Abstract
Natural killer (NK) cells have a specialized function in peripheral organs, which is determined by the organ-specific niches. We have attempted to explore whether lung NK cells display a particular phenotype according to their function in the unique pulmonary environment in health or during respiratory infection in mice. In healthy mice, higher frequencies of NK cells among lymphocytes were detected in the lung than in other tissues (lymph node, bone marrow, spleen, blood and liver), and lung NK cells maintained a more mature phenotype, implying that lung NK cells were critical for the pulmonary immune response. However, lung NK cells expressed higher levels of inhibitory receptors and lower levels of activating receptors, migration/adhesion-associated molecules and co-stimulatory molecules than splenic NK cells, implying that lung NK cells were quiescent, and the activation of lung NK cells was tightly regulated by the pulmonary environment in health. During respiratory infection, lung NK cells could be activated and express functional molecules (CD107a and interferon-γ) to take part in the response to infection quickly. These results suggested that the unique pulmonary environment promotes the development of NK cells with a lung-specific phenotype.
Keywords: function, lung, natural killer cells, phenotype
Introduction
Natural killer (NK) cells are innate lymphocytes that originate from bone marrow (BM) precursors.1,2 As an important part of the innate immune system and the body's first line of defence, NK cells can rapidly respond to infection and regulate the subsequent adaptive immune response.3 The activation of NK cells is regulated by a range of activating and inhibitory receptors. In mice, activating receptors expressed by NK cells include natural cytotoxicity receptor NKp46 and C-type lectin like receptors NKG2D and NKG2C, as well as activating members of the Ly49 family, which commonly recognize host or pathogen glycoproteins. Inhibitory receptors, including most members of the Ly49 family and CD94-NKG2A dimer, commonly recognize classic and non-classic MHC-I molecules. The activation of NK cells is manipulated by the balance of activating receptors and inhibitory receptors.3
Consistent with their role in immune defence and surveillance, NK cells are widely distributed in lymphoid and non-lymphoid tissues.4 However, the frequencies of NK cells in lymphocytes in different tissues are not homogeneous,4 and NK cells specialize in their function in appropriate tissues.5 The respiratory tract is a special mucosal surface that can be easily invaded by many pathogens. In the lung, NK cells make up a higher percentage of resident lymphocytes than in other tissues,4 and the ability of NK cells to respond to infection is critical for maintaining pulmonary homeostasis.6,7 However, under steady-state conditions, NK cells must be tolerant to environmental antigens and commensal organisms.8,9 As a result, NK cells may tip the balance between health and pathology in the lung.
In the unique environment of the lung, which can regulate pulmonary immune responses,10,11 lung NK cells specialize in their function5 and display a particular phenotype. Under steady-state conditions, there is a high frequency of NK cells within the total resident lymphocyte population in the lung, and lung NK cells have a more mature phenotype, which might help NK cells to respond rapidly to infection. However, the lung NK cells express higher levels of inhibitory receptors and lower levels of activating receptors, adhesion/migration-associated molecules and co-stimulatory molecules when compared with NK cells from any other tissues, which is beneficial for maintaining pulmonary homeostasis under steady-state conditions. Further studies demonstrate that, when it comes to respiratory infection, lung NK cells can be activated and express functional molecules rapidly to respond to infection.
Materials and methods
Viral strain and growth condition
Mouse-adapted influenza A/PR/8/34 strain (H1N1) was a gift from Prof. Hong Meng (Institute of Basic Medicine, Shangdong Academy of Medical Sciences, China) and was amplified in allantoic cavities of 10-day-old specific pathogen-free embryonated chicken eggs (Merial-Vital Laboratory Animal Technology Co. Ltd., Beijing, China). Allantoic fluids were harvested 72 hr after inoculation and stored at −80° until needed. The haemagglutination (HA) titre of each sample was 10 240 HA unit/ml.
Bacterial strain and growth condition
The Staphylococcus aureus strain (NCTC-8325) was a gift from Prof. Baolin Sun (School of Life Sciences, University of Science and Technology of China). The Klebsiella pneumoniae strain (ATCC-700603) was a gift from the Department of Microbiology, Anhui Medical University. The S. aureus and K. pneumoniae cultures were grown from frozen stocks to mid-exponential phase of growth (optical density at 600 nm ≍ 0·75) in tryptic soy broth medium at 37° with shaking (250 rpm). Bacteria were washed with chilled non-pyrogenic saline, then resuspended in non-pyrogenic saline at appropriate concentrations and kept on ice until infection.
Animals
Male C57BL/6 and BALB/c mice were purchased from the Shanghai Experimental Animal Centre, Chinese Science Academy (Shanghai, China). All mice were maintained at an animal facility under specific pathogen-free conditions and were used at 6–10 weeks of age (body weight 20–25 g). Animal care and experimental procedures were followed in accordance with the experimental animal guidelines of the University of Science and Technology of China. BALB/c mice were used to detect the percentage of NK cells, and C57BL/6 mice were used to detect the percentage and phenotype of NK cells and perform functional assays.
Mouse infection
C57BL/6 mice were intraperitoneally anaesthetized with sodium pentobarbital (50 μg/g body weight) before they were intranasally infected with 0·1 Haemagglutination units of influenza virus (PR8) or 1 × 107 colony-forming units of S. aureus or K. pneumoniae, respectively, in 50 μl sterile saline. Control mice received 50 μl sterile saline alone. At days 0, 1, 2 and 3 after infection, lymphocytes were isolated from the lung and spleen. Expression of CD69 was detected on NK cells. In the experiment on NK function detection, at day 2 after K. pneumoniae infection, lymphocytes were isolated from lung, and then the expressions of CD107a on the surface and interferon-γ (IFN-γ) in the cytosol of NK cells were determined.
Isolation of lymphocytes
Mice were killed, and inguinal lymph nodes (LN), femurs, spleen, blood, liver and lung were collected from mice. For LN lymphocytes, the inguinal LN was passed through a 200-gauge stainless steel mesh, and then cells were washed twice and counted. For BM and spleen lymphocytes, the BM and spleen were passed through a 200-gauge stainless steel mesh. After the red blood cells were lysed, cells were washed twice and counted. For blood lymphocytes, blood was collected in heparin-sodium-containing tubes and centrifuged. The cells were resuspended in PBS, overlaid on 70% Percoll (GE Healthcare, Uppsala, Sweden) and then centrifuged at 750 g for 30 min at room temperature. Cells were collected from the PBS/70% Percoll interface, washed twice and counted. Liver lymphocytes were isolated as described previously.12 Briefly, livers were passed through a 200-gauge stainless steel mesh. The cells were resuspended in 40% Percoll, overlaid on 70% Percoll and then centrifuged at 750 g for 30 min at room temperature. Cells were collected from the 40/70% Percoll interface, washed twice and counted. Lung lymphocytes were isolated as previously described with minor modifications.13 In brief, lungs were excised and minced, then digested for 60 min at 37° with RPMI-1640 containing 0·1% collagenase I (Sigma, St Louis, MO) and 5% fetal calf serum. The large pieces of lung were removed by filtration through gauze. Lymphocytes were prepared by density gradient centrifugation with 40% and 70% Percoll. Cells were collected from the 40/70% Percoll interface, washed twice and counted. Specifically, for detecting the expressions of the Ly49 family on lung NK cells, lung lymphocytes were isolated similarly to liver lymphocytes.
Flow cytometric analysis
After blocking the Fc receptor with anti-mouse CD16/CD32, single-cell suspensions were incubated with the indicated fluorescently labelled monoclonal antibodies at 4° for 30 min in PBS (containing 0·1% sodium azide and 1% bovine serum albumin), and then washed twice. For CD107a and intracellular IFN-γ staining, cells were stimulated for 4 hr at 37° with YAC-1 cells in the presence of monensin and anti-CD107a antibody, after staining for extracellular markers, cells were fixed, permeabilized and stained with phycoerythrin-conjugated anti-IFN-γ or isotype control. Samples were analysed by flow cytometry (Becton Dickinson, Franklin Lakes, NJ) and using FlowJo software. Cells were gated according to forward scatter and side scatter, and cell types were identified by their phenotype: NK cells, CD3− DX5+ in BALB/c mice and CD3− NK1.1+ in C57BL/6 mice. Absolute numbers of NK cells were calculated from the frequency of NK cells and the total number of lymphocytes in the lung and spleen. The anti-mouse monoclonal antibodies used for flow cytometry included the following: NK1.1 (PK136), CD49b (DX5), CD3e (145-2C11), CD27 (LG.3A10), CD11b (M1/70), CD122 (Tm-β1), Ly49C/I/F/H (14B11), CD43 (S7), CD51 (RMV-7), CD94 (18d3), NKG2A (16A11), Ly49G2 (AT-8), NKp46 (29A1.4), NKG2D (CX5), Ly49H(3D10), CD69 (H1.2F3), CD244 (2B4), CD11a (2D7), CD18 (C71/16), CD54 (3E2), CD2 (RM2-5), CD48 (OX-78), CD29 (HMB1-1), CD62L (MEL-14), CD44 (IM7), CD11c (HL3), CD73 (TY/11.8), CCR5 (C34-3448), CXCR2 (242216), PDL1 (MIH5), PD1 (J43), CRACC (polyclonal), OX40 (OX86), CTLA-4 (VC10-4F10-11), CD86 (GL1), B220 (RA3-6B2), CD1d (1B1), Ly6C (AL-21), KLRG1 (2F1), CD107a (1D4B), IFN-γ (XMG1.2). All antibodies and isotype controls were purchased form BD Biosciences (Franklin Lakes, NJ), R&D Systems (Minneapolis, MN), or eBioscience (San Diego, CA).
Statistical analysis
Results were analysed by using two-tailed Student's t-test or Mann–Whitney U-test. All experimental data are expressed as the mean ± standard error of the mean (SEM) and P-values < 0·05 were considered statistically significant.
Results
The frequency of NK cells in lymphocytes in the lung is higher than in other tissues both in C57BL/6 and BALB/c mice
As an important part of the innate immune system, NK cells are widely distributed in the body.4 As both C57BL/6 and BALB/c mice are commonly used as models of lung disease, we studied the distribution of NK cells in both strains. First, we defined NK cells as CD3− NK1.1+ in C57BL/6 mice and CD3− DX5+ in BALB/c mice that lack NK1.1 expression. The frequency of NK cells in lymphocytes in different tissues was detected by flow cytometry. Consistent with previous research,4,14 NK cells were widely distributed in lymphoid and non-lymphoid tissues, and the frequencies of NK cells in lymphocytes were not similar in the different tissues of C57BL/6 mice and BALB/c mice (Fig. 1a). The frequency of NK cells in lymphocytes in the lung (∼ 10%) was highest among all tissues; next was the liver (∼ 6%), followed by blood (3–4%), spleen (3–4%), BM (∼ 2%) and LN (∼ 0·5%) (Fig. 1b). However, the frequency of NK cells in lymphocytes from the same tissue was not significantly different between C57BL/6 and BALB/c mice (Fig. 1). Together, these results implied that the higher NK-cell frequency in the lung may be of significance.
Figure 1.
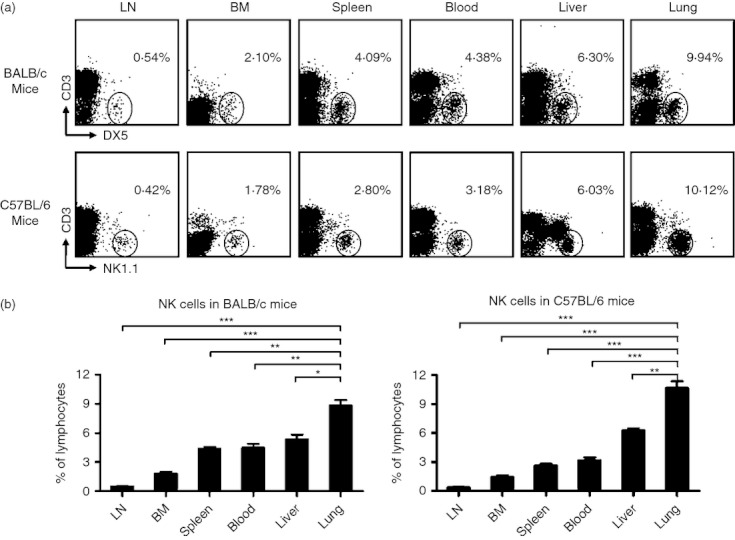
The frequency of natural killer (NK) cells in lymphocytes in the lung is higher than in other tissues in both C57BL/6 and BALB/c mice. Lymphocytes were isolated from different tissues in normal BALB/c and C57BL/6 mice. (a) Flow cytometry assay determined the frequency of NK cells in lymphocytes in different tissues [lymph node (LN), bone marrow (BM), spleen, blood, liver and lung). NK cells were gated as CD3− DX5+ in BALB/c mice and CD3− NK1.1+ in C57BL/6 mice. Dot plots are representative of three independent experiments using six mice per group. (b) The frequencies of NK cells in lymphocytes are shown as mean ± SEM. Mann–Whitney U-test; *P < 0·05; **P < 0·01; ***P < 0·001.
NK cells in the lung maintain a more mature phenotype than NK cells in other tissues under steady-state conditions
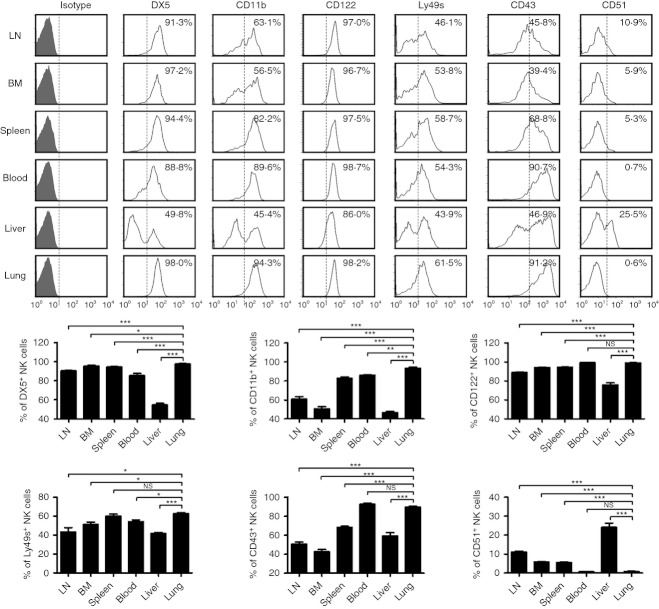
The diversity of the tissue distribution of lymphocytes is important for tissue-specific immune responses.3,4,15 Therefore, the tissue distribution of NK cells may imply their specific role in immune responses.5 According to the expression of CD27 and CD11b, mouse NK cells are subdivided into four subsets: CD11blow CD27low (double-negative, DN), CD11blow CD27high, CD11bhigh CD27high (double-positive, DP), and CD11bhigh CD27low, and the developmental relationship among these four subsets following the pathway DN → CD11blow CD27high → DP → CD11bhigh CD27low15,16. To study the phenotype of lung NK cells in a comprehensive manner, C57BL/6 mice were chosen as the research object in the next experiments. More than 70% of lung NK cells were from the CD11bhigh CD27low NK-cell subset under steady-state conditions, which was higher than the CD11bhigh CD27low NK-cell subset in blood (65·19%), spleen (46·20%), liver (31·15%), LN (25·25%), BM (17·85%), respectively (Fig. 2a,b), suggesting that most of the lung NK cells are in a more mature state. Furthermore, the mature surface markers, including DX5, CD11b, CD122, Ly49s and CD43, were also more prevalent on lung NK cells, and the immature surface marker CD51 was less prevalent on lung NK cells than on NK cells from other tissues (Fig. 3). Together, these data indicated that lung NK cells possess a more mature phenotype under steady-state conditions.
Figure 2.
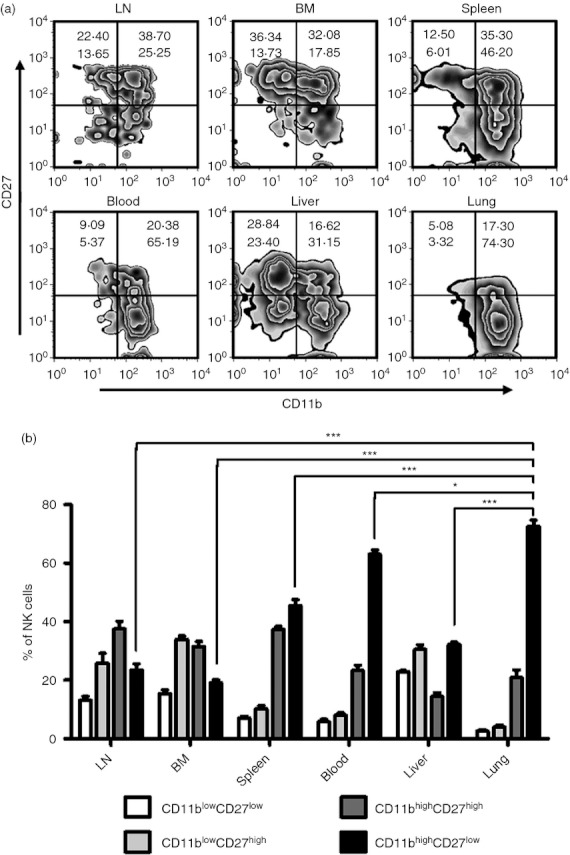
Most lung natural killer (NK) cells have CD11bhigh CD27low phenotype. Lymphocytes were isolated from different tissues in normal C57BL/6 mice. (a) Flow cytometry assay determined the expression of CD27 and CD11b on NK cells (CD3− NK1.1+) from different tissues. Zebra plots are representative of three independent experiments using three mice per group. (b) The frequencies of NK cells subsets are shown as mean ± SEM. LN, lymph node; BM, bone marrow. *P < 0·05; ***P < 0·001.
Figure 3.
Lung natural killer (NK) cells have a mature phenotype. Flow cytometry assay determined the expression of DX5, CD11b, CD122, Ly49s, CD43 and CD51 on NK cells (CD3− NK1.1+) from different tissues. Histograms are representative of three independent experiments using three mice per group, the shadow histogram is isotope control. The frequencies of DX5+, CD11b+, CD122+, Ly49s+, CD43+ and CD51+ NK cells from different tissues are shown as mean ± SEM. LN, lymph node; BM, bone marrow. *P < 0·05; **P < 0·01; ***P < 0·001; NS, not significant.
Lung NK cells are under tighter restrictions in normal conditions
The respiratory tract is a major route of entry for pathogens into the body, and the immune response in the lung must be tightly regulated to prevent excessive activation.5 In our study, we found that the proportions of NK cells expressing the inhibitory receptor CD94-NKG2A were increased in the lungs (Fig. 4a). Moreover, the proportions of lung NK cells expressing the activating receptors NKp46 and NKG2D and surface expression of the activation marker CD69 on lung NK cells were lower than in the spleen and BM (Fig. 4b,c). In addition, the expression of CD244, which can act as an activating or an inhibitory receptor, was also lower on lung NK cells (Fig. 4c). However, lung, spleen and BM NK cells expressed similar levels of the inhibitory receptor Ly49G2 and the activating receptor Ly49H (data not shown). Together, these data indicated that lung NK cells may need to overcome a higher threshold of inhibition to become activated than spleen or BM NK cells.
Figure 4.
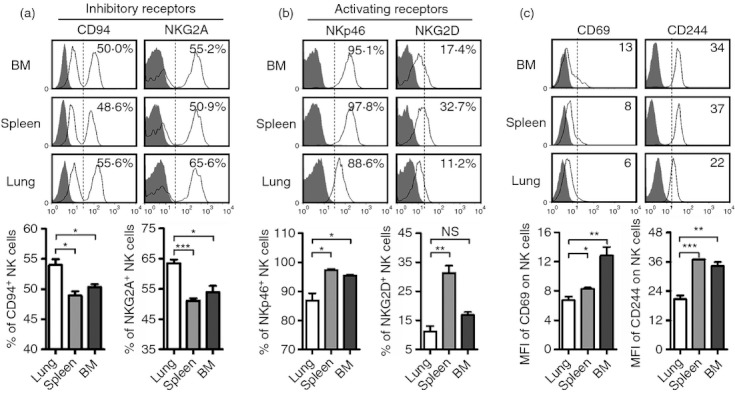
Lung natural killer (NK) cells express higher levels of inhibitory receptors and lower levels of activating receptors. Lymphocytes were isolated from bone marrow (BM), spleen and lung in normal C57BL/6 mice. (a) Flow cytometry assay determined the expression of inhibitory receptors (CD94 and NKG2A) on NK cells (CD3− NK1.1+), and the frequencies are shown as mean ± SEM. (b) Flow cytometry assay determined the expression of activating receptors (NKp46 and NKG2D) on NK cells, and the frequencies are shown as mean ± SEM. (c) Flow cytometry assay determined the expression of CD69 and CD244 on NK cells, and the mean fluorescence intensity (MFI) values are shown as mean ± SEM. Histograms are representative of three independent experiments using three mice per group, the shadow histogram is the isotype control. *P < 0·05; **P < 0·01; ***P < 0·001; NS, not significant.
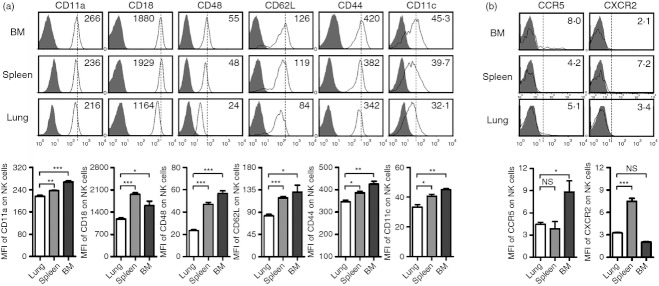
The expression of various adhesion molecules and chemokine receptors can regulate the migration of NK cells.4 It has been reported that the ability of NK cells to migrate gradually decreased following the maturity of NK cells.4,15 Indeed, the expression of adhesion molecules, including CD11a, CD18, CD48, CD62L, CD44 and CD11c (Fig. 5a) and the chemokine receptors CCR5 and CXCR2 (Fig. 5b) was lower on lung NK cells than on NK cells from spleen or BM. However, the expressions of adhesion molecules, including CD54, CD2, CD29 and CD73, on NK cells from lung, spleen and BM were similar (data not shown). Together, these data indicated that the battle field for lung NK cells may mainly focus on the lung tissue, and that the mature lung NK cells may not be able to influence other tissues during respiratory infections.
Figure 5.
Lung natural killer (NK) cells express lower levels of adhesion/migration-associated molecules. Lymphocytes were isolated from bone marrow (BM), spleen and lung in normal C57BL/6 mice. (a, b) Flow cytometry assay determined the expression of adhesion/migration-associated molecules CD11a, CD18, CD48, CD62L, CD44, CD11c (a), CCR5 and CXCR2 (b) on NK cells (CD3− NK1.1+). Histograms are representative of three independent experiments using three mice per group, the shadow histogram is the isotype control. The mean fluorescence intensity (MFI) values are shown as mean ± SEM. *P < 0·05; **P < 0·01; ***P < 0·001; NS, not significant.
As the first line of defence against infections and tumours, NK cells not only play critical roles in eliminating pathogens and killing target cells, but also interact with other immune cells, such as macrophages, dendritic cells and adaptive immune cells. The interaction between NK cells and other immune cells depends on cytokines and direct cell contact.17–20 Under steady-state conditions, lung NK cells expressed lower levels of activating co-stimulatory molecules CD86, B220 and CD1d than spleen or BM NK cells (Fig. 6a). The expression of PDL1 and PD1 on lung NK cells was also lower, but the functional relevance of these molecules on NK cells remains to be fully elucidated (Fig. 6b). In addition, the expression of CRACC, OX40 and CTLA-4 by NK cells from different tissues was similar (data not shown). All of these findings indicate that lung NK cells may be under tighter restriction in normal conditions.
Figure 6.
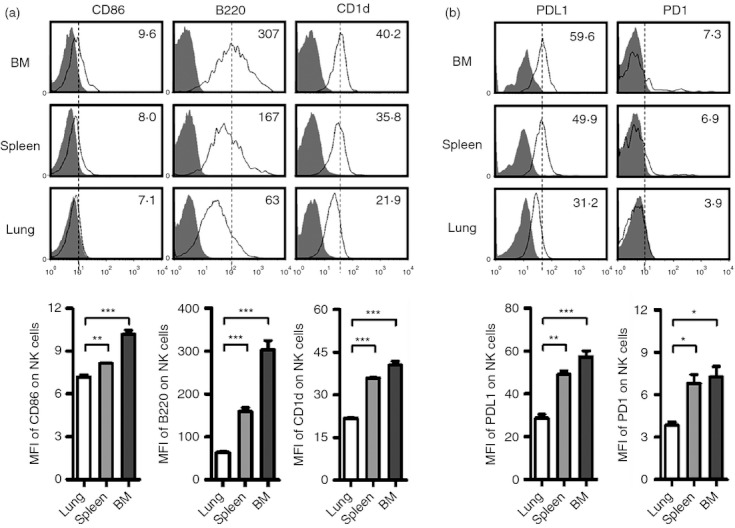
Lung natural killer (NK) cells express lower levels of co-stimulatory molecules. Lymphocytes were isolated from bone marrow (BM), spleen and lung in normal C57BL/6 mice. (a, b) Flow cytometry assay determined the expression of co-stimulatory molecules CD86, B220, CD1d (a), PDL1 and PD1 (b) on NK cells (CD3− NK1.1+). Histograms are representative of three independent experiments using three mice per group, the shadow histogram is the isotype control. The mean fluorescence intensity (MFI) values are shown as mean ± SEM. *P < 0·05; **P < 0·01; ***P < 0·001.
Lung NK cells are activated and gain functions rapidly after respiratory infection
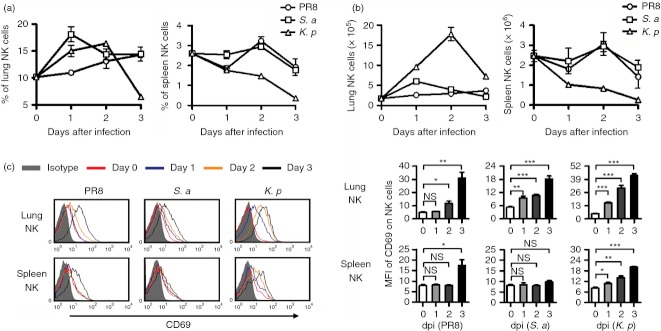
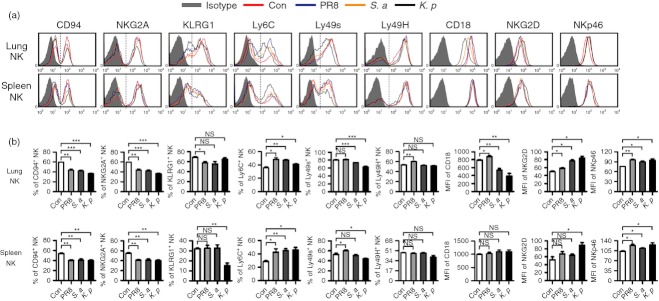
The respiratory tract is a unique environment in which the immune response must be precisely regulated. In health, the respiratory tract must tolerate harmless environmental antigens and commensal bacteria. However, in respiratory infection, the immune system in the lung must be activated rapidly.5 In our study, we found that the frequency and number of NK cells in the lung were increased quickly from day 1 after virus (A/PR8 influenza virus) and bacteria (S. aureus or K. pneumoniae) infection. At the same time, the frequency and number of NK cells in the spleen were decreased (Fig. 7a,b), implying that the increase of lung NK cells may be explained by recruitment from other tissues during infection. Furthermore, the expression of activation marker CD69 on lung NK cells gradually increased from day 0 to day 3 after virus and bacteria infection, and lung NK cells were activated faster than splenic NK cells (Fig. 7c). Furthermore, we also found that these pathogens could up-regulate the expression of the activating receptor NKG2D and NKp46 on lung NK cells, with a greater increase in NKG2D expression after K. pneumoniae infection. At the same time, these pathogens could decrease the proportions of CD94+ and NKG2A+ lung NK cells, and the decreases in CD94+ and NKG2A+ lung NK cells were greater after K. pneumoniae infection. The proportion of KLRG1+ lung NK cells was decreased after influenza virus infection, and the proportion of Ly6C+ lung NK cells was increased after infection with these pathogens. Interestingly, influenza virus infection could increase the proportion of CD18+ and Ly49H+ lung NK cells, but S. aureus and K. pneumoniae infection decreased the proportion of CD18+ and Ly49s+ lung NK cells. In addition, overall, the pulmonary infection of these pathogens could also increase the expression of activating molecules and decrease the expression of inhibitory molecules on splenic NK cells (Fig. 8). Therefore, lung NK cells underwent major changes in the expression of surface molecules and were activated rapidly after respiratory infection, yet respiratory infection could also influence the phenotypes of NK cells in other tissues.
Figure 7.
Lung natural killer (NK) cells expand and are activated rapidly after respiratory infection. C57BL/6 mice were infected intranasally with 0·1 haemagglutination units of influenza A virus (PR8 strain), 1 × 107 colony-forming units (CFU) of Staphylococcus aureus (S. a) or 1 × 107 CFU of Klebsiella pneumoniae (K. p), respectively. At days 0, 1, 2 and 3 post-infection, mice were killed, and lymphocytes were isolated from lung and spleen. (a, b) The changes of the frequency (a) and number (b) of NK cells in the lung and spleen are shown. (c) Flow cytometry assay determined the expression of CD69 on NK cells (CD3− NK1.1+), and the mean fluorescence intensity values are shown as mean ± SEM. Histograms are representative of three independent experiments using three mice per group at each time-point, the shadow histogram is the isotype control. dpi, days post-infection. *P < 0·05; **P < 0·01; ***P < 0·001; NS, not significant.
Figure 8.
Increased natural killer (NK) cell function after respiratory infection. C57BL/6 mice were infected intranasally with 0·1 haemagglutination units of influenza A virus (PR8 strain), 1 × 107 colony-forming units (CFU) of Staphylococcus aureus (S. a) or 1 × 107 CFU of Klebsiella pneumoniae (K. p), respectively. Mice were killed, and lymphocytes were isolated from lung and spleen at day 2 post-infection. (a) Flow cytometry assay determined the expression of CD94, NKG2A, KLRG1, Ly6C, Ly49s, Ly49H, CD18, NKG2D and NKp46 on NK cells (CD3− NK1.1+). Histograms are representative of three independent experiments using three mice per group, the shadow histogram is isotype control. (b) The frequencies or the mean fluorescence intensity values are shown as mean ± SEM. *P < 0·05; **P < 0·01; ***P < 0·001; NS, not significant.
Activation of lung NK cells was more noticeable in K. pneumoniae infection than in influenza virus and S. aureus infection (Fig. 7c), so we next chose the mouse model of K. pneumoniae infection to study whether lung NK cells could gain functions during infection. We found that, accompanying the activation of lung NK cells, expression of CD107a and IFN-γ in lung NK cells was also increased at day 2 after K. pneumoniae infection (Fig. 9a). Furthermore, the expression of CD107a and IFN-γ was increased both in CD27high and CD27low NK cells after K. pneumoniae infection, but CD27high NK cells expressed more CD107a and IFN-γ than CD27low NK cells (Fig. 9b). Hence, lung NK cells can be activated and express functional molecules rapidly in response to harmful pathogens during respiratory infection.
Figure 9.
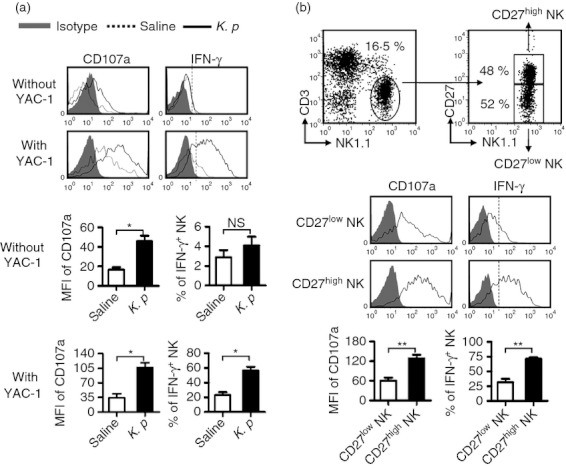
Lung natural killer (NK) cells gain functions rapidly after respiratory infection. C57BL/6 mice were infected intranasally with 1 × 107 colony-forming units (CFU) of K. pneumoniae. At day 2 post-infection, mice were killed, and lymphocytes were isolated from lung and stimulated for 4 hr with YAC-1 cells or not in presence of monensin and anti-CD107a antibody, cells were then surface-stained and intracellular interferon-γ (IFN-γ) was revealed. (a) Flow cytometry assay determined the expression of CD107a and production of IFN-γ on NK cells. The frequencies or the mean fluorescence intensity (MFI) values are shown as mean ± SEM. (b) The MFI values of CD107a on CD27high and CD27low NK cells and the frequencies of IFN-γ+ NK cells in CD27high and CD27low NK cells are shown as mean ± SEM. Histograms are representative of three independent experiments using three mice per group, the shadow histogram is isotype control. *P < 0·05; **P < 0·01; NS, not significant.
Discussion
Lung NK cells display unique phenotypic and functional properties.5 As shown in our study, NK cells made up about 10% of the total resident lymphocytes in the lung and had a more mature phenotype (CD11bhigh CD27low) in mice. However, lung NK cells expressed higher levels of inhibitory receptors (CD94-NKG2A) and lower levels of activating receptors (NKG2D) in homeostasis, implying that lung NK cells can perform their function immediately during infection, but their functions might be suppressed in the unique lung environment in homeostasis. The more mature phenotype and the lower expression of adhesion molecules (CD11a, CD18, CD48, CD62L, CD44 and CD11c) and chemokine receptors (CCR5 and CXCR2) suggest a restricted ability for lung NK cells to migrate, and the battle field of lung NK cells may be restricted to the lungs.
Natural killer cells originate from the BM and go through a complex maturation process that includes the expression of integrin and chemokine receptors,21 the migration from the BM and LN to blood, spleen and liver,22 and the acquisition of tissue-specific effector functions.4,5 The frequency of NK cells in lymphocytes is greatest in the lung among all tissues, and one reason for that is that bronchial epithelial cells can spontaneously produce interleukin-15 which is crucial for the survival of NK cells.23 Surprisingly, in our study, we found that the phenotype of blood NK cells was similar to that of lung NK cells. Two reasons for this are suggested: first, the migration of NK cells from BM to lung goes through blood and second, NK-cell recirculation occurs between the lung and the blood.4 However, the activities of NK cells from the lung and the blood are different. Some studies have shown that lung NK cells have lower cytotoxic capacity than blood NK cells.9, 24
In our study, the number of NK cells in the lung was increased after infection with respiratory pathogen. Previous studies have demonstrated that, within days or hours of infection, NK cells are recruited into the lung from the peripheral blood and become activated to secrete cytokines.6,7 In our study, we also found that, accompanying the increase of lung NK cells, the number of splenic NK cells was decreased during respiratory infection, implying that the increase of lung NK cells may come from peripheral NK-cell recruitment. However, we also found that the expression of CD107a and IFN-γ on lung CD27low NK cells was increased after K. pneumoniae infection, implying that the resident lung NK cells are also activated to proliferate. Hence, the proliferation of resident NK cells may also be responsible for the increase of lung NK cells during respiratory infection. New approaches need to be built to distinguish resident and recruited lung NK cells in vivo, which may help us to clarify this question.
The lung is an organ for air exchange, and various pathogens in the atmosphere enter into the respiratory tract during breathing. The unique function and environment of the lung determine the character of the lung immune system, which must rapidly respond to harmful pathogens.5 The higher frequency of mature NK cells in the lung was possibly developed to meet this requirement. It has been reported that lung NK cells not only respond rapidly to acute pulmonary infection but are also important in regulating chronic infection and inflammation. In our study, we found that lung NK cells were activated rapidly in the early stages of bacterial or viral infections, and their functions were normal. However, the lung immune system must be tolerant to harmless environmental antigens and commensal organisms in homeostasis. Some studies have shown that the functions of NK cells from bronchoalveolar lavage fluid and lung tissue are suppressed.24 In our study, when compared with NK cells from any other tissues, lung NK cells expressed higher levels of inhibitory receptors and lower levels of activating receptors, which also indicated that the activation of lung NK cells may need to overcome a higher threshold of inhibition. Alveolar macrophages that can produce interleukin-10 and transforming growth factor-β to restrain the inflammatory response in the lung may be responsible for this inhibitory effect on the activation of lung NK cells.25
As an important part of the innate immune system, NK cells play critical roles in protecting the host against tumours and pathogens.26,27 However, increasing evidence suggests that NK-cell activation can lead to tissue injury during the inflammatory response. For instance, NK cells activated by poly I:C and d-galactosamine injection can cause overproduction of IFN-γ, which causes damage to hepatocytes,28 and NK cells are also involved in the lung immune injury at the early stage of respiratory syncytial virus infection.29 Inspired by the findings in our study, we may control the extent of NK-cell activation using genetic methods to regulate the expression of activating and inhibitory receptors on NK cells, which may provide a therapy to improve the host's immune response or to control disease caused by excessive NK-cell immune response.
As described above, the unique lung environment needs NK cells to activate rapidly during infection, but the activation of lung NK cells must overcome a higher threshold of inhibition. The mechanisms by which the respiratory immune system regulates the activation of lung NK cells at the early stage of infection deserves further study.
Acknowledgments
This work was supported by the Natural Science Foundation of China (#31021061) and the Ministry of Science & Technology of China (2010CB945303). We thank Prof. Hong Meng (Institute of Basic Medicine, Shangdong Academy of Medical Sciences, China) for providing mouse-adapted influenza A/PR/8/34 strain (H1N1). We thank Prof. Baolin Sun (School of Life Sciences, University of Science and Technology of China) for providing Staphylococcus aureus strain (NCTC-8325). The Klebsiella pneumoniae strain (ATCC-700603) was a gift from the Department of Microbiology, Anhui Medical University.
References
- 1.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–14. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 2.Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol. 2001;31:1900–9. doi: 10.1002/1521-4141(200106)31:6<1900::aid-immu1900>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 4.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128:151–63. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein-Streilein J, Guffee J, Fan W. Locally and systemically derived natural killer cells participate in defense against intranasally inoculated influenza virus. Reg Immunol. 1988;1:100–5. [PubMed] [Google Scholar]
- 7.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003;112:1862–70. doi: 10.1172/JCI18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordignon C, Villa F, Vecchi A, Giavazzi R, Introna M, Avallone R, Mantovani A. Natural cytotoxic activity in human lungs. Clin Exp Immunol. 1982;47:437–44. [PMC free article] [PubMed] [Google Scholar]
- 9.Weissman DN, deShazo RD, Banks DE. Modulation of natural killer cell function by human alveolar macrophages. J Allergy Clin Immunol. 1986;78(4 Pt 1):571–7. doi: 10.1016/0091-6749(86)90073-4. [DOI] [PubMed] [Google Scholar]
- 10.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–52. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 11.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2009;296:L936–46. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Sun R, Wei H, Dong Z, Gao B, Tian Z. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. J Hepatol. 2006;44:446–54. doi: 10.1016/j.jhep.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa E, Nakazawa M, Yoshinari M, Minami M. Role of tumor necrosis factor-related apoptosis-inducing ligand in immune response to influenza virus infection in mice. J Virol. 2005;79:7658–63. doi: 10.1128/JVI.79.12.7658-7663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein-Streilein J, Bennett M, Mann D, Kumar V. Natural killer cells in mouse lung: surface phenotype, target preference, and response to local influenza virus infection. J Immunol. 1983;131:2699–704. [PubMed] [Google Scholar]
- 15.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 16.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–96. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 17.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–8. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–64. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 20.Andoniou CE, Coudert JD, Degli-Esposti MA. Killers and beyond: NK-cell-mediated control of immune responses. Eur J Immunol. 2008;38:2938–42. doi: 10.1002/eji.200838882. [DOI] [PubMed] [Google Scholar]
- 21.Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, Caligiuri MA, Evans SS. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400–8. [PubMed] [Google Scholar]
- 22.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–59. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 23.Ge N, Nishioka Y, Nakamura Y, et al. Synthesis and secretion of interleukin-15 by freshly isolated human bronchial epithelial cells. Int Arch Allergy Immunol. 2004;135:235–42. doi: 10.1159/000081309. [DOI] [PubMed] [Google Scholar]
- 24.Robinson BW, Pinkston P, Crystal RG. Natural killer cells are present in the normal human lung but are functionally impotent. J Clin Invest. 1984;74:942–50. doi: 10.1172/JCI111513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wissinger E, Goulding J, Hussell T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol. 2009;21:147–55. doi: 10.1016/j.smim.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Miller JS. Biology of natural killer cells in cancer and infection. Cancer Invest. 2002;20:405–19. doi: 10.1081/cnv-120001185. [DOI] [PubMed] [Google Scholar]
- 27.Shereck E, Satwani P, Morris E, Cairo MS. Human natural killer cells in health and disease. Pediatr Blood Cancer. 2007;49:615–23. doi: 10.1002/pbc.21158. [DOI] [PubMed] [Google Scholar]
- 28.Hou X, Zhou R, Wei H, Sun R, Tian Z. NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis. Hepatology. 2009;49:940–9. doi: 10.1002/hep.22725. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Zhu H, Sun R, Wei H, Tian Z. Natural killer cells are involved in acute lung immune injury caused by respiratory syncytial virus infection. J Virol. 2012;86:2251–8. doi: 10.1128/JVI.06209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]