Abstract
Methylglyoxal (MG) is a reactive dicarbonyl metabolite formed during glucose, protein and fatty acid metabolism. In hyperglycaemic conditions, increased MG level has been linked to the development of diabetes and its vascular complications at the macrovascular and microvascular levels where inflammation plays a role. To study the mechanism of MG-induced inflammation in vivo, we applied MG locally to healthy mice and used intravital microscopy to investigate the role of endothelial cell adhesion molecules in MG-induced leucocyte recruitment in cremasteric microvasculature. Administration of MG (25 and 50 mg/kg) to the tissue dose-dependently induced leucocyte recruitment at 4·0–5·5 hr, with 84–92% recruited cells being neutrophils. Such MG treatment up-regulated the expression of endothelial cell adhesion molecules P-selectin, E-selectin, intercellular adhesion molecule-1, but not vascular cell adhesion molecule-1. Activation of the nuclear factor-κB signalling pathway contributed to MG-induced up-regulation of these adhesion molecules and leucocyte recruitment. The role of the up-regulated endothelial cell adhesion molecules in MG-induced leucocyte recruitment was determined by applying specific functional blocking antibodies to MG-treated animals and observing changes in leucocyte recruitment parameters. Our data demonstrate that the up-regulation of P-selectin, E-selectin and intercellular adhesion molecule-1 contributes to the increased leucocyte rolling flux, reduced leucocyte rolling velocity, and increased leucocyte adhesion, respectively. Our results reveal the role of endothelial cell adhesion molecules in MG-induced leucocyte recruitment in microvasculature, an inflammatory condition related to diabetic vascular complications.
Keywords: adhesion molecules, endothelial cells, leucocyte recruitment, methylglyoxal, nuclear factor-κB
Introduction
Vascular dysfunction is a main feature of diabetic complications and involves both micro- and macro-angiopathy.1 The progression of diabetic vascular complications results in cardiovascular disease, chronic renal failure, retinal damage, neuropathy and poor wound healing. The pathological changes in the macro- and micro-vasculature in diabetes are linked to inflammation.2–5 In blood vessels and tissues, an abnormal elevation of the highly reactive glycolytic by-product methylglyoxal (MG) increases oxidative stress and the generation of advanced glycation end-products,6–8 which lead to vascular inflammation.9 However, the mechanism of vascular inflammation induced by MG itself in diabetes and diabetic vascular complications is unclear.
Methylglyoxal is a reactive carbonyl metabolite formed during glucose, protein and fatty acid metabolism.6 In physiological conditions, MG is endogenously produced by various metabolic pathways.10,11 It is one of the most powerful glycation agents of proteins and other important cellular components such as DNA and key enzymes. Increased MG levels have been reported in diabetic patients and animals.12,13 Besides its strong glycation capability, an increased MG level is involved in the inflammatory response in diabetes by up-regulating the expression of inflammatory mediators.14–17 An increased plasma MG level is related to the expression of cytokines in patients with diabetic nephropathy and patients with type 2 diabetes mellitus.12,14 Yamawaki et al.18 showed that MG is a strong inducer of inflammation in human endothelial cells. It has also been shown that MG activates various signalling pathways such as nuclear factor-κB (NF-κB), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways in endothelial cells and leucocytes.18–20 The activation of these pathways further triggers downstream inflammatory cascade events,18,21,22 such as the production of cytokines and chemokines and up-regulation of cell adhesion molecules, which mediate leucocyte recruitment. Hence, elevated MG may play a role in inducing inflammation in patients with diabetes.
Leucocyte recruitment from the blood stream into the extravascular space is essential for developing an appropriate inflammatory response to injury or infection. In many tissues, this process follows a well-defined cascade of events, beginning with the capture of free-flowing blood leucocytes to the vessel wall, and followed by rolling and adhesion of leucocytes to the inflamed endothelium and, then by leucocyte transendothelial migration. Studies have shown that various cytokines, chemokines and adhesion molecules are essential for this process.23,24 In the initial phase of the adhesion cascade, leucocyte rolling is mediated by members of the selectin family (L-selectin on leucocytes, and P-selectin and E-selectin on activated endothelial cells), and adhesion by intercellular adhesion molecule-1 (ICAM-1), which binds to β2 integrins, such as lymphocyte function-associated antigen 1 and Mac-1 on the leucocyte membrane. This latter interaction results in the arrest and firm adhesion of the leucocytes to the endothelium, and is required for the subsequent leucocyte transendothelial migration. Although the process of leucocyte–endothelial cell interaction has been extensively investigated, the mechanism of MG-induced inflammatory response in macrovasculature and microvasculature in diabetic vascular complications is not clear. The questions of whether and which adhesion molecules are up-regulated and which particular step in the recruitment process is affected by their up-regulation remain unanswered.
Previous studies demonstrated that the expression of ICAM-1, E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in the blood vessels is increased in spontaneously hypertensive rats.25,26 The serum concentrations of VCAM-1 and ICAM-1 are increased in patients with systemic vascular inflammation, type 2 diabetes and cardiovascular diseases.27–30 As these diseases are related to hyperglycaemia, inflammation and increased MG formation, it is reasonable to infer that there is a potential relationship between MG production, endothelial cell adhesion molecule expression and inflammation. In this study, we used intravital microscopy to investigate the effects of MG on leucocyte recruitment and the role of endothelial cell adhesion molecules P-selectin, E-selectin and ICAM-1 in MG-induced leucocyte recruitment. We evaluated the changes in recruitment parameters (leucocyte rolling, adhesion and emigration) in the microvasculature of the cremaster muscle at different time-points after intrascrotal injection of different doses of MG. We also explored the involvement of the NF-κB signalling pathway in this process. Based on the time–course data, we established an MG-induced inflammation model and investigated the mechanism of MG-induced leucocyte recruitment. The expression of P-selectin, E-selectin, ICAM-1 and VCAM-1 after MG treatment was determined by immunohistochemistry. The role of each up-regulated adhesion molecule was determined by using functional blocking antibodies. The role of NF-κB involved in MG-induced leucocyte recruitment was investigated by using a specific inhibitor.
Materials and methods
Animals
Male C57BL/6 mice between 8 and 12 weeks old were obtained from Charles River Canada (Saint-Constant, QC, Canada). All animal protocols were approved by the University of Saskatchewan Committee on Animal Care and Supply, and met the standards of the Canadian Council on Animal Care.
Cell culture
EA.hy926 cell, a hybrid human umbilical vein endothelial cell line, was obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured in Dulbecco's modified Eagle's medium (Cellgro, Manassas, VA) with 10% fetal bovine serum (Hyclone, Logan, UT) and 100 U/ml penicillin–streptomycin (Amresco, Solon, OH) with 5% CO2 and maximal humidity at 37°. Cells between passage 3 and 6 were used for the experiments.
MG administration
The MG (Sigma-Aldrich, Oakville, ON, Canada) was dissolved in saline. To give MG doses of 0, 1, 5, 25 and 50 mg/kg, 200 μl of the MG solution was injected into the right side of the scrotum using a 30-G needle beneath the scrotum skin without puncturing any underlying tissue (intrascrotal injection). At various time-points after the MG injection, the mice were prepared for intravital microscopy (described below). Intrascrotal injection of 500 ng recombinant murine tumour necrosis factor-α (TNF-α; R & D Systems, Minneapolis, MN) in 200 μl saline was used as positive control for immunohistochemistry studies.
MG assay
Methylglyoxal levels in plasma and cremaster muscle tissue were measured by a specific and sensitive HPLC method as described previously.31,32 Briefly, MG was derivatized with o-phenylenediamine (o-PD) to specifically form 2-methylquinoxaline. Samples were incubated in the dark for 24 hr with PCA solution (1 m HClO4, 4 mm Na2S2O5, 0·1 mm EDTA) and 100 mm o-PD at room temperature. 2-Methylquinoxaline and quinoxaline internal standard (5-methylquinoxaline) were quantified on a Hitachi D-7000 HPLC system (Hitachi, Ltd., Mississauga, ON, Canada) via a Nova-Pak® C18 column (3·9 × 150 mm, and 4-μm particle diameter; Waters, Milford, MA). The protein concentrations in the samples were determined by bicinchoninic acid assay kit (Sigma-Aldrich).
Intravital microscopy
Mice were anaesthetized by intraperitoneal injection of a mixture of 10 mg/kg xylazine (Bayer Inc., Toronto, ON, Canada) and 200 mg/kg ketamine hydrochloride (Bioniche Inc., Belleville, ON, Canada). The right jugular vein was cannulated for the administration of additional anaesthetics and the antibodies. An incision was made in the scrotal skin to expose the left cremaster muscle, which was then carefully separated from the associated fascia. A lengthwise incision was made on the ventral surface of the cremaster muscle. The testicle and epididymis were separated from the underlying muscle and reintroduced into the abdominal cavity. The muscle was then spread out over an optically clear viewing pedestal, secured along the edges with 5-0 suture, and superfused with 37° bicarbonate buffered saline (pH 7·4). The cremaster microcirculation was observed on a TV monitor through an intravital microscope (BX61WI; Olympus, Tokyo, Japan) using 10 × eyepieces and a 20 × objective lens. Single unbranched venules (25–35 μm in diameter) were selected for study and images of the microcirculation were recorded using a video camera (model DXC-990, Sony, Tokyo, Japan) and video recorder (model LRH-890; LG, Seoul, South Korea). To minimize variability, the same section of cremasteric venule was observed throughout the experiment. The number of rolling, adherent and emigrated leucocytes was determined offline during video playback analysis. Rolling leucocytes were defined as cells moving at a velocity less than that of erythrocytes within a given vessel. The flux of rolling cells was measured as the number of rolling cells passing a given point in the venule per minute. The rolling velocity was determined as 100-μm length of distance on the venule divided by the average time that the first 20 rolling cells covered this distance at the recording time-point. A leucocyte was considered to be adherent if it remained stationary for ≥ 30 seconds, and total leucocyte adhesion was quantified as the number of adherent cells within a 100-μm length of venule in 5 min. Leucocyte emigration was defined as the number of cells in the extravascular space within an area of 443 × 286 μm2 (two fields of view on a TV monitor) on both sides of the venule. Only cells adjacent to and clearly outside the vessel under study were counted as emigrated.33
Histological examination
Haematoxylin and eosin (H & E) staining: H & E staining of paraffin sections was used to determine the subtypes of emigrated leucocytes. After intravital microscopy, the cremaster muscle was collected and fixed for 16 hr in 4% paraformaldehyde (Sigma-Aldrich) in PBS solution (pH 7·4). After fixation, the tissue was processed by an automatic vacuum tissue processor (model RVG/1; Belair, Springfield, NJ) for dehydration, clearing and infiltration with embedding medium. The tissue was then embedded into paraffin blocks and sliced at 5-μm thickness by a microtome. H & E staining was carried out as previously described.34 After H & E staining, cremasteric venules (25–35 μm diameter) were selected under a microscope, the emigrated cells in the same area (443 × 286 μm2) as in the intravital microscopy experiment were observed and counted. The subtypes of leucocytes recruited in the extravascular space were determined by their morphology under the microscope.
Immunohistochemistry: Immunohistochemistry of frozen sections of cremaster muscle was used to determine the expression of adhesion molecules. After intravital microscopy, the cremaster muscle was collected and fixed for 16 hr by 4% buffered paraformaldehyde solution. After fixation, the tissue was dehydrated using hypertonic sucrose solution (10% 4 hr, 15% 4 hr, and 30% 16 hr). Then the tissue was embedded in OCT compound and sectioned on a cryostat microtome (model HM 500; Microm, Walldorf, Germany) at 7 μm. The blocks were kept at −70° until use.
Tissue slices were mounted on microscope slides and washed in TBST (50 mm Tris–HCl, 150 mm NaCl, 0·1% Triton X-100, pH 7·4) twice (10 min each) for permeabilization, and then in TBS (50 mm Tris–HCl, 150 mm NaCl, pH 7·4) three times. The sections were immersed in 0·3% (v/v) H2O2 in TBS for 15 min to remove the endogenous peroxidase activity. After washing twice with TBS, blocking solution [10% goat serum (Abcam, Cambridge, MA) plus 5% BSA in TBS] was added for 2 hr. After blocking, sections were incubated for 16 hr in a humidity chamber at 4° with diluted primary antibodies: anti-E-selectin (UZ6; Abcam) at 1 : 100, anti-P-selectin (polyclonal; LifeSpan BioSciences, Seattle, WA) at 1 : 50, anti-ICAM-1 (YN1/1.7.4; Abcam) at 1 : 100 or anti-VCAM-1 (MVCAM.A (429); Abcam) at 1 : 100. After washing with TBS three times (10 min each), the diluted secondary antibodies horseradish peroxidase-conjugated goat anti-rabbit (Abcam) at 1 : 200 and horseradish peroxidase-conjugated goat anti-rat (Abcam) at 1 : 200 were applied for 1 hr in a humidity chamber at room temperature. Slides were washed with TBS three times (10 min each), developed with diaminobenzidine chromogen for 3 min, and rinsed in distilled water for 5 min. Coverslips were mounted with permanent mounting medium.
Western blotting
After treatment with PBS (control), MG or TNF-α, EA.hy926 cells were harvested and lysed on ice for 30 min in RIPA buffer (50 mm Tris–HCl pH 8·0, 150 mm NaCl, 1% nonidet P-40, 0·5% sodium deoxycholate, 0·1% SDS and protease inhibitors cocktail). The lysate was centrifuged at 10 000 g for 10 min, and the supernatant was collected, mixed with 4× sample loading buffer (200 mm Tris–HCl pH 6·8, 50% glycerol, 2% SDS, 20% β-mercaptoethanol, 0·04% bromophenol blue), boiled for 5 min and centrifuged at 10 000 g for 5 min before loading. The same amount of proteins in cell lysates were separated on 7·5% SDS–PAGE, electrotransferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA), blocked with 5% non-fat milk in TBS-Tween buffer for 1·5 hr at room temperature, and incubated overnight at 4° with the primary antibodies against P-selectin (1 : 1000; Lifespan BioSciences), E-selectin (1 : 1000; Abcam), ICAM-1 (1 : 1000; Abcam), and β-actin (1 : 2000; Santa Cruz, CA), and then incubated with horseradish peroxidase-conjugated secondary antibody (Abcam) for 1 hr at room temperature. After extensive washing, the bands were visualized with enhanced chemiluminescence reagents (GE Healthcare Life Sciences, Princeton, NJ) and exposed to X-ray film (Kodak scientific imaging film, ON, Canada).
Functional blocking study
Mice were injected with MG and prepared for intravital microscopy as above. The functional blocking antibody was injected intravenously 5 min after the start of intravital microscopy. The antibodies were: anti-E-selectin antibody (9A9, 100 μg/mouse; a gift from Dr Paul Kubes, University of Calgary, AB, Canada), anti-P-selectin antibody (RB40.34, 25 μg/mouse; BD Pharmingen, San Jose, CA), anti-ICAM-1 antibody (YN1/1.7.4, 100 μg/mouse; eBioscience, San Diego, CA), rat anti-mouse negative control IgG1 (for E-selectin and P-selectin; BD Pharmingen) and rat anti-mouse negative control IgG2b (for ICAM-1; eBioscience).
Administration of NF-κB inhibitor
BAY 11-7082 (Sigma-Aldrich), a specific NF-κB inhibitor, was first dissolved in DMSO as a 30 mg/ml stock solution, the appropriate amount of which was dissolved in 0·4 ml saline and injected into the animal at 20 mg/kg intraperitoneally 30 min before MG administration. The same concentration of DMSO was used in the vehicle control group.
Statistical analysis
Data are expressed as mean ± SEM from at least three independent experiments. Statistical differences between mean values in two groups were analysed by Student's t-test, and the differences among more than two groups were analysed by one-way analysis of variance and Tukey's post-hoc test. P < 0·05 was considered statistically significant.
Results
MG levels in plasma and cremaster muscle after exogenous MG administration
We measured MG levels in plasma and cremaster muscle 4 hr after MG intrascrotal injection. Table 1 shows that as MG dose increased (0–50 mg/kg), the mean MG levels in plasma dose-dependently increased from 0·934 to 1·660 μm, and the mean MG levels in the local tissue increased from 0·999 nmol/mg protein to 3·878 nmol/mg protein. Local injection of MG significantly increased MG levels in plasma and local tissue in a dose-dependent manner. The MG levels in plasma and tissue in our model are consistent with those in previously established acute MG-treated animal models.35,36
Table 1.
The levels of methylglyoxal (MG) in plasma and local tissue
| Amount of MG detected by HPLC | ||
|---|---|---|
| MG doses | Plasma (μm) | Cremaster muscle (nmol/mg protein) |
| 0 mg/kg | 0·934 ± 0·055 | 0·999 ± 0·040 |
| 1 mg/kg | 1·051 ± 0·061 | 1·476 ± 0·135* |
| 5 mg/kg | 1·170 ± 0·061* | 2·494 ± 0·007* |
| 25 mg/kg | 1·486 ± 0·109* | 3·149 ± 0·172* |
| 50 mg/kg | 1·660 ± 0·096* | 3·878 ± 0·166* |
The MG levels in plasma and cremaster muscle were analysed by HPLC after 4-hr MG local treatment. The values are mean ± SEM (n = 3).
P < 0·05 compared with saline-treated control group (0 mg/kg).
Dose–response effects of MG on leucocyte recruitment
To determine the effect of a local MG increase on leucocyte recruitment in microvasculature, we examined leucocyte recruitment after intrascrotal injection of various doses of MG (1, 5, 25 and 50 mg/kg). Figure 1 shows leucocyte recruitment in cremaster muscle at 4·0–5·5 hr after local administration of MG. In response to increasing doses of MG, leucocyte rolling flux did not increase until the dose of MG reached 25 mg/kg, and leucocyte rolling velocity was dose-dependently decreased when MG was 5 mg/kg or higher. The adhesion and emigration of leucocytes were increased in an MG dose-dependent manner. As the MG dose increased, the leucocyte adhesion and emigration increased from 2 cells to > 10 cells, and from 0 to > 8 cells, respectively. Low-dose MG treatment at 1 or 5 mg/kg showed no significant statistical change compared with the saline control group. For 25 and 50 mg/kg MG treatment groups, significant differences were always observed on rolling flux, rolling velocity, adhesion and emigration. These results indicate that MG induces a dose-dependent increase of leucocyte recruitment.
Figure 1.
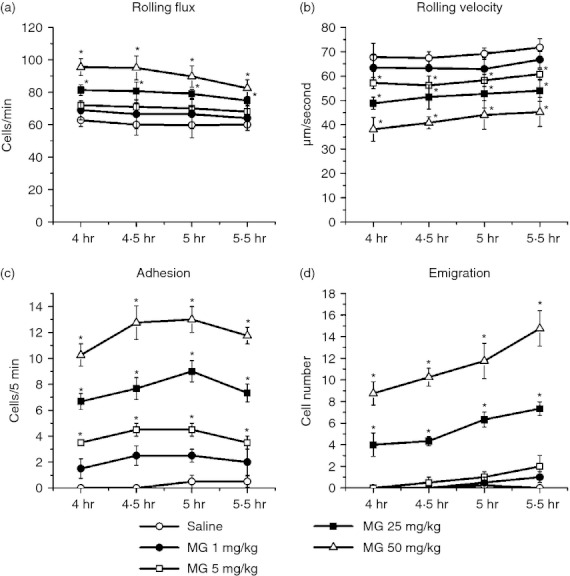
Dose–response effect of methylglyoxal (MG) on leucocyte recruitment in cremasteric postcapillary venules. MG at different doses (dissolved in 200 μl saline) was injected intrascrotally, and the mouse cremaster muscle was prepared for intravital microscopy at 4 hr. Leucocyte rolling flux (a), rolling velocity (b), number of adherent leucocytes (c) and the number of emigrated leucocytes (d) were determined at 4·0–5·5 hr after MG treatment. Values are means ± SEM (n = 4). *, P < 0·05 compared with saline-treated control group.
The time–course of leucocyte recruitment after MG treatment
To investigate the kinetics of leucocyte recruitment after MG treatment, we examined leucocyte recruitment 4, 8, 16 or 24 hr after 25 or 50 mg/kg MG. The results showed that the effects of MG treatment on leucocyte recruitment peaked at 8 hr (Fig. 2). For both 25 and 50 mg/kg treatment groups, the lowest rolling velocity, and highest adhesion and emigration, were all observed in 8 hr. After 8 hr, the rolling velocity increased, and the adhesion and emigration decreased, towards the untreated level. These data indicate that MG treatment induces rapid leucocyte recruitment in local tissue, and the peak response occurs at 8 hr.
Figure 2.
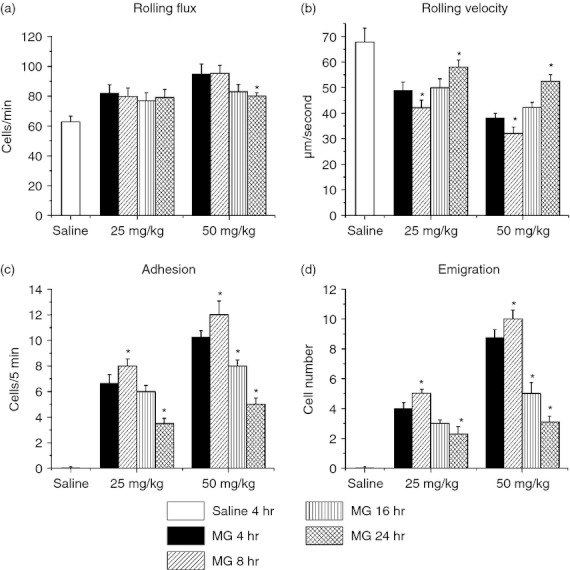
Time–course effect of methylglyoxal (MG) on leucocyte recruitment in cremasteric postcapillary venules. MG, 25 or 50 mg/kg (dissolved in 200 μl saline), was injected intrascrotally, and the mouse cremaster muscle was prepared for intravital microscopy at 4, 8, 16 or 24 hr. Leucocyte rolling flux (a), rolling velocity (b), number of adherent leucocytes (c) and the number of emigrated leucocytes (d) were determined. The 4-hr saline group is shown as the control. Values are means ± SEM (n = 3). *P < 0·05 compared with the 4-hr MG treatment group.
Neutrophils predominantly recruited by acute MG treatment
To determine whether MG induces a typical acute inflammation response in cremaster muscle, we measured the percentage of neutrophils and other subtypes of leucocytes in emigrated cells 5·5 hr after the intrascrotal injection of 25 or 50 mg/kg MG. The majority (∼ 84–92%) of the emigrated leucocytes were neutrophils, ∼ 7–14% were lymphocytes and monocytes, while eosinophils and basophils accounted for < 2% (Fig. 3 and Table 2). Our results reveal that MG local treatment causes a typical, neutrophil-dominant, acute inflammation response in the cremaster muscle.
Figure 3.

Haematoxylin & eosin (H & E) staining of cremaster muscle sections. After 5·5 hr of methylglyoxal (MG) or saline treatment, the cremaster muscles were collected and processed as described in the Materials and methods. After H & E staining, cremasteric venules (25–35 μm diameter) and adjacent tissues were examined under the microscope, and the emigrated leucocytes in the same area as intravital microscopy were counted (Table 2). The subtypes of leucocytes in the extravascular space were determined by their morphology (magnification: 400×).
Table 2.
The subtypes and percentage of recruited leucocytes after methylglyoxal (MG) treatment
| The percentage of each subtype of recruited leucocytes | ||||
|---|---|---|---|---|
| Dose and time of MG treatment | Eosinophils (%) | Basophils (%) | Neutrophils (%) | Lymphocytes/Monocytes (%) |
| 25 mg/kg, 5·5 hr | 0·38 | 0·49 | 91·78 | 7·34 |
| 50 mg/kg, 5·5 hr | 0·91 | 0·85 | 84·24 | 14·00 |
After Haematoxylin & Eosin staining, the emigrated leucocytes in the same area as intravital microscopy experiment were examined and counted. The subtypes of cells in the extravenular space were determined by their morphology under microscope. For each treatment group, > 500 leucocytes in extravascular space were counted.
The acute inflammation model induced by MG
We found that MG treatment for 8 hr or longer increased tissue damage such as scrotum swelling and interstitial tissue stickiness, which were more apparent at 16 and 24 hr, and that 25 mg/kg MG local treatment for 4·0–5·5 hr induces a typical acute inflammation response with a high percentage of recruitment cells being neutrophils (Fig. 1 and Table 2). In the following experiments, we chose 25 mg/kg MG local treatment for 4 hr to study the role of endothelial cell adhesion molecules in MG-induced leucocyte recruitment.
Up-regulation of endothelial adhesion molecule expression by MG treatment
By using immunohistochemistry, we determined the expression of endothelial cell adhesion molecules P-selectin, E-selectin, ICAM-1 and VCAM-1 on cremasteric postcapillary venules. After 25 mg/kg MG treatment for 4·0–5·5 hr, the expression of P-selectin (Fig. 4), E-selectin (Fig. 5) and ICAM-1 (Fig. 6) were increased, whereas VCAM-1 expression did not change (Fig. 7).
Figure 4.
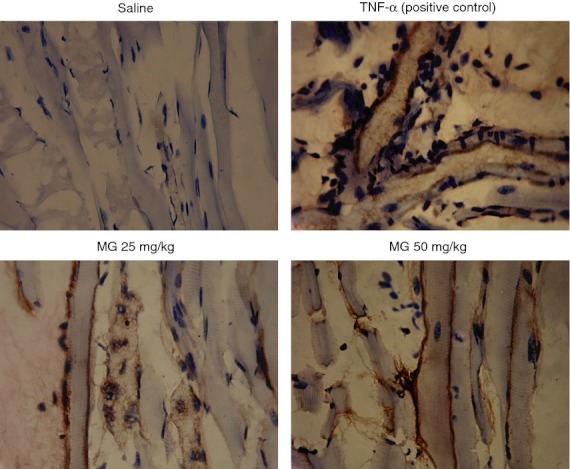
Immunohistochemistry staining of P-selectin in cremasteric endothelium after methylglyoxal (MG) treatment. MG at 25 or 50 mg/kg (dissolved in 200 μl saline) was injected intrascrotally for 5·5 hr, and the mouse cremaster muscles were collected and processed as described in the Materials and methods. The brown staining on the endothelial cells of cremasteric postcapillary venules reveals the expression of P-selectin. Saline and 500 ng tumour necrosis factor-α (TNF-α) were used as negative and positive controls, respectively (magnification: 400×).
Figure 5.
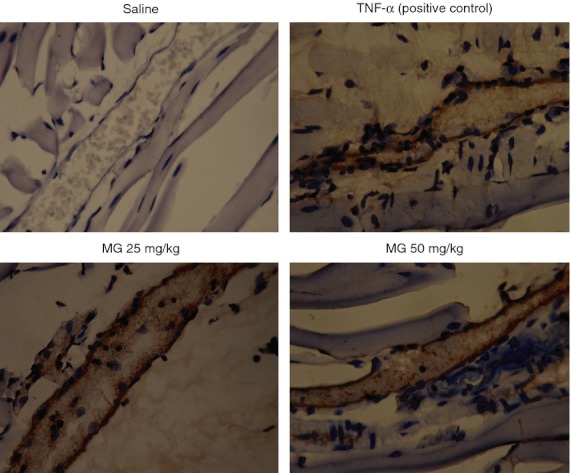
Immunohistochemistry staining of E-selectin on cremasteric endothelium after methylglyoxal (MG) treatment. MG at 25 or 50 mg/kg (dissolved in 200 μl saline) was injected intrascrotally for 5·5 hr, and the mouse cremaster muscles were collected and processed as described in the Materials and methods. The brown staining on the endothelial cells of cremasteric postcapillary venules reveals the expression of E-selectin. Saline and 500 ng tumour necrosis factor-α (TNF-α) were used as negative and positive controls, respectively (magnification: 400×).
Figure 6.
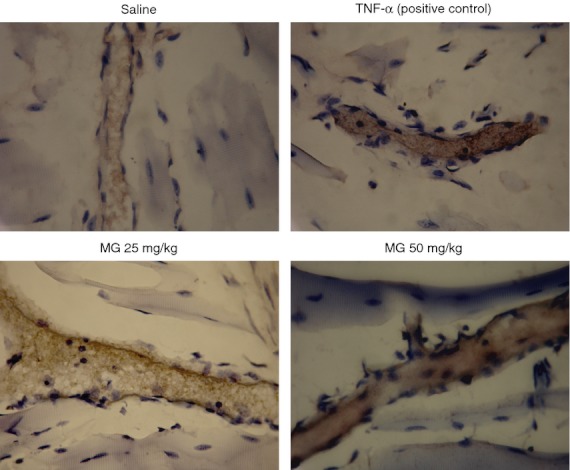
Immunohistochemistry staining of intracellular adhesion molecule 1 (ICAM-1) on cremasteric endothelium after methylglyoxal (MG) treatment. MG at 25 or 50 mg/kg (dissolved in 200 μl saline) was injected intrascrotally for 5·5 hr, and the mouse cremaster muscles were collected and processed as described in the Materials and methods. The brown staining on the endothelial cells of cremasteric postcapillary venules reveals the expression of ICAM-1. Saline and 500 ng tumour necrosis factor-α (TNF-α) were used as negative and positive controls, respectively (magnification: 400×).
Figure 7.
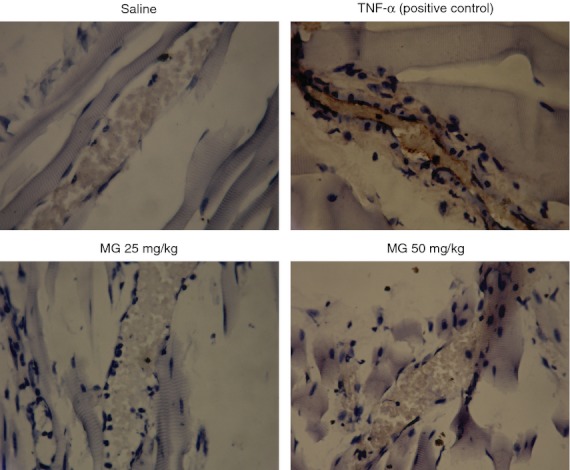
Immunohistochemistry staining of vascular cell adhesion molecule 1 (VCAM-1) on cremasteric endothelium after methylglyoxal (MG) treatment. MG at 25 or 50 mg/kg (dissolved in 200 μl saline) was injected intrascrotally for 5·5 hr, and the mouse cremaster muscles were collected and processed as described in the Materials and methods. Saline and 500 ng tumour necrosis factor-α (TNF-α) were used as negative and positive controls, respectively. The brown staining on the endothelial cells of cremasteric postcapillary venules reveals the expression of VCAM-1 in the TNF-α-treated group. Both MG treatment groups show no apparent change of VCAM-1 expression after 5·5 hr MG treatment compared with saline control group (magnification: 400×).
The direct activation effect of MG on endothelial cells
To investigate whether MG is directly activating endothelial cells, we tested the effect of MG on the expression of endothelial adhesion molecules using an in vitro system. Cultured EA.hy926 endothelial cells were treated with 100 μm MG, PBS or 20 ng/ml TNF-α for 4 hr, and the expression of P-selectin, E-selectin and ICAM-1 was determined by Western blot. Figure 8 shows that MG treatment increased the expression of these adhesion molecules in cultured EA.hy926 endothelial cells, demonstrating that the effect of MG was directly on the endothelial cells.
Figure 8.
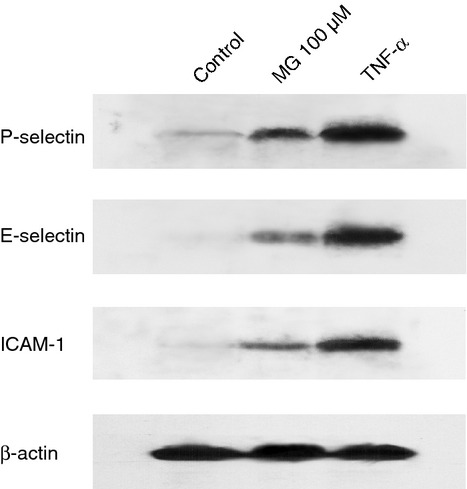
Methylglyoxal (MG)-induced expression of P-selectin, E-selectin and intracellular adhesion molecule 1 (ICAM-1) in cultured endothelial cells. EA.hy926 endothelial cells were cultured to 90% confluence and treated with PBS (negative control), 100 μm MG or 20 ng/ml tumour necrosis factor-α (TNF-α; positive control) for 4 hr. The expression of P-selectin, E-selectin and ICAM-1 in cell lysates was determined by Western blot.
The role of P-selectin, E-selectin and ICAM-1 in MG-induced leucocyte recruitment
To investigate the role of P-selectin, E-selectin and ICAM-1 during MG-induced leucocyte recruitment, we administered the functional blocking antibodies against these adhesion molecules 4 hr after MG treatment, and determined leucocyte rolling velocity, rolling flux, adhesion and emigration by intravital microscopy over the next 1·5 hr.
Suppression of MG-induced increase of leucocyte rolling flux by P-selectin blockade
Figure 9 illustrates that after anti-P-selectin antibody injection, the leucocyte rolling flux dropped to 0, indicating that the rolling of leucocytes on the venular wall depends on the functions of P-selectin. As there were no leucocytes rolling on the venular wall after P-selectin blockade, the rolling velocity was unable to be determined, and the further increases of leucocyte adhesion and emigration after MG were subsequently prevented (Fig. 9c,d). These data indicate that MG-induced P-selectin up-regulation results in more leucocytes rolling on the endothelium, which increases the interactions between leucocytes and endothelial cells.
Figure 9.
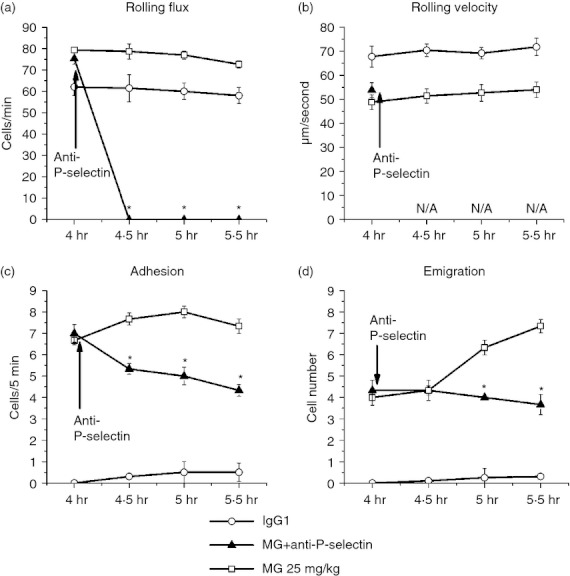
Effect of P-selectin blocking antibody on leucocyte recruitment in cremasteric postcapillary venules after methylglyoxal (MG) treatment. Mice were treated with MG (25 mg/kg) as described in the legend of Fig. 1, and leucocyte recruitment in cremaster muscle was measured at 4·0–5·5 hr using intravital microscopy. After baseline measurement at 4 hr, 25 μg anti-P-selectin antibody was infused intravenously (arrow). The leucocyte rolling flux (a), rolling velocity (b), number of adherent leucocytes (c) and the number of emigrated leucocytes (d) after antibody treatment were determined. Values are means ± SEM (n = 3). *P < 0·05 compared with 25 mg/kg MG-treated group without antibody.
Restoration of MG-induced reduction of leucocyte rolling velocity by E-selectin blockade
The intravital microscopy data demonstrate that anti-E-selectin antibody treatment significantly blocked the MG-induced decrease in rolling velocity, whereas the rolling flux showed no significant change compared with the animals treated with the same dose of MG only (Fig. 10). After blocking the functions of E-selecin, the rolling velocity increased back to the normal level and the adhesion and emigration were significantly decreased. Increasing the leucocyte rolling velocity reduces the time for leucocyte–endothelial cell interactions and decreases the subsequent increase of adhesion and emigration of leucocytes after MG treatment (Fig. 10c,d). This result indicates that after MG treatment, the expression of E-selectin is important for the decrease in leucocyte rolling velocity.
Figure 10.
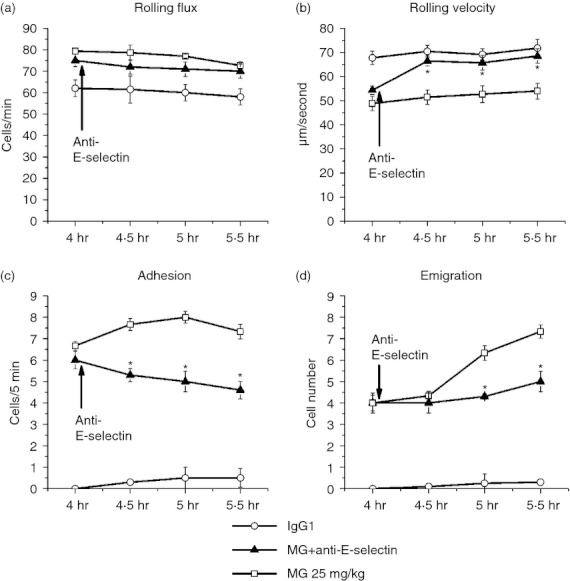
Effect of E-selectin blocking antibody on leucocyte recruitment in cremasteric postcapillary venules after methylglyoxal (MG) treatment. Mice were treated with MG (25 mg/kg) as described in the legend of Fig. 1, and leucocyte recruitment in cremaster muscle was measured at 4·0–5·5 hr using intravital microscopy. After baseline measurement at 4 hr, 100 μg anti-E-selectin antibody was infused intravenously (arrow). The leucocyte rolling flux (a), rolling velocity (b), number of adherent leucocytes (c) and the number of emigrated leucocytes (d) after antibody treatment were determined. Values are means ± SEM (n = 3). *P < 0·05 compared with 25 mg/kg MG-treated group without antibody.
Decreased MG-induced leucocyte adhesion by ICAM-1 blockade
After anti-ICAM-1 antibody administration, the adhesion cell number induced by MG dropped from ∼ 7 to < 3, while the rolling velocity and rolling flux were not significantly changed (Fig. 11). As adhesion was significantly decreased by anti-ICAM-1 treatment, the increased leucocyte emigration induced by MG was suppressed, and the emigration cell number remained ∼ 4. These data indicate that ICAM-1 plays an important role in MG-induced adhesion of leucocytes to endothelial cells, and may be crucial for MG-induced leucocyte emigration.
Figure 11.
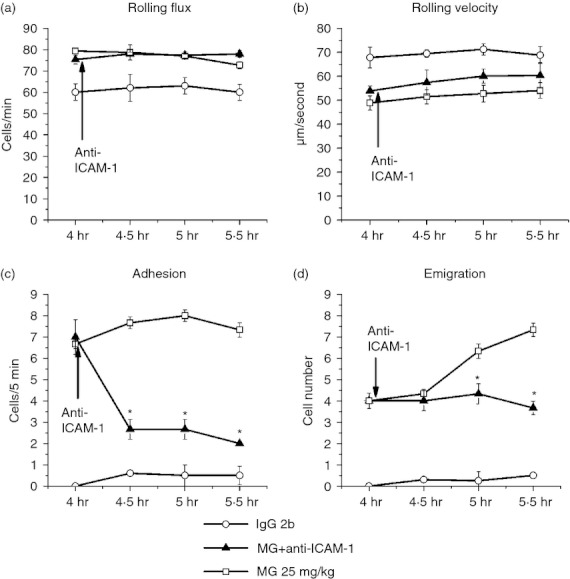
Effect of intracellular adhesion molecule 1 (ICAM-1) blocking antibody on leucocyte recruitment in cremasteric postcapillary venules after methylglyoxal (MG) treatment. Mice were treated with MG (25 mg/kg) as described in the legend of Fig. 1, and leucocyte recruitment in cremaster muscle was measured at 4·0–5·5 hr using intravital microscopy. After baseline measurement at 4 hr, 100 μg anti-ICAM-1 antibody was infused intravenously (arrow). The leucocyte rolling flux (a), rolling velocity (b), number of adherent leucocytes (c) and the number of emigrated leucocytes (d) after antibody treatment were determined. Values are means ± SEM (n = 3). *P < 0·05 compared with 25 mg/kg MG-treated group without antibody.
The contribution of NF-κB signalling pathway to MG-induced adhesion molecule up-regulation and leucocyte recruitment
To determine whether the NF-κB pathway is involved in MG-induced adhesion molecule up-regulation, we applied a specific NF-κB inhibitor, BAY 11-7082, 30 min before MG administration to observe the changes on adhesion molecule expression and leucocyte recruitment. Figure 12 shows that BAY 11-7082 pre-treatment significantly suppressed the expression of MG-induced endothelial adhesion molecules P-selectin, E-selectin and ICAM-1, and attenuated MG-induced leucocyte recruitment. The immunostaining of P-selectin, E-selectin and ICAM-1 were was lighter in the 25 mg/kg MG + NF-κB inhibitor-treated group than in the 25 mg/kg MG-treated group (Fig. 12a). The MG-induced leucocyte adhesion and emigration were also significantly reduced by BAY 11-7082 treatment (Fig. 12d,e). Our data indicate that activation of the NF-κB pathway is involved in MG-induced endothelial adhesion molecule up-regulation and leucocyte recruitment.
Figure 12.
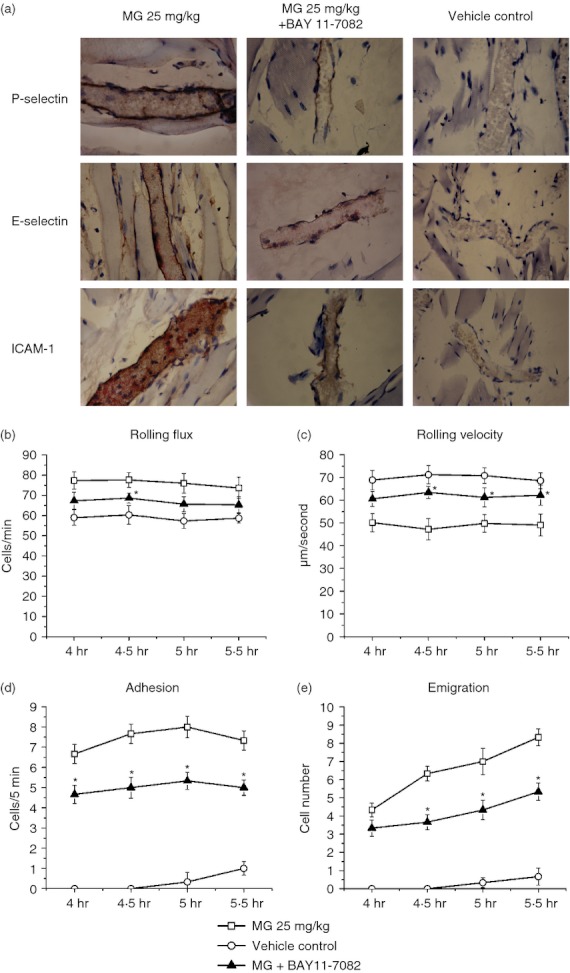
Effect of nuclear factor-κB (NF-κB) inhibition on methylglyoxal (MG) -induced endothelial adhesion molecule expression and leucocyte recruitment. BAY11-7082 at 20 mg/kg was injected to the mice 30 min before 25 mg/kg MG administration. The vehicle control group was injected with the same concentration of the solvent for BAY11-7082. After 4-hr MG injection, the mouse cremaster muscle was prepared for intravital microscopy. The muscle samples were then collected and processed for immunostaining after 1·5 hr intravital microscopy. The brown staining on the endothelial cells of cremasteric postcapillary venules reveals the expression levels of P-selectin, E-selectin and intracellular adhesion molecule 1 (ICAM-1; magnification: 400×) in the upper, middle and lower panels, respectively (a). The inhibitory effects of BAY11-7082 on MG-induced leucocyte rolling flux (b), rolling velocity (c), the number of adherent leucocytes (d) and the number of emigrated leucocytes (e) were determined. Values in (b) to (e) are means ± SEM (n = 3). *P < 0·05 compared with 25 mg/kg MG treatment group without BAY 11-7082.
Discussion
In the present study, using intravital microscopy, immunohistochemistry and functional blocking antibodies in vivo, we investigated the role of endothelial cell adhesion molecules P-selectin, E-selectin, ICAM-1 in leucocyte recruitment induced by exogenous MG. We applied MG locally to healthy mice to induce leucocyte recruitment in the cremasteric microvasculature. MG-induced leucocyte recruitment is neutrophil-dominant, MG dose-dependent and time-dependent; the decrease of leucocyte rolling velocity, and the increases of leucocyte rolling flux, adhesion and emigration are MG dose-dependent. MG treatment up-regulates the expression of endothelial cell adhesion molecules P-selectin, E-selectin and ICAM-1, but not VCAM-1. Functional blocking studies confirmed that the expression of P-selectin and E-selectin was responsible for the increased leucocyte rolling flux and decreased leucocyte rolling velocity, respectively, and ICAM-1 up-regulation was important for leucocyte adhesion. We show that the activation of NF-κB is involved and contributes to MG-induced endothelial adhesion molecule expression and leucocyte recruitment and that the effect of MG is, at least in part, directly through its action on endothelial cells.
Accumulating data from clinical and experimental studies suggest that increased formation of MG is linked to the development of diabetic vascular complications and the dysfunction of various cells, including endothelial cells, neutrophils and vascular smooth muscle cells that are associated with vascular damage.8,14,37 Plasma MG levels in healthy humans are 1 μm or less but are twofold to sixfold higher in diabetic patients.12,38 However, plasma MG concentrations as high as 400 μm have been reported in patients with poorly controlled diabetes.39 It has been suggested that local MG concentration in tissues could be much higher than its plasma levels18,40 although the exact local concentration of MG in diabetic patients is unclear. It was reported that cultured cells may produce larger amounts of MG (up to 310 μm in cell extracts).41 In this study, we used MG local treatment to study the acute effect of MG on leucocyte recuitment, and determined the optimal dose and treatment time for MG based on intravital microscopy. We observed that systemic (intraperitoneal) MG treatment for 4 hr induced leucocyte recruitment in a similar way to the recruitment response elicited by local MG treatment but the systemic MG had to be at a higher dose to have the same magnitude of recruitment response as local MG treatment (see Supplementary material, Fig. S1). Therefore, we used 25 mg/kg MG local injection for 4 hr as the acute inflammation model to study the role of endothelial cell adhesion molecules in MG-induced leucocyte recruitment. We found that in 25 mg/kg MG-treated mice, plasma MG level was lower than 1·60 μm at all the time-points (5 min to 4 hr) that were tested (Y. Su and L. Liu, unpublished observations) and even in the 50 mg/kg group, the plasma MG level (Table 1) was still lower than it was reported in diabetic patients.6,12 We also found that a low dose of paraformaldehyde, a biologically active chemical with active aldehyde group (similar to MG) in solutions, induced leucocyte recruitment similar to MG but less potent than MG at 4 hr, whereas the 4-hr treatment with biologically inactive d-glucose did not elicit leucocyte recruitment (see Supplementary material, Fig. S2), suggesting that the effect of MG in inducing leucocyte recruitment is possibly the result of the presence of the biologically active aldehyde group in its molecule. This suggests that the effect of MG may be very local, that leucocyte recruitment induced by local MG treatment may not be the same as the systemic MG effect, and that there is a clear relationship between local MG dose and the degree of leucocyte recruitment in the tissue.
In this study, we focused on the role of endothelial cell adhesion molecules in MG-induced leucocyte recruitment. The up-regulated endothelial cell adhesion molecules are the biomarkers of endothelial activation and dysfunction42,43 and the indication of the progression of diabetic complications in type 1 and type 2 diabetes.44,45,46 It has been revealed that the role of adhesion molecules is important in the early stage of diabetic vascular complications.47,48 Evidence from patients also indicates that the increased expression of adhesion molecules in kidneys is directly associated with endothelial dysfunction, renal tubular damage, and the progression of diabetic nephropathy.49,50 We found up-regulated expression of P-selectin, E-selectin and ICAM-1, but not of VCAM-1, after acute MG local treatment. Because neutrophils are the first cells to emigrate to the inflammation site, it is not surprising that they are the dominant leucocytes being recruited in tissue after acute MG treatment. We noted that after acute MG treatment, the level of VCAM-1 did not change. VCAM-1 is important for the recruitment of leucocytes other than neutrophils, such as lymphocytes, monocytes, eosinophils and basophils. In our results, these cells consisted of only a small percentage of the recruited leucocytes (Table 2). It has been shown that the level of VCAM-1 is increased with the progression of diabetic complications in patients with type 1 or type 2 diabetes.51 The unchanged VCAM-1 level in our experiment may be the result of the short treatment time and/or relatively low MG dose. Indeed, our experiments found evidence of increased percentages of recruited lymphocytes, monocytes, eosinophils and basophils with the increasing MG dose (Table 2). Whether VCAM-1 expression and functions are increased as the time and dose of MG treatment increases warrants further investigation.
In this study, the role of each MG-up-regulated endothelial cell adhesion molecule was determined by functional blocking studies. Our data suggest that acute MG treatment increases leucocyte rolling flux by up-regulating P-selectin expression, reduces leucocyte rolling velocity by up-regulating E-selectin expression, and increases cell adhesion by up-regulating ICAM-1 expression. All of these lead to the increased leucocyte–endothelial cell interactions that result in leucocyte emigration. How MG increases the expression or functions of these endothelial cell adhesion molecules is still unclear, but our data reveal that the NF-κB signalling pathway appears to be involved. The activation of various intracellular signalling pathways including NF-κB is known to up-regulate the expression of inflammatory cytokines and chemokines and endothelial adhesion molecules.52 Some studies have shown clear evidence that MG activates other signalling pathways such as JNK and p38 MAPK in endothelial cells.18,53 Other studies have demonstrated that, in various cell types, MG induces the expression and production of cytokines such as TNF-α and IL-8, which are pro-inflammatory and able to induce leucocyte recruitment.16,17,54,55 In addition, research data from clinical studies show that MG levels are elevated in patients with type 2 diabetes mellitus and patients with diabetic nephropathy and are related to the increased expression of inflammatory cytokines such as TNF-α, IL-8 and IL-6.12,14 Therefore, it is likely that, in our model system, MG up-regulates the expression of endothelial cell adhesion molecules and induces leucocyte recruitment in the tissue at least in part through the activation of the NF-κB signalling pathway in endothelial cells.
In conclusion, our present study describes the role of endothelial cell adhesion molecules P-selectin, E-selectin and ICAM-1 in exogenous MG-induced leucocyte recruitment in local tissue. We demonstrate that increased MG in local tissue dose-dependently reduces leucocyte rolling velocity, increases leucocyte rolling flux and promotes cell adhesion by up-regulating the expression of P-selectin, E-selectin and ICAM-1, respectively, and NF-κB is involved in these functional responses induced by MG. The MG-induced up-regulation of endothelial cell adhesion molecules may be important in leucocyte infiltration in the early stage of diabetic vascular complications and that revealing the mechanisms of MG-induced inflammation may unveil the mystery of vascular complications and immune dysfunctions in diabetes and may provide a new clue for possible therapeutic strategies.
Acknowledgments
The authors thank Arlene Drimmie and Karen Yuen for their expert technical help in histology and immunohistochemistry experiments. This work is supported by a research grant to L. Liu from the Canadian Institutes of Health Research (CIHR). L. Liu is a recipient of a CIHR New Investigator Award. Y. Su is supported by a scholarship from the China Scholarship Council.
Glossary
- AGEs
advanced glycation end-products
- ICAM-1
intercellular adhesion molecule-1
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MG
methylglyoxal
- NF-κB
nuclear factor-κB
- TNF-α
tumour necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
Disclosure
The author declare having no competing interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Systemic methylglyoxal (MG) treatment-induced leucocyte recruitment in cremasteric postcapillary venules.
Figure S2. The effect of methylglyoxal (MG), d-glucose and paraformaldehyde on leucocyte recruitment.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Cooper ME, Gilbert RE, Jerums G. Diabetic vascular complications. Clin Exp Pharmacol Physiol. 1997;24:770–5. doi: 10.1111/j.1440-1681.1997.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 2.Aleksandrovskii YA. Antithrombin III, C1 inhibitor, methylglyoxal, and polymorphonuclear leukocytes in the development of vascular complications in diabetes mellitus. Thromb Res. 1992;67:179–89. doi: 10.1016/0049-3848(92)90137-y. [DOI] [PubMed] [Google Scholar]
- 3.Colwell GA. Inflammation and diabetic vascular complications. Diabetes Care. 1999;22:1927–8. doi: 10.2337/diacare.22.12.1927. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson J, Bengtsson E, Fredrikson GN, Bjorkbacka H. Inflammation and immunity in diabetic vascular complications. Curr Opin Lipidol. 2008;19:519–24. doi: 10.1097/MOL.0b013e32830f47cd. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2:96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai K, Wu L. Methylglyoxal and advanced glycation endproducts: new therapeutic horizons? Recent Pat Cardiovasc Drug Discov. 2007;2:89–99. doi: 10.2174/157489007780832498. [DOI] [PubMed] [Google Scholar]
- 7.Berlanga J, Cibrian D, Guillen I, et al. Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin Sci (Lond) 2005;109:83–95. doi: 10.1042/CS20050026. [DOI] [PubMed] [Google Scholar]
- 8.Chang T, Wang R, Wu L. Methylglyoxal-induced nitric oxide and peroxynitrite production in vascular smooth muscle cells. Free Radic Biol Med. 2005;38:286–93. doi: 10.1016/j.freeradbiomed.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Gawlowski T, Stratmann B, Stirban AO, Negrean M, Tschoepe D. AGEs and methylglyoxal induce apoptosis and expression of Mac-1 on neutrophils resulting in platelet–neutrophil aggregation. Thromb Res. 2007;121:117–26. doi: 10.1016/j.thromres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J, Untereiner A, Wu L. Oxidative stress and aging: is methylglyoxal the hidden enemy? Can J Physiol Pharmacol. 2010;88:273–84. doi: 10.1139/Y10-001. [DOI] [PubMed] [Google Scholar]
- 11.Chang T, Wu L. Methylglyoxal, oxidative stress, and hypertension. Can J Physiol Pharmacol. 2006;84:1229–38. doi: 10.1139/y06-077. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Meng QH, Gordon JR, Khandwala H, Wu L. Proinflammatory and proapoptotic effects of methylglyoxal on neutrophils from patients with type 2 diabetes mellitus. Clin Biochem. 2007;40:1232–9. doi: 10.1016/j.clinbiochem.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Wang R, Desai K, Wu L. Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovasc Res. 2011;92:494–503. doi: 10.1093/cvr/cvr239. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Randell E, Han Y, Adeli K, Krahn J, Meng QH. Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin Biochem. 2011;44:307–11. doi: 10.1016/j.clinbiochem.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Price CL, Hassi HO, English NR, Blakemore AI, Stagg AJ, Knight SC. Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med. 2010;14:1806–15. doi: 10.1111/j.1582-4934.2009.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster L, Abordo EA, Thornalley PJ, Limb GA. Induction of TNF alpha and IL-1 beta mRNA in monocytes by methylglyoxal- and advanced glycated endproduct-modified human serum albumin. Biochem Soc Trans. 1997;25:250S. doi: 10.1042/bst025250s. [DOI] [PubMed] [Google Scholar]
- 17.Kuntz S, Kunz C, Rudloff S. Carbonyl compounds methylglyoxal and glyoxal affect interleukin-8 secretion in intestinal cells by superoxide anion generation and activation of MAPK p38. Mol Nutr Food Res. 2010;54:1458–67. doi: 10.1002/mnfr.200900408. [DOI] [PubMed] [Google Scholar]
- 18.Yamawaki H, Saito K, Okada M, Hara Y. Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1510–7. doi: 10.1152/ajpcell.00252.2008. [DOI] [PubMed] [Google Scholar]
- 19.Akhand AA, Hossain K, Mitsui H, et al. Glyoxal and methylglyoxal trigger distinct signals for MAP family kinases and caspase activation in human endothelial cells. Free Radic Biol Med. 2001;31:20–30. doi: 10.1016/s0891-5849(01)00550-0. [DOI] [PubMed] [Google Scholar]
- 20.Biswas S, Ray M, Misra S, Dutta DP, Ray S. Selective inhibition of mitochondrial respiration and glycolysis in human leukaemic leucocytes by methylglyoxal. Biochem J. 1997;323(Pt 2):343–8. doi: 10.1042/bj3230343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams JL, Badger AM, Kumar S, Lee JC. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Prog Med Chem. 2001;38:1–60. doi: 10.1016/s0079-6468(08)70091-2. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Zheng H, Zhao J, Lin L, Li C, Liu J, Pan Y. Porphyromonas gingivalis induces intracellular adhesion molecule-1 expression in endothelial cells through the nuclear factor-kappaB pathway, but not through the p38 MAPK pathway. J Periodontal Res. 2011;46:31–8. doi: 10.1111/j.1600-0765.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 23.Butcher EC. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 24.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–46. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Ke Y, Zhu CY, Tang N, Tian DK, Gao YH, Zheng JP, Bian K. Inflammatory reaction versus endogenous peroxisome proliferator-activated receptors expression, re-exploring secondary organ complications of spontaneously hypertensive rats. Chin Med J (Engl) 2008;121:2305–11. [PubMed] [Google Scholar]
- 26.Illoh K, Campbell C, Illoh O, Diehl J, Cherry J, Elkhaloun A, Chen Y, Hallenbeck J. Mucosal tolerance to E-selectin and response to systemic inflammation. J Cereb Blood Flow Metab. 2006;26:1538–50. doi: 10.1038/sj.jcbfm.9600308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruno CM, Valenti M, Bertino G, Ardiri A, Bruno F, Cunsolo M, Pulvirenti D, Neri S. Plasma ICAM-1 and VCAM-1 levels in type 2 diabetic patients with and without microalbuminuria. Minerva Med. 2008;99:1–5. [PubMed] [Google Scholar]
- 28.Jublanc C, Beaudeux JL, Aubart F, Raphael M, Chadarevian R, Chapman MJ, Bonnefont-Rousselot D, Bruckert E. Serum levels of adhesion molecules ICAM-1 and VCAM-1 and tissue inhibitor of metalloproteinases, TIMP-1, are elevated in patients with autoimmune thyroid disorders: relevance to vascular inflammation. Nutr Metab Cardiovasc Dis. 2010;10:817–22. doi: 10.1016/j.numecd.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Papayianni A, Alexopoulos E, Giamalis P, Gionanlis L, Belechri AM, Koukoudis P, Memmos D. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: association with inflammation, dyslipidaemia, and vascular events. Nephrol Dial Transplant. 2002;17:435–41. doi: 10.1093/ndt/17.3.435. [DOI] [PubMed] [Google Scholar]
- 30.Kressel G, Trunz B, Bub A, Hulsmann O, Wolters M, Lichtinghagen R, Stichtenoth DO, Hahn A. Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis. 2009;202:263–71. doi: 10.1016/j.atherosclerosis.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Dhar A, Desai K, Liu J, Wu L. Methylglyoxal, protein binding and biological samples: are we getting the true measure? J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1093–100. doi: 10.1016/j.jchromb.2009.02.055. [DOI] [PubMed] [Google Scholar]
- 32.Dhar A, Dhar I, Desai KM, Wu L. Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br J Pharmacol. 2010;161:1843–56. doi: 10.1111/j.1476-5381.2010.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Cara DC, Kaur J, Raharjo E, Mullaly SC, Jongstra-Bilen J, Jongstra J, Kubes P. LSP1 is an endothelial gatekeeper of leukocyte transendothelial migration. J Exp Med. 2005;201:409–18. doi: 10.1084/jem.20040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Driscoll J, Ryan JP. A modified haematoxylin and eosin stain for histological sections of lymph nodes. J Clin Pathol. 1978;31:700. doi: 10.1136/jcp.31.7.700-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhar A, Dhar I, Jiang B, Desai KM, Wu L. Chronic methylglyoxal infusion by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes. 2011;60:899–908. doi: 10.2337/db10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhar A, Desai KM, Wu L. Alagebrium attenuates acute methylglyoxal-induced glucose intolerance in Sprague-Dawley rats. Br J Pharmacol. 2010;159:166–75. doi: 10.1111/j.1476-5381.2009.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhar A, Dhar I, Desai KM, Wu L. Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br J Pharmacol. 2010;161:1843–56. doi: 10.1111/j.1476-5381.2010.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLellan AC, Thornalley PJ, Benn J, Sonksen PH. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 1994;87:21–9. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- 39.Lapolla A, Reitano R, Seraglia R, Sartore G, Ragazzi E, Traldi P. Evaluation of advanced glycation end products and carbonyl compounds in patients with different conditions of oxidative stress. Mol Nutr Food Res. 2005;49:685–90. doi: 10.1002/mnfr.200400093. [DOI] [PubMed] [Google Scholar]
- 40.Randell EW, Vasdev S, Gill V. Measurement of methylglyoxal in rat tissues by electrospray ionization mass spectrometry and liquid chromatography. J Pharmacol Toxicol Methods. 2005;51:153–7. doi: 10.1016/j.vascn.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Chaplen FW, Fahl WE, Cameron DC. Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1998;95:5533–8. doi: 10.1073/pnas.95.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balciunas M, Bagdonaite L, Samalavicius R, Baublys A. Markers of endothelial dysfunction after cardiac surgery: soluble forms of vascular-1 and intercellular-1 adhesion molecules. Medicina (Kaunas) 2009;45:434–9. [PubMed] [Google Scholar]
- 43.Schram MT, Stehouwer CD. Endothelial dysfunction, cellular adhesion molecules and the metabolic syndrome. Horm Metab Res. 2005;37(Suppl 1):49–55. doi: 10.1055/s-2005-861363. [DOI] [PubMed] [Google Scholar]
- 44.Song Y, Manson JE, Tinker L, et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007;56:1898–904. doi: 10.2337/db07-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dogruel N, Kirel B, Akgun Y, Us T. Serum soluble endothelial-cell specific adhesion molecules in children with insulin-dependent diabetes mellitus. J Pediatr Endocrinol Metab. 2001;14:287–93. doi: 10.1515/jpem.2001.14.3.287. [DOI] [PubMed] [Google Scholar]
- 46.Skyrme-Jones RA, Meredith IT. Soluble adhesion molecules, endothelial function and vitamin E in type 1 diabetes. Coron Artery Dis. 2001;12:69–75. doi: 10.1097/00019501-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Hagiwara S, Matsumoto M, et al. Effects of eicosapentaenoic acid on the early stage of type 2 diabetic nephropathy in KKA(y)/Ta mice: involvement of anti-inflammation and antioxidative stress. Metabolism. 2006;55:1590–8. doi: 10.1016/j.metabol.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Fornoni A, Ijaz A, Tejada T, Lenz O. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev. 2008;4:10–7. doi: 10.2174/157339908783502361. [DOI] [PubMed] [Google Scholar]
- 49.Wong CK, Ho AW, Tong PC, Yeung CY, Chan JC, Kong AP, Lam CW. Aberrant expression of soluble co-stimulatory molecules and adhesion molecules in type 2 diabetic patients with nephropathy. J Clin Immunol. 2008;28:36–43. doi: 10.1007/s10875-007-9137-4. [DOI] [PubMed] [Google Scholar]
- 50.Bavbek N, Kargili A, Kaftan O, Karakurt F, Kosar A, Akcay A. Elevated concentrations of soluble adhesion molecules and large platelets in diabetic patients: are they markers of vascular disease and diabetic nephropathy? Clin Appl Thromb Hemost. 2007;13:391–7. doi: 10.1177/1076029607303615. [DOI] [PubMed] [Google Scholar]
- 51.Soedamah-Muthu SS, Chaturvedi N, Schalkwijk CG, Stehouwer CD, Ebeling P, Fuller JH. Soluble vascular cell adhesion molecule-1 and soluble E-selectin are associated with micro- and macrovascular complications in Type 1 diabetic patients. J Diabetes Complications. 2006;20:188–95. doi: 10.1016/j.jdiacomp.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 53.Akhand AA, Hossain K, Kato M, Miyata T, Du J, Suzuki H, Kurokawa K, Nakashima I. Glyoxal and methylglyoxal induce lyoxal and methyglyoxal induce aggregation and inactivation of ERK in human endothelial cells. Free Radic Biol Med. 2001;31:1228–35. doi: 10.1016/s0891-5849(01)00702-x. [DOI] [PubMed] [Google Scholar]
- 54.Pal A, Bhattacharya I, Bhattacharya K, Mandal C, Ray M. Methylglyoxal induced activation of murine peritoneal macrophages and surface markers of T lymphocytes in sarcoma-180 bearing mice: involvement of MAP kinase, NF-kappa B signal transduction pathway. Mol Immunol. 2009;46:2039–44. doi: 10.1016/j.molimm.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Fukunaga M, Miyata S, Liu BF, et al. Methylglyoxal induces apoptosis through activation of p38 MAPK in rat Schwann cells. Biochem Biophys Res Commun. 2004;320:689–95. doi: 10.1016/j.bbrc.2004.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


