to invade epithelial cells is reverted by the effector protein SptP. This protein restores the normal cytoskeleton structure after bacterial entry has been completed (Fig. 1).93 Given that SptP is also secreted by the T3SS-1 into epithelial cells,93 it is likely that some of the Salmonella effector proteins require a temporal regulation. It has been suggested that this regulation is achieved by both hierarchical injection94,95 and differential degra-dation of these proteins by the proteasome of the host cells.96
Salmonella-induced inflammation at the intestine
Once S. Typhimurium invades host intestinal epithelial cells, a transcriptional re-programming occurs in these cells,97–99 which includes expression of several pro-inflammatory molecules99–101 and the activation of MyD88-dependent Toll-like receptor signalling processes in epithelial and local immune cells.102–108 This inflammatory environment promotes the recruitment of neutrophils, macrophages and DCs to the LP.109–111 Recent studies have shown that effector proteins secreted by the T3SS-1, such as SopE and SopB, can also promote an inflammatory response in epithelial cells (Fig. 1).97,101 These proteins mediate the induction of inflammation through mitogen-activated protein kinase signalling and nuclear factor-κB activation, independently of the TLR signalling.97 During this process, epithelial cells secrete inflammatory cytokines, such as interleukin-8 (IL-8), CCL2 and CCL20, further promoting the recruitment of more immune cells.99,101,112,113 These inflammatory processes induced by S. Typhimurium help intestinal invasion, LP colonization and the spreading to other hosts by means of diarrhoea.109,110
T3SS-1 also promotes inflammation by an alternative pathway that requires the activation of NLR-containing inflammasomes, specifically NLRP3 and NLRC4, which induce caspase-1 activity and the concomintant secretion of IL-1β and IL-18 (Fig. 1B).114,115 A recent report suggests that the T3SS-1 protein PrgJ binds directly to the NAIP2 protein, promoting the activation of the NLRC4 inflammasome.116 Although it seems possible that these inflammatory responses can facilitate the dissemination of S. Typhimurium towards internal organs in the host,117 recent studies have shown that NLRC4 inflammasome activation is a protective mechanism to discriminate against commensal and pathogenic bacteria.115,118 According to this theory, mice lacking the NLRP3 and NLRC4 inflammasome are more susceptible to S. Typhimurium infection than control mice, showing higher bacterial loads in the liver, spleen and mesenteric lymph nodes after oral infection.114
Flagellin, the main component of bacterial flagella, has also been involved in S. Typhimurium-induced inflammation in the LP (Fig. 1b). It has been described that this protein can also be translocated by T3SS-1 into the host cytosol and bind directly to the intracellular receptors NAIP5 and NAIP6, which activate the NLRC4 inflammasome.116,119–121 This promotes cell death by pyroptosis122 and caspase-1-dependent secretion of cytokines, such as IL-1β, IL-8, IL-18 and tumour necrosis factor-α.120,122–124, Furthermore, it has been suggested that inflammation induced by flagellin can play an important role in S. Typhimurium-induced enterocolitis.125–128 However, a recent report has described that aflagellated strains of S. Typhimurium showed increased proliferation in Peyer's patches and mesenteric lymph nodes, as compared with wild-type strains.129 Although these aflagellated strains failed to induce early inflammation, they promoted an enhanced secretion of IL-1β, interferon-γ and tumour necrosis factor-α later during infection.129 Previous reports have also described that aflagellated strains can cause a more severe systemic infection in mice than do wild-type strains.130,131 These findings also suggest that the inflammation induced by flagellin through Toll-like receptor 5 seems to reduce S. Typhimurium dissemination to deeper organs.104,129
Salmonella Typhimurium seems also able to prevent an excessive inflammatory response at the intestinal epithelium by the injection of another effector protein, known as AvrA.132 Several reports have shown that this protein prevents IK-B degradation133,134 and other recent studies indicate that AvrA injection through the T3SS-1 might also block the Jun N-terminal kinase pathway.135–137 Both signalling pathways promote the transcription of genes coding for inflammatory mediators. It has been also reported that AvrA might contribute to stabilize tight junctions to prevent inflammatory damage on epithelial cells.138 It is possible that, because of all the functions of AvrA, this protein counteracts pro-inflammatory effector proteins secreted by S. Typhimurium into epithelial cells. Such a molecular regulatory circuit might contribute to avoiding sustained inflammation upon Salmonella infection.
Role of DCs in Salmonella invasion of LP
Several studies have suggested that S. Typhimurium, even in the absence of T3SS-1, remains capable of translocating to the LP.58,139 Furthermore, it has been shown that these strains require functional DCs to be translocated through the sub-epithelial dome.58 Consistent with this notion, depletion of DCs from the LP can prevent the invasion of these T3SS-1 mutant strains.58 However, the capture of virulent S. Typhimurium by DCs seems to be a process tightly regulated by effector proteins secreted through the T3SS-1. Our recent studies suggest that S. Typhimurium strains lacking functional T3SS-1 are taken up more efficiently by DCs than are wild-type strains.140 It seems that active translocation of effector molecules through the T3SS-1 would prevent an excessive entry of bacteria to DCs, which could be required for controlling the amount of intracellular bacteria in these cells.140 It is likely that a massive capture of S. Typhimurium by transepithelial DCs would promote an immune response that could contribute to restricting Salmonella replication and dissemination. Hence, virulent S. Typhimurium may optimize this process by finely regulating the entry to epithelial and DCs to translocate to the LP.
Salmonella survival inside phagocytic cells
Inside phagocytic cells S. Typhimurium resides in Salmonella-containing vacuoles (SCV).42,45,47,141,142 Inside these compartments, intracellular Salmonella survives and is protected from several anti-bacterial molecules. Several studies have shown that S. Typhimurium can survive for up to 24 hr inside murine DCs and locate at a specific sub-cellular area inside DCs, near to the trans-Golgi network.143 In contrast with what has been observed in macrophages, S. Typhimurium seem unable to replicate in a significant manner inside these cells.42,45,47,52,53,144 Several virulent proteins encoded by SPI-2 allow S. Typhimurium to survive and localize inside DCs.45,52,143 These proteins are secreted by T3SS-2, which can transverse the vacuolar membrane and inject Salmonella effector proteins directly into the host cell cytoplasm.52,145,146 Accordingly, the deletion of SPI-2 or genes encoding the T3SS-2 reduces the ability of the bacterium to survive inside DCs and reduces its virulence in mice.42
In DCs, some Salmonella effectors can subvert DC function by altering the cellular trafficking and preventing fusion of SCV with lysosomes.147 One of the effector proteins that contributes to avoiding SCV fusion with lysosomes in DCs is SpiC, which prevents vesicular trafficking in target cells.42,148 This effector protein specifically binds a host protein known as Hook-3, which links the Golgi apparatus to the microtubules.148 In addition, SpiC acts as a regulator for the assembly of T3SS-2 and the translocation of other virulence factors into host cell cytoplasm.145,149 Accordingly, Salmonella strains lacking SpiC are unable to secrete other effector proteins to the DC cytoplasm and are targeted for lysosome degradation.143 For this reason, SpiC-deficient strains are attenuated in mice.42,150
Another effector protein secreted by T3SS-2 that is required for survival inside DCs is SifA. This protein modulates the SCV integrity in macrophages and promotes the formation of Salmonella-induced filaments.151–153 SifA also binds to SipA and Kinesin-interacting protein (or SKIP), which antagonizes the Rab9 protein and prevents kinesin-1 recruitment to SCV.154–157 Strains of S. Typhimurium lacking SifA escape from SCV and fail to replicate inside the cytoplasm of macrophages.153 In support of this notion, it has been shown that SifA-deficient bacteria escape from SCV to the DC cytoplasm and fail to co-localize with lysosomal markers.144 However, another report describes how SifA mutants still reside in an intact SCV and co-localize with the lysosomal marker Lamp-1 within DCs.143 The reason for the discrepancies observed in these studies has not been resolved. There are additional T3SS-2-secreted effector proteins that are injected by S. Typhimurium into the cytoplasm of DCs, such as SseJ, SseF, SspH2 and PipB2, and that contribute to intracellular survival and subversion of DC function.143 However, further studies are required to define whether S. Typhimurium strains lacking these effector proteins show reduced survival inside DCs, increased fusion with lysosomes and reduced virulence in mice.
Salmonella interference with host adaptive immunity
A hallmark of S. Typhimurium is its capacity to prevent the processing and presentation of bacterial antigens by DCs to T cells, both on class I and class II MHC molecules (Fig. 2).42,47,144,158–161 Importantly, suppression of antigen presentation by S. Typhimurium is restricted to antigens expressed by this bacterium, because DCs infected with Salmonella remain capable of presenting bystander soluble antigens.42,143 However, one study showed that the presentation of non-bacterial antigens might be also affected by S. Typhimurium infection.42,51
Figure 2.
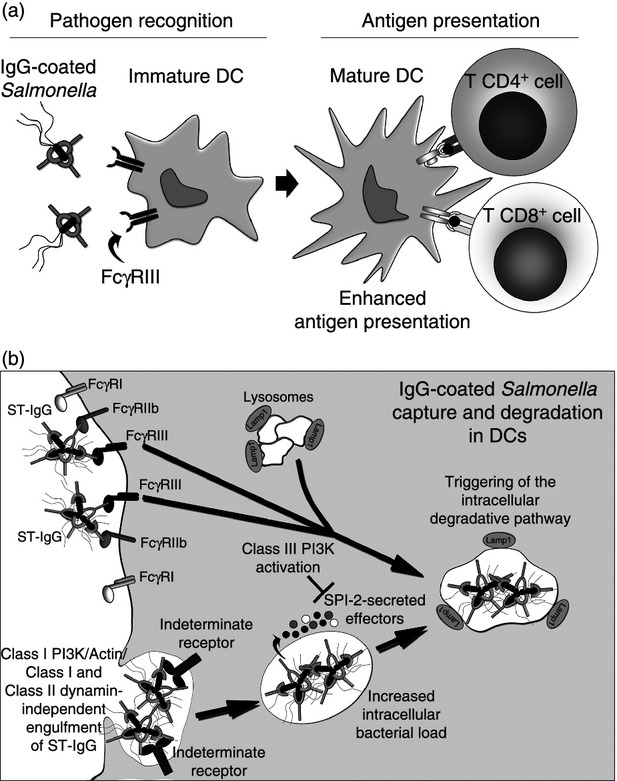
Fcγ receptor-mediated enhancement of T-cell priming by dendritic cells (DCs) after recognition and degradation of Salmonella-derived antigens. (a) After IgG opsonization, Salmonella can be recognized by FcγRIII expressed on the surface of DCs. Then, these cells are able to present bacterial-derived antigens MHC-I and MHC-II to CD8+ and CD4+ T cells, respectively. IgG opsonization can restore antigen presentation by DCs infected with Salmonella. (b) IgG-opsonized Salmonella engages FcγRIII on the surface of the DCs, triggering an intracellular class III phosphatidyl inositol 3 kniase (PI3K) -dependent degradation pathway, which enhances fusion of the Salmonella-containing vacuoles (SCV) with lysosomes (Lamp1+). SCV-containing IgG-coated bacteria are created after internalization of elevated numbers of Salmonella complexes in an FcγRs/class I PI3K/Actin/Class I and Class II and dynamin-independent mechanism. Internalization is driven by an unidentified receptor, which could recognize bacterial surface molecules unblocked by IgG-opsonization. Once inside the SCV, Salmonella still remain able to secrete Salmonella pathogenicity island 2 (SPI-2) -derived virulence determinants. As a result of IgG opsonization, antigen capture, degradation and presentation by DCs are significantly enhanced.
It has also been described that S. Typhimurium infection reduces the amount of MHC molecules expressed on the surface of both mouse and human DCs.143,162 Studies in this area suggest that this evasion mechanism is the result of the negative effect of bacteria effector proteins in both endosomal trafficking and lysosomal degradation of the bacterium.42,47,158,163 This effect results in reduced availability of bacterial antigens loaded on MHC molecules and poor activation of T cells.47,52,142 Additionally, studies in human DCs suggest that S. Typhimurium infection promotes degradation of HLA-DR molecules by poly-ubiquitination.162
In agreement with the previous notion, attenuated strains of S. Typhimurium lacking SPI-2, T3SS-2 or T3SS-2-secreted effector proteins fail to prevent antigen presentation by DCs (Fig. 2).42,51 These bacterial strains show a significant impairment in their intracellular survival inside DCs and become attenuated in mice.42,51 The TTSS-2-secreted effector proteins required for avoiding antigen presentation include SlrP, SspeH2, PipB2, SopD and SifA.143 The S. Typhimurium strains lacking these genes are unable to prevent the activation of T cells by Salmonella-infected DCs.143 Importantly, other Salmonella serovars, such as S. Enteritidis and S. Typhi, are also unable to prevent antigen presentation by murine DCs.53 These Salmonella serovars are less virulent in mice and similar to SPI-2 or T3SS-2 mutant strains, these bacteria are targeted for lysosomal degradation in murine DCs.53 Therefore, the capacity of Salmonella to prevent antigen presentation might be a feature of host restriction.
Importantly, we have described how impairment of antigen presentation would depend on effector proteins secreted largely by T3SS-2, because S. Typhimurium strains lacking T3SS-1 remain capable of preventing antigen presentation on MHC molecules by DCs.140 In addition, we have observed that prevention of antigen presentation, might be fundamental for S. Typhimurium to cause systemic disease in mice, as SPI-2 or T3SS-2 mutant strains fail to colonize internal organs and cause a lethal infection.42 In contrast, either T3SS-1 or SPI-1 mutant strains show mild attenuation and remain capable of causing systemic infection.164
Salmonella interactions with Fcγ receptors and its role in T-cell activation by DCs
Although virulent strains of S. Typhimurium are able to prevent antigen presentation by host DCs, we have described how the presence of anti-Salmonella antibodies in the infected host is an efficient way to counteract these evasion mechanisms that prevent the activation of adaptive immunity. This phenomenon takes place because IgG-opsonized virulent Salmonella can be both taken up and processed by Fcγ receptors expressed by DCs. It is widely accepted that Fcγ receptor engagement can significantly increases the capacity of DCs to degrade and present bacterial antigens on MHC molecules and activate T cells.47,141 It has been suggested that enhancement of processing and presentation of Salmonella antigens is mainly mediated by FcγRIII, a low-affinity activating Fcγ receptor expressed by DCs (Fig. 2).141 We have shown that binding of Salmonella-IgG to FcγRIII results in the fusion of the SCV with DC lysosomes and subsequently in the degradation of bacteria for antigen presentation, perhaps because of the activation of specific signalling pathways.141,47 Although opsonization with IgG does not prevent secretion of effector proteins by T3SS-1 and T3SS-2,165 FcγRIII signalling seems to overcome the effect of the T3SS-2 secreted proteins that are responsible for evading the degradation of captured Salmonella. Furthermore, enhancement of Salmonella degradation and antigen presentation induced by FcγRIII requires class III phosphatidyl inositol 3-kinase (PI3K) activity on DCs, as it can be reverted by the PI3K inhibitor Wortmannin (Fig. 2).141 This molecule not only reduces presentation of bacterial antigens to T cells, but also increases the intracellular survival of either virulent or attenuated Salmonella in DCs.141
In addition, we have shown that Salmonella-specific IgGs increase bacterial engulfment by DCs, so abrogating the uptake evasion displayed by SPI-1.165 However, neither FcγRI, FcγRIIb nor FcγRIII are employed by DCs to capture these large immune complexes (Fig. 2).165 Our data suggest that antigen presentation enhancement displayed by IgG-coated Salmonella-infected DCs is orchestrated by an FcγRIII-dependent degradation and an FcγRs-independent internalization.165 Hence, IgG on the surface of Salmonella could be engaging FcγRIII, promoting intracellular degradation and, at the same time, another still undefined receptor could be recognizing different bacterial components promoting internalization. This new receptor (or cluster of receptors) does not require class I PI3K, an actin cytoskeleton or class I/class II dynamin because specific inhibitors for these molecules failed to prevent bacterial internalization by DCs (Fig. 2).165 Hence, despite the finding that anti capture SPI-1-derived effectors could still be translocated by IgG-coated Salmonella into the DC cytoplasm, an alternative internalization mechanism could effectively enhance Salmonella uptake.140,165 Enhanced bacterial uptake increases the amount of intracellular antigen available to be further processed. This could explain why DCs present on their surfaces elevated the numbers of bacterial-derived antigens on both class I and class II MHC molecules that can prime naive T cells, enhancing host adaptive immunity (Fig 2).141
In conclusion, our observations suggest that a previously acquired antibody response against S. Typhimurium might contribute to counteract the virulence mechanisms displayed by this pathogen, due to the targeting of bacteria to activating Fcγ receptors and to the enhancement of the DCs capacity to capture, degrade and present bacterial antigens to T cells.
Concluding remarks
Salmonella Typhimurium has successfully evolved molecular mechanisms to alter DC function and exploit the immune response to cause a successful infection in the host. Studies performed in the past decade allowed us to conclude that effector molecules encoded by SPI-1 and SPI-2 contribute to exploiting the inflammation induced by DCs and other immune cells at the intestinal mucosa to replicate and spread to new hosts. Further, Salmonella has evolved molecular mechanisms to prevent activation of T cells by DCs and cause an uncontrolled systemic disease. The characterization of the strategies used by S. Typhimurium to evade host immunity could contribute to generate new prophylactic tools aimed at improving DC function and to promote immune responses to counteract this pathogen virulence. Studies describing Fcγ receptor-independent uptake separate from Fcγ receptor-mediated intracellular degradation of Salmonella by DCs could provide new insights for improved immunotherapies and vaccines against salmonellosis, using DCs and antibodies as a new therapeutic approaches.
Acknowledgments
The authors were supported by grants FONDECYT no. 1070352, FONDECYT no. 1050979, FONDECYT no. 1040349, FONDECYT no. 1100926, FONDECYT no. 1110397, FONDECYT no. 1100971, FONDECYT no. 1110604, and the Millennium Institute on Immunology and Immunotherapy (P09-016-F). SAR is a CONICYT fellow and AMK is a Chaire De La Région Pays De La Loire De Chercheur Étranger D'excellence. SMB is the Country Liaison for the American Society for Microbiology in Chile.
Disclosures
The authors declare no conflict of interest.
References
- 1.Reinicke AT, et al. Dendritic cell cross-priming is essential for immune responses to Listeria monocytogenes. PLoS ONE. 2009;4:e7210. doi: 10.1371/journal.pone.0007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 3.Rescigno M. Dendritic cells and the complexity of microbial infection. Trends Microbiol. 2002;10:425–61. doi: 10.1016/s0966-842x(02)02425-3. [DOI] [PubMed] [Google Scholar]
- 4.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 5.Lee HK, et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359–70. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manickasingham S, Reis e Sousa C. Microbial and T cell-derived stimuli regulate antigen presentation by dendritic cells in vivo. J Immunol. 2000;165:5027–34. doi: 10.4049/jimmunol.165.9.5027. [DOI] [PubMed] [Google Scholar]
- 7.Bueno SM, et al. Molecular interactions between dendritic cells and Salmonella: escape from adaptive immunity and implications on pathogenesis. Crit Rev Immunol. 2005;25:389–403. doi: 10.1615/critrevimmunol.v25.i5.40. [DOI] [PubMed] [Google Scholar]
- 8.Iruretagoyena MI, et al. Inhibition of nuclear factor-κB enhances the capacity of immature dendritic cells to induce antigen-specific tolerance in experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther. 2006;318:59–67. doi: 10.1124/jpet.106.103259. [DOI] [PubMed] [Google Scholar]
- 9.Guo Z, et al. Fas signal links innate and adaptive immunity by promoting dendritic-cell secretion of CC and CXC chemokines. Blood. 2005;106:2033–41. doi: 10.1182/blood-2004-12-4831. [DOI] [PubMed] [Google Scholar]
- 10.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–12. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Webb TJ, et al. The phenotype and function of lung dendritic cells. Crit Rev Immunol. 2005;25:465–91. doi: 10.1615/critrevimmunol.v25.i6.20. [DOI] [PubMed] [Google Scholar]
- 12.Cruickshank SM, et al. Characterization of colonic dendritic cells in normal and colitic mice. World J Gastroenterol. 2005;11:6338–47. doi: 10.3748/wjg.v11.i40.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman RM, et al. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979;149:1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 15.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 16.Turley SJ, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–7. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 17.Ebner S, et al. Expression of maturation-/migration-related molecules on human dendritic cells from blood and skin. Immunobiology. 1998;198:568–87. doi: 10.1016/S0171-2985(98)80079-X. [DOI] [PubMed] [Google Scholar]
- 18.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 19.Michelsen KS, et al. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J Biol Chem. 2001;276:25680–6. doi: 10.1074/jbc.M011615200. [DOI] [PubMed] [Google Scholar]
- 20.Means TK, et al. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–75. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 21.Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–7. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–80. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 23.Schnare M, et al. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr Biol. 2000;10:1139–42. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- 24.Sheng KC, et al. Dendritic cells: activation and maturation – applications for cancer immunotherapy. Curr Med Chem. 2005;12:1783–800. doi: 10.2174/0929867054367248. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez PA, et al. Modulation of immunological synapse by membrane-bound and soluble ligands. Cytokine Growth Factor Rev. 2007;18:19–31. doi: 10.1016/j.cytogfr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Huang Q, et al. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–5. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 27.Gillet-Hladky S, et al. Induction of high expression of CCR7 and high production of IL-12 in human monocyte-derived dendritic cells by a new bacterial component: LCOS 1013. Int Immunopharmacol. 2008;8:1151–63. doi: 10.1016/j.intimp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 28.von Meyenn F, et al. Toll-like receptor 9 contributes to recognition of Mycobacterium bovis Bacillus Calmette–Guérin by Flt3-ligand generated dendritic cells. Immunobiology. 2006;211:557–65. doi: 10.1016/j.imbio.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Loof TG, Goldmann O, Medina E. Immune recognition of Streptococcus pyogenes by dendritic cells. Infect Immun. 2008;76:2785–92. doi: 10.1128/IAI.01680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jong EC, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168:1704–9. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 31.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–31. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller M, et al. Intestinal colonization of IL-2 deficient mice with non-colitogenic B. vulgatus prevents DC maturation and T-cell polarization. PLoS ONE. 2008;3:e2376. doi: 10.1371/journal.pone.0002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milling SW, et al. Regulation of intestinal immunity: effects of the oral adjuvant Escherichia coli heat-labile enterotoxin on migrating dendritic cells. Eur J Immunol. 2007;37:87–99. doi: 10.1002/eji.200636199. [DOI] [PubMed] [Google Scholar]
- 34.Zhao C, et al. Salmonella typhimurium infection triggers dendritic cells and macrophages to adopt distinct migration patterns in vivo. Eur J Immunol. 2006;36:2939–50. doi: 10.1002/eji.200636179. [DOI] [PubMed] [Google Scholar]
- 35.Bueno SM, et al. T cell immunity evasion by virulent Salmonella enterica. Immunol Lett. 2007;111:14–20. doi: 10.1016/j.imlet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 36.House D, et al. Typhoid fever: pathogenesis and disease. Curr Opin Infect Dis. 2001;14:573–8. doi: 10.1097/00001432-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Jones BD, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–61. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 38.Pang T, et al. Typhoid fever – important issues still remain. Trends Microbiol. 1998;6:131–3. doi: 10.1016/s0966-842x(98)01236-0. [DOI] [PubMed] [Google Scholar]
- 39.Conlan JW, North RJ. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect Immun. 1992;60:5164–71. doi: 10.1128/iai.60.12.5164-5171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salcedo SP, et al. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 2001;3:587–97. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 41.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–80. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobar JA, et al. Virulent Salmonella enterica serovar typhimurium evades adaptive immunity by preventing dendritic cells from activating T cells. Infect Immun. 2006;74:6438–48. doi: 10.1128/IAI.00063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia K, et al. Autophagy genes protect against Salmonella typhimurium infection and mediate insulin signaling-regulated pathogen resistance. Proc Natl Acad Sci U S A. 2009;106:14564–9. doi: 10.1073/pnas.0813319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastroeni P, et al. A dynamic view of the spread and intracellular distribution of Salmonella enterica. Nat Rev Microbiol. 2009;7:73–80. doi: 10.1038/nrmicro2034. [DOI] [PubMed] [Google Scholar]
- 45.Albaghdadi H, et al. Selectively reduced intracellular proliferation of Salmonella enterica serovar typhimurium within APCs limits antigen presentation and development of a rapid CD8 T cell response. J Immunol. 2009;183:3778–87. doi: 10.4049/jimmunol.0900843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawley TD, et al. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006;2:e11. doi: 10.1371/journal.ppat.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tobar JA, Gonzalez PA, Kalergis AM. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fcγ receptors on dendritic cells. J Immunol. 2004;173:4058–65. doi: 10.4049/jimmunol.173.6.4058. [DOI] [PubMed] [Google Scholar]
- 48.Marcus SL, et al. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2000;2:145–56. doi: 10.1016/s1286-4579(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 49.Hensel M, et al. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–74. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 50.Hansen-Wester I, Hensel M. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 2001;3:549–59. doi: 10.1016/s1286-4579(01)01411-3. [DOI] [PubMed] [Google Scholar]
- 51.Cheminay C, Mohlenbrink A, Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J Immunol. 2005;174:2892–9. doi: 10.4049/jimmunol.174.5.2892. [DOI] [PubMed] [Google Scholar]
- 52.Jantsch J, et al. Intracellular activities of Salmonella enterica in murine dendritic cells. Cell Microbiol. 2003;5:933–45. doi: 10.1046/j.1462-5822.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 53.Bueno SM, et al. The capacity of Salmonella to survive inside dendritic cells and prevent antigen presentation to T cells is host specific. Immunology. 2008;124:522–33. doi: 10.1111/j.1365-2567.2008.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardt WD, et al. STyphimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–26. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- 55.Stender S, et al. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol Microbiol. 2000;36:1206–21. doi: 10.1046/j.1365-2958.2000.01933.x. [DOI] [PubMed] [Google Scholar]
- 56.Mirold S, et al. Isolation of a temperate bacteriophage encoding the type III effector protein SopE from an epidemic Salmonella typhimurium strain. Proc Natl Acad Sci U S A. 1999;96:9845–50. doi: 10.1073/pnas.96.17.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chakravortty D, Hansen-Wester I, Hensel M. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med. 2002;195:1155–66. doi: 10.1084/jem.20011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hapfelmeier S, et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med. 2008;205:437–50. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eichelberg K, Galan JE. Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1) -encoded transcriptional activators InvF and hilA. Infect Immun. 1999;67:4099–105. doi: 10.1128/iai.67.8.4099-4105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rappl C, Deiwick J, Hensel M. Acidic pH is required for the functional assembly of the type III secretion system encoded by Salmonella pathogenicity island 2. FEMS Microbiol Lett. 2003;226:363–72. doi: 10.1016/S0378-1097(03)00638-4. [DOI] [PubMed] [Google Scholar]
- 61.Lober S, et al. Regulation of Salmonella pathogenicity island 2 genes by independent environmental signals. Int J Med Microbiol. 2006;296:435–47. doi: 10.1016/j.ijmm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Bustamante VH, et al. HilD-mediated transcriptional cross-talk between SPI-1 and SPI-2. Proc Natl Acad Sci U S A. 2008;105:14591–6. doi: 10.1073/pnas.0801205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cotter PA, DiRita VJ. Bacterial virulence gene regulation: an evolutionary perspective. Annu Rev Microbiol. 2000;54:519–65. doi: 10.1146/annurev.micro.54.1.519. [DOI] [PubMed] [Google Scholar]
- 64.Collazo CM, Galan JE. The invasion-associated type-III protein secretion system in Salmonella – a review. Gene. 1997;192:51–9. doi: 10.1016/s0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- 65.Kubori T, et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–5. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 66.Nikolaus T, et al. SseBCD proteins are secreted by the type III secretion system of Salmonella pathogenicity island 2 and function as a translocon. J Bacteriol. 2001;183:6036–45. doi: 10.1128/JB.183.20.6036-6045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lawhon SD, et al. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol. 2002;46:1451–64. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- 68.Lucas RL, et al. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar typhimurium. J Bacteriol. 2000;182:1872–82. doi: 10.1128/jb.182.7.1872-1882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steele-Mortimer O, et al. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell Microbiol. 2002;4:43–54. doi: 10.1046/j.1462-5822.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhou D, et al. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol. 2001;39:248–59. doi: 10.1046/j.1365-2958.2001.02230.x. [DOI] [PubMed] [Google Scholar]
- 71.Scherer CA, Cooper E, Miller SI. The Salmonella type III secretion translocon protein SspC is inserted into the epithelial cell plasma membrane upon infection. Mol Microbiol. 2000;37:1133–45. doi: 10.1046/j.1365-2958.2000.02066.x. [DOI] [PubMed] [Google Scholar]
- 72.Waterman SR, Holden DW. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol. 2003;5:501–11. doi: 10.1046/j.1462-5822.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 73.Ochman H, et al. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci U S A. 1996;93:7800–4. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shea JE, et al. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun. 1999;67:213–9. doi: 10.1128/iai.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blanc-Potard AB, et al. The SPI-3 pathogenicity island of Salmonella enterica. J Bacteriol. 1999;181:998–1004. doi: 10.1128/jb.181.3.998-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Golubeva YA, Slauch JM. Salmonella enterica serovar Typhimurium periplasmic superoxide dismutase SodCI is a member of the PhoPQ regulon and is induced in macrophages. J Bacteriol. 2006;188:7853–61. doi: 10.1128/JB.00706-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho TD, et al. Identification of GtgE, a novel virulence factor encoded on the Gifsy-2 bacteriophage of Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:5234–9. doi: 10.1128/JB.184.19.5234-5239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Figueroa-Bossi N, et al. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol Microbiol. 2001;39:260–71. doi: 10.1046/j.1365-2958.2001.02234.x. [DOI] [PubMed] [Google Scholar]
- 79.Quiroz TS, et al. Excision of an unstable pathogenicity island in Salmonella enterica serovar Enteritidis is induced during infection of phagocytic cells. PLoS ONE. 2011;6:e26031. doi: 10.1371/journal.pone.0026031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Craig M, Slauch JM. Phagocytic superoxide specifically damages an extracytoplasmic target to inhibit or kill Salmonella. PLoS ONE. 2009;4:e4975. doi: 10.1371/journal.pone.0004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo A, et al. FimH alleles direct preferential binding of Salmonella to distinct mammalian cells or to avian cells. Microbiology. 2009;5:1623–33. doi: 10.1099/mic.0.026286-0. [DOI] [PubMed] [Google Scholar]
- 82.Link C, et al. An SopB-mediated immune escape mechanism of Salmonella enterica can be subverted to optimize the performance of live attenuated vaccine carrier strains. Microbes Infect. 2006;8:2262–9. doi: 10.1016/j.micinf.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 83.Gerlach RG, et al. Salmonella Pathogenicity Island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell Microbiol. 2007;9:1834–50. doi: 10.1111/j.1462-5822.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 84.Jepson MA, Kenny B, Leard AD. Role of sipA in the early stages of Salmonella typhimurium entry into epithelial cells. Cell Microbiol. 2001;3:417–26. doi: 10.1046/j.1462-5822.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 85.Higashide W, et al. Involvement of SipA in modulating actin dynamics during Salmonella invasion into cultured epithelial cells. Cell Microbiol. 2002;4:357–65. doi: 10.1046/j.1462-5822.2002.00196.x. [DOI] [PubMed] [Google Scholar]
- 86.Wood MW, et al. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a Sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–38. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 87.Bakshi CS, et al. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J Bacteriol. 2000;182:2341–4. doi: 10.1128/jb.182.8.2341-2344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raffatellu M, et al. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype typhimurium invasion of epithelial cells. Infect Immun. 2005;73:146–54. doi: 10.1128/IAI.73.1.146-154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bourdet-Sicard R, Tran Van Nhieu G. Actin reorganization by SipA and Salmonella invasion of epithelial cells. Trends Microbiol. 1999;7:309–10. doi: 10.1016/s0966-842x(99)01548-6. [DOI] [PubMed] [Google Scholar]
- 90.Lara-Tejero M, Galan JE. Salmonella enterica serovar typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect Immun. 2009;77:2635–42. doi: 10.1128/IAI.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Francis CL, et al. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–42. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 92.Ginocchio CC, et al. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–24. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 93.Fu Y, Galan JE. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–7. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 94.Winnen B, et al. Hierarchical effector protein transport by the Salmonella Typhimurium SPI-1 type III secretion system. PLoS ONE. 2008;3:e2178. doi: 10.1371/journal.pone.0002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Engelenburg SB, Palmer AE. Quantification of real-time Salmonella effector type III secretion kinetics reveals differential secretion rates for SopE2 and SptP. Chem Biol. 2008;15:619–28. doi: 10.1016/j.chembiol.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kubori T, Galan JE. Temporal regulation of salmonella virulence effector function by proteasome-dependent protein degradation. Cell. 2003;115:333–42. doi: 10.1016/s0092-8674(03)00849-3. [DOI] [PubMed] [Google Scholar]
- 97.Bruno VM, et al. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 2009;5:e1000538. doi: 10.1371/journal.ppat.1000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eckmann L, et al. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J Biol Chem. 2000;275:14084–94. doi: 10.1074/jbc.275.19.14084. [DOI] [PubMed] [Google Scholar]
- 99.Izadpanah A, et al. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–9. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 100.Eckmann L, Kagnoff MF. Cytokines in host defense against Salmonella. Microbes Infect. 2001;3:1191–200. doi: 10.1016/s1286-4579(01)01479-4. [DOI] [PubMed] [Google Scholar]
- 101.Elewaut D, et al. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–66. [PubMed] [Google Scholar]
- 102.Chieppa M, et al. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ewaschuk JB, et al. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun. 2007;75:2572–9. doi: 10.1128/IAI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vijay-Kumar M, et al. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am J Pathol. 2006;169:1686–700. doi: 10.2353/ajpath.2006.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 106.Uematsu S, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–74. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 107.Tallant T, et al. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-κB and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 2004;4:33. doi: 10.1186/1471-2180-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosenstiel P, et al. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J Immunol. 2007;178:8203–11. doi: 10.4049/jimmunol.178.12.8203. [DOI] [PubMed] [Google Scholar]
- 109.Tam MA, et al. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol Rev. 2008;225:140–62. doi: 10.1111/j.1600-065X.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 110.Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 111.Tukel C, et al. Neutrophil influx during non-typhoidal salmonellosis: who is in the driver's seat? FEMS Immunol Med Microbiol. 2006;46:320–9. doi: 10.1111/j.1574-695X.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 112.Burkey TE, et al. Expression of Toll-like receptors, interleukin 8, macrophage migration inhibitory factor, and osteopontin in tissues from pigs challenged with Salmonella enterica serovar Typhimurium or serovar Choleraesuis. Vet Immunol Immunopathol. 2007;115:309–19. doi: 10.1016/j.vetimm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 113.Hobert ME, et al. Cdc42 and Rac1 regulate late events in Salmonella typhimurium-induced interleukin-8 secretion from polarized epithelial cells. J Biol Chem. 2002;277:51025–32. doi: 10.1074/jbc.M210466200. [DOI] [PubMed] [Google Scholar]
- 114.Broz P, et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–55. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Franchi L, et al. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol. 2012;13:449–56. doi: 10.1038/ni.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kofoed EM, Vance RE. NAIPs: Building an innate immune barrier against bacterial pathogens: NAIPs function as sensors that initiate innate immunity by detection of bacterial proteins in the host cell cytosol. Bioessays. 2012;34:589–98. doi: 10.1002/bies.201200013. [DOI] [PubMed] [Google Scholar]
- 117.Monack DM, et al. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J Exp Med. 2000;192:249–58. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–32. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lightfield KL, et al. Critical function for NAIP5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–8. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006;7:569–75. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 121.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 122.Zeng H, et al. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G96–108. doi: 10.1152/ajpgi.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat Immunol. 2006;7:576–82. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 124.Simon R, Samuel CE. Interleukin-1β secretion is activated comparably by FliC and FljB flagellins but differentially by wild-type and DNA adenine methylase-deficient salmonella. J Interferon Cytokine Res. 2008;28:661–6. doi: 10.1089/jir.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gewirtz AT, et al. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stecher B, et al. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun. 2004;72:4138–50. doi: 10.1128/IAI.72.7.4138-4150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lodes MJ, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zeng H, et al. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171:3668–74. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 129.Fournier B, et al. Toll-like receptor 5-dependent regulation of inflammation in systemic Salmonella enterica Serovar typhimurium infection. Infect Immun. 2009;77:4121–9. doi: 10.1128/IAI.00656-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lockman HA, Curtiss 3rdR. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun. 1990;58:137–43. doi: 10.1128/iai.58.1.137-143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schmitt CK, et al. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect Immun. 2001;69:5619–25. doi: 10.1128/IAI.69.9.5619-5625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hardt WD, Galan JE. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc Natl Acad Sci U S A. 1997;94:9887–92. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ye Z, et al. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am J Pathol. 2007;171:882–92. doi: 10.2353/ajpath.2007.070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Collier-Hyams LS, et al. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-κB pathway. J Immunol. 2002;169:2846–50. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 135.Jones RM, et al. Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host Microbe. 2008;3:233–44. doi: 10.1016/j.chom.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 136.Du F, Galan JE. Selective inhibition of type III secretion activated signaling by the Salmonella effector AvrA. PLoS Pathog. 2009;5:e1000595. doi: 10.1371/journal.ppat.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu H, Jones RM, Neish AS. The Salmonella effector AvrA mediates bacterial intracellular survival during infection in vivo. Cell Microbiol. 2012;14:28–39. doi: 10.1111/j.1462-5822.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liao AP, et al. Salmonella type III effector AvrA stabilizes cell tight junctions to inhibit inflammation in intestinal epithelial cells. PLoS ONE. 2008;3:e2369. doi: 10.1371/journal.pone.0002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hapfelmeier S, et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174:1675–85. doi: 10.4049/jimmunol.174.3.1675. [DOI] [PubMed] [Google Scholar]
- 140.Bueno SM, et al. Salmonella pathogenicity island 1 differentially modulates bacterial entry to dendritic and non-phagocytic cells. Immunology. 2010;130:273–87. doi: 10.1111/j.1365-2567.2009.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Herrada AA, et al. Immune complex-induced enhancement of bacterial antigen presentation requires Fcγ receptor III expression on dendritic cells. Proc Natl Acad Sci U S A. 2007;104:13402–7. doi: 10.1073/pnas.0700999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garcia-Del Portillo F, et al. Interaction of Salmonella enterica serotype Typhimurium with dendritic cells is defined by targeting to compartments lacking lysosomal membrane glycoproteins. Infect Immun. 2000;68:2985–91. doi: 10.1128/iai.68.5.2985-2991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Halici S, et al. Functional analysis of the Salmonella pathogenicity island 2-mediated inhibition of antigen presentation in dendritic cells. Infect Immun. 2008;76:4924–33. doi: 10.1128/IAI.00531-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Petrovska L, et al. Salmonella enterica serovar Typhimurium interaction with dendritic cells: impact of the sifA gene. Cell Microbiol. 2004;6:1071–84. doi: 10.1111/j.1462-5822.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 145.Freeman JA, et al. SpiC is required for translocation of Salmonella pathogenicity island 2 effectors and secretion of translocon proteins SseB and SseC. J Bacteriol. 2002;184:4971–80. doi: 10.1128/JB.184.18.4971-4980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kuhle V, Hensel M. SseF and SseG are translocated effectors of the type III secretion system of Salmonella pathogenicity island 2 that modulate aggregation of endosomal compartments. Cell Microbiol. 2002;4:813–24. doi: 10.1046/j.1462-5822.2002.00234.x. [DOI] [PubMed] [Google Scholar]
- 147.Vazquez-Torres A, et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–8. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 148.Shotland Y, Kramer H, Groisman EA. The Salmonella SpiC protein targets the mammalian Hook3 protein function to alter cellular trafficking. Mol Microbiol. 2003;49:1565–76. doi: 10.1046/j.1365-2958.2003.03668.x. [DOI] [PubMed] [Google Scholar]
- 149.Yu XJ, et al. SpiC is required for secretion of Salmonella Pathogenicity Island 2 type III secretion system proteins. Cell Microbiol. 2002;4:531–40. doi: 10.1046/j.1462-5822.2002.00211.x. [DOI] [PubMed] [Google Scholar]
- 150.Buckner MM, et al. A comprehensive study of the contribution of Salmonella enterica serovar Typhimurium SPI2 effectors to bacterial colonization, survival, and replication in typhoid fever, macrophage, and epithelial cell infection models. Virulence. 2011;2:208–16. doi: 10.4161/viru.2.3.15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guignot J, et al. Microtubule motors control membrane dynamics of Salmonella-containing vacuoles. J Cell Sci. 2004;7:1033–45. doi: 10.1242/jcs.00949. [DOI] [PubMed] [Google Scholar]
- 152.Boucrot E, et al. Salmonella typhimurium SifA effector protein requires its membrane-anchoring C-terminal hexapeptide for its biological function. J Biol Chem. 2003;278:14196–202. doi: 10.1074/jbc.M207901200. [DOI] [PubMed] [Google Scholar]
- 153.Beuzon CR, et al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–49. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Diacovich L, et al. Interaction between the SifA virulence factor and its host target SKIP is essential for Salmonella pathogenesis. J Biol Chem. 2009;284:33151–60. doi: 10.1074/jbc.M109.034975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ohlson MB, et al. Structure and function of Salmonella SifA indicate that its interactions with SKIP, SseJ, and RhoA family GTPases induce endosomal tubulation. Cell Host Microbe. 2008;4:434–46. doi: 10.1016/j.chom.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jackson LK, et al. The Salmonella virulence protein SifA is a G protein antagonist. Proc Natl Acad Sci U S A. 2008;105:14141–6. doi: 10.1073/pnas.0801872105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Boucrot E, et al. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 2005;308:1174–8. doi: 10.1126/science.1110225. [DOI] [PubMed] [Google Scholar]
- 158.Srinivasan A, et al. Low-dose Salmonella infection evades activation of flagellin-specific CD4 T cells. J Immunol. 2004;173:4091–9. doi: 10.4049/jimmunol.173.6.4091. [DOI] [PubMed] [Google Scholar]
- 159.Alaniz RC, et al. Salmonella typhimurium coordinately regulates FliC location and reduces dendritic cell activation and antigen presentation to CD4+ T cells. J Immunol. 2006;177:3983–93. doi: 10.4049/jimmunol.177.6.3983. [DOI] [PubMed] [Google Scholar]
- 160.Salazar-Gonzalez RM, et al. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J Immunol. 2007;179:6169–75. doi: 10.4049/jimmunol.179.9.6169. [DOI] [PubMed] [Google Scholar]
- 161.McSorley SJ, et al. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–77. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 162.Lapaque N, et al. Salmonella regulates polyubiquitination and surface expression of MHC class II antigens. Proc Nat Acad Sci USA. 2009;106:14052–7. doi: 10.1073/pnas.0906735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Uchiya K, et al. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–33. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Murray RA, Lee CA. Invasion genes are not required for Salmonella enterica serovar typhimurium to breach the intestinal epithelium: evidence that salmonella pathogenicity island 1 has alternative functions during infection. Infect Immun. 2000;68:5050–5. doi: 10.1128/iai.68.9.5050-5055.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Riquelme SA, Bueno SM, Kalergis AM. IgG keeps virulent Salmonella from evading dendritic cell uptake. Immunology. 2012;136:291–305. doi: 10.1111/j.1365-2567.2012.03578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]