Abstract
BACKGROUND AND PURPOSE
The shearing forces generated by flow generally evoke dilatation in systemic vessels but constriction in the cerebral circulation. The aim of this study was to determine the effects of flow on the conduit artery delivering blood to the brain in the rat, that is, the carotid artery.
EXPERIMENTAL APPROACH
Carotid artery segments were mounted in a pressure myograph and pressurized to 100 mmHg. Changes in vessel diameter to flow (0.5–10 mL·min−1 for 2–10 min) at constant pressure were then measured using a video dimension analyser.
KEY RESULTS
Following the induction of tone, the onset of flow evoked a transient dilatation followed by a powerful constriction that was sustained until the termination of flow. Endothelial denudation or treatment with indomethacin, NG-nitro-L-arginine methyl ester, or the combination of apamin and TRAM−34 showed that the initial flow-mediated dilatation arose from the combined actions of endothelium-derived NO and endothelium-derived hyperpolarizing factor (EDHF). The flow-mediated constriction, which increased in magnitude with increasing flow rate and duration of flow, was also endothelium dependent, but was unaffected by treatment with superoxide dismutase, BQ-123, indomethacin, HET0016 or carbenoxolone. Flow-mediated constriction therefore appeared not to involve superoxide anion, endothelin-1, a COX product, 20-HETE or gap-junctional communication.
CONCLUSIONS AND IMPLICATIONS
Although a weak, transient flow-mediated dilatation is observed in the rat carotid artery, the dominant response to flow is a powerful and sustained constriction. Whether this flow-mediated constriction in the carotid artery serves as an extracranial mechanism to regulate cerebral blood flow remains to be determined.
Keywords: endothelium, flow-mediated constriction, flow-mediated dilatation, endothelium-derived hyperpolarizing factor (EDHF), nitric oxide
Introduction
Endothelial cells in the vascular wall are subjected to a variety of distinct haemodynamic forces, including the longitudinal shearing force of the flowing blood and the radial cyclic strain induced by the pulsatile changes in blood pressure occurring during the cardiac cycle (Busse and Fleming, 2003). These forces have a profound influence on endothelial phenotype through the regulated expression of multiple genes controlling anti-atherogenic, anti-inflammatory, anti-apoptotic and anti-proliferative functions (Bongrazio et al., 2000; Chen et al., 2001; Wasserman et al., 2002; Lamack and Friedman, 2007). In addition, these forces have a more immediate function in regulating vascular tone and hence, blood flow and pressure. In the majority of systemic arteries and arterial beds, shearing forces acting on the luminal surface elicit flow-mediated dilatation, resulting from the release and actions of either one or a combination of the endothelium-derived vasodilators, NO, endothelium-derived hyperpolarizing factor (EDHF) and prostacyclin (Sun et al., 1999; Dube and Canty, 2001; Huang et al., 2001; Fujioka et al., 2004; Bellien et al., 2006). Although flow-mediated dilatation has also been observed in rat and rabbit cerebral arteries (Fujii et al., 1991; Gaw and Bevan, 1993), reports of flow-mediated constriction appear to predominate in rat and cat cerebral arteries (Madden and Christman, 1999; Bryan et al., 2001a, b; New et al., 2003). In pig cerebral arteries, both flow-mediated dilatation and constriction have been reported, depending on flow rate (Shimoda et al., 1996). Flow-mediated constriction has also been observed in pig and cat small pulmonary arteries (Shimoda et al., 1997; Liu et al., 1998) and in the rabbit femoral artery (Hoogerwerf et al., 1992; Clifford et al., 2010). Furthermore, flow-mediated dilatation in rat gracilis muscle arterioles and venules is reversed to constriction following the development of hyperhomocysteinemia (Bagi et al., 2002; Racz et al., 2010).
The cerebral circulation is somewhat unusual in that large proximal arteries, such as the middle cerebral artery, are important determinants of vascular resistance, rather than the small arteries typical of systemic vascular beds (Bohlen, 1987; Faraci and Heistad, 1990; 1998). Cerebral microvascular pressure is therefore greatly influenced by the tone of large feeder arteries, and flow-mediated constriction of these has been regarded as a mechanism that protects small, thin-walled intracranial vessels from high pressures that might otherwise provoke haemorrhage (Faraci and Heistad, 1998; New et al., 2003).
As the carotid arteries are the main conduits to the brain, we wished to determine if these too might play a role in cerebrovascular protection by exhibiting flow-mediated constriction. Indeed, in this study we showed that while rat carotid artery exhibits a weak, flow-mediated dilator response, this is dominated by a much more powerful and sustained flow-mediated constriction.
Methods
Flow-mediated vasodilatation and constriction in the rat carotid artery assessed by pressure myography
All animal care and experimental procedures complied with the UK Home Office regulations. Female Wistar rats (83 in total) weighing 150–200 g were killed by an overdose of CO2. The carotid arteries were removed, cleared of adhering fat and connective tissue, and trimmed to 1.5 cm long segments. All the studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
Flow-mediated dilatation and constriction in the carotid artery was measured at constant pressure using a pressure myograph equipped with a video dimension analyser (Danish Myo Technology, model 11OP). The video dimension analyser measured only outer diameter because the vessels were not transparent. The carotid artery segments were cannulated at both ends and mounted in the heated (37°C) microscope stage bath (volume 10 mL) filled with Krebs solution comprising (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24, glucose 11, and gassed with 95% O2 and 5% CO2. The vessels were then pressurized to an inflow pressure of 100 mmHg by infusion with oxygenated Krebs delivered via the inflow cannula and by raising the height of the end of a 2 m long, 3 mm internal diameter, silicone rubber outflow tube held on a rigid scaffold. Vessels were allowed to equilibrate in the absence of any flow for 1 h before experiments were begun. Flow-mediated dilator and constrictor responses were elicited by inducing flow using a peristaltic pump (Gilson Minipuls model 3). Periods of flow ranged from 2 to 10 min at flow rates of 0.5–10 mL·min−1. Except where explicitly stated, pressure was held constant at 100 mmHg by lowering the height of the outflow tubing during flow and raising it again at the end of flow; it took ∼15 s to stabilize pressure when flow was initiated or stopped. Where indicated, some experiments were also conducted at 25, 50, 75 and 125 mmHg. A pulse dampener was employed to reduce the pulse pressure fluctuations caused by the peristaltic pump and ensure flow was laminar; this was placed in the inflow circuit between the pump and myograph and consisted of a sealed glass chamber (10 mL volume) with an inflow tube at the bottom and outflow at the 5 mL mark, giving a cushioning volume of air above the perfusion fluid. This also acted as a bubble trap, preventing bubbles of gas reaching the tissue.
In some experiments, the endothelium was removed by pinning the carotid artery segments at both ends on a silicone resin-filled Petri dish and gently inserting into the lumen a 500 µm diameter stainless steel wire five times. Pinning ensured that arterial segments were not subjected to longitudinal stretch when the luminal surface was being abraded. Endothelial denudation was deemed to have been successful by the absence of flow-mediated dilatation.
Experimental protocols
As will be seen in the Results section, induction of flow had virtually no effect on vessel diameter in pressurized carotid artery segments at resting levels of tone. Preliminary experiments demonstrated, however, that a constant low level of tone (∼50 µm constriction) induced using the thromboxane-mimetic U46619 (5–20 nM) provided ideal conditions for examining flow-mediated constriction; raising tone to an intermediate level (∼100 µm constriction) using 5–30 nM U46619 permitted examination of biphasic flow-mediated responses, consisting of an initial dilatation followed by constriction; and raising tone yet further to ∼75% of the maximum achievable (∼300 µm constriction) using 30–150 nM U46619 permitted examination of flow-mediated dilatation against a stable baseline without flow-mediated constriction obscuring the response.
In some experiments, superoxide dismutase (SOD, 100 u·mL−1), catalase (1200 u·mL−1), BQ-123 (1 µM), indomethacin (3 µM), HET0016 (3 µM) and carbenoxolone (100 µM) were used to determine, respectively, the potential involvement of superoxide anion, hydrogen peroxide, endothelin, COX products, 20-HETE or gap-junctional communication in the flow-mediated constrictor response. In these and all other experiments, the drugs were added to both the bath and perfusion fluid for a minimum of 1 h to ensure adequate diffusion into the vessel. A further set of experiments was conducted to determine the role of calcium ions in the flow-mediated constrictor response. In these, the standard Krebs solution was replaced with one lacking calcium, but containing the calcium chelator, EGTA, 1 mM.
In other experiments, NG-nitro-L-arginine methyl ester (L-NAME; 100 µM), indomethacin (3 µM) or the combination of apamin (0.5 µM) and TRAM-34 (10 µM) were used to determine, respectively, the potential involvement of NO, COX products and EDHF in the flow-mediated dilator response. In yet other experiments, all four of these blocking agents were combined to determine if inhibition of the endothelium-derived vasodilator agents affected the magnitude of flow-mediated constriction.
At the end of every experiment, papaverine (300 µM) was added to dilate fully the vessels and enable calibration of flow-mediated dilator responses.
All drug and molecular target nomenclature conforms to the Br. J. Pharmacol. Guide to Receptors and Channels (Alexander et al., 2011).
Drugs and chemicals
Apamin, indomethacin, L-NAME, papaverine hydrochloride, SOD (from bovine erythrocytes), TRAM-34 and U46619 were all obtained from Sigma, Poole, UK. Catalase (bovine liver) was obtained from Calbiochem UK and BQ-123 and carbenoxolone disodium from Tocris UK. HET0016 was obtained from Cayman Chemical, Ann Arbor, MI, USA. All drugs were dissolved in 0.9% saline, with the following exceptions: BQ-123 (1 mM stock) in distilled water; indomethacin (10 mM stock) in Na2CO3 (3 mM); TRAM-34 (10 mM stock) and HET0016 (10 mM stock) in dimethylsulfoxide (DMSO); and U46619 (1 mM stock) in 50% ethanol. Control experiments showed that at the concentrations employed, these solvents themselves had no effect on the carotid artery.
Data analysis
Changes in vessel outer diameter, reflecting flow-mediated constrictor and dilator responses, were measured in µm, although in some cases the latter were converted to a percentage of the maximum dilatation achieved by papaverine (300 µM), which was added at the end of each experiment. Data are expressed as the mean ± SEM of n separate observations, each from a separate tissue. Graphs were drawn and statistical comparisons were made using Student's t-test or one-way ANOVA and Bonferroni's post-test, as appropriate, with the aid of a computer programme, Prism (GraphPad, San Diego, CA, USA). A probability (P) less than or equal to 0.05 was considered significant.
Results
Effects of tone on flow-mediated responses
Following pressurization at 100 mmHg, rat carotid artery segments had a resting outer diameter of 1081 ± 9 µm (n= 33). When papaverine (300 µM) was added at the end of each experiment to produce complete relaxation, the diameter was not significantly different (1100 ± 9 µm), suggesting a low level of myogenic tone (19 ± 4 µm constriction).
In the absence of any vasoconstrictor agent, the induction of flow at 5 mL·min−1 for 5 min at constant pressure elicited a barely perceptible constriction of 10 ± 4 µm (n= 5; Figure 1). The sequential addition of L-NAME (100 µM), indomethacin (3 µM), then apamin (0.5 µM) and TRAM-34 (10 µM), to block any opposing dilator effects of NO, prostanoids or EDHF, had no effect on the magnitude of this constriction. In the continued presence of these blocking agents, and following the induction of ∼10% maximal tone (48 ± 5 µm constriction) using U46619 (5–20 nM), flow elicited a small transient dilatation followed by a more powerful, slowly developing constriction (87 ± 2 µm) that was sustained until the termination of flow. While this basal level of tone was necessary to uncover flow-mediated constriction, the presence of the blocking agents was not; in their absence flow (5 mL·min−1 for 5 min) elicited a similar constrictor response (102 ± 9 µm, n= 13). The use of phenylephrine to induce a low level of tone similarly uncovered the ability of flow to induce constriction (data not shown).
Figure 1.
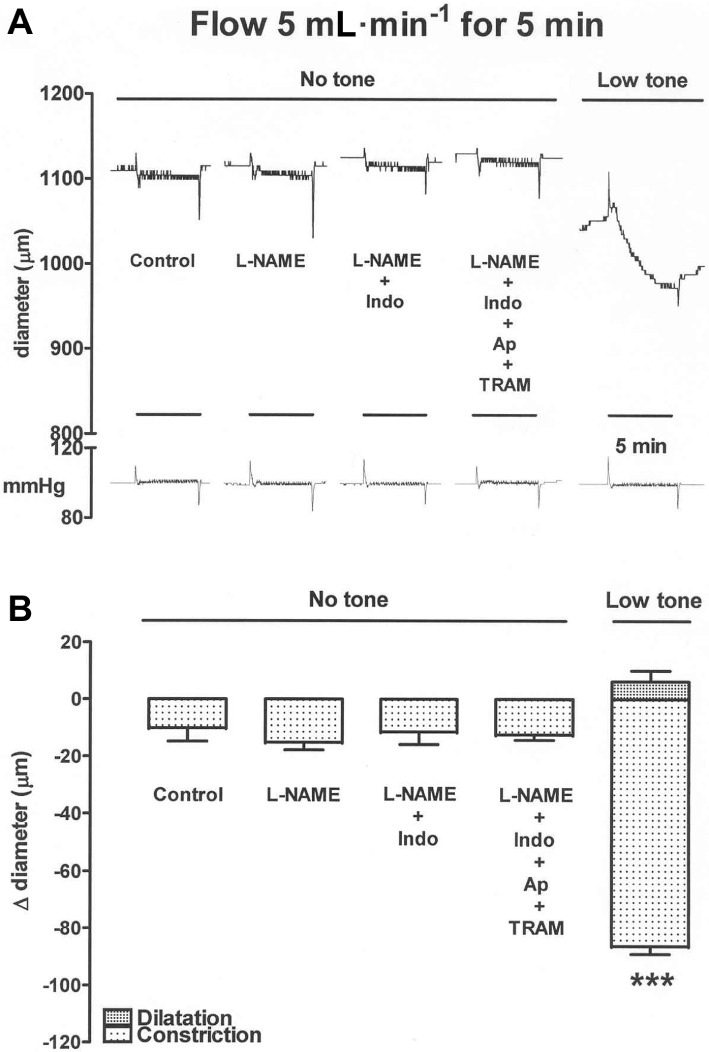
An experimental trace (A) and the results from several experiments (B) showing that in the absence of tone, flow for 5 min at 5 mL·min−1 elicited only a barely detectable constrictor response in rat carotid artery segments. The sequential addition of L-NAME (100 µM), indomethacin (Indo, 3 µM), then apamin (Ap, 0.5 µM) and TRAM-34 (10 µM), to block any opposing dilator effects of NO, prostanoids or EDHF, had no effect on the magnitude of this constriction. However, following induction of low-level tone using U46619, flow elicited a small transient dilatation followed by a more powerful constriction that was sustained until the termination of flow. The 5 min periods of flow are indicated by the time bars. The pressure trace (in mmHg) shows that flow was induced while maintaining constant pressure. Data are presented as the mean ± SEM of five observations. ***P < 0.001 indicates a significant difference from control.
Effects of SOD, catalase, BQ-123, indomethacin, HET0016, carbenoxolone, Ca2+-free solution, endothelial denudation and distending pressure on flow-mediated constriction
In the presence of ∼10% maximal tone (58 ± 17 µm; n= 8) induced using U46619 (10–50 nM), flow at 5 mL·min−1 for 5 min elicited robust constriction of 91 ± 6 µm (Figure 2). This flow-mediated constriction was unaffected by SOD (100 u·mL−1), either alone or in combination with catalase (1200 u·mL−1), by the ETA-receptor blocker BQ-123 (1 µM), indomethacin (3 µM), HET0016 (3 µM) or carbenoxolone (100 µM). Conducting experiments in Ca2+-free solution (containing EGTA, 1 mM) or following endothelial denudation did, however, significantly impair flow-mediated constriction.
Figure 2.
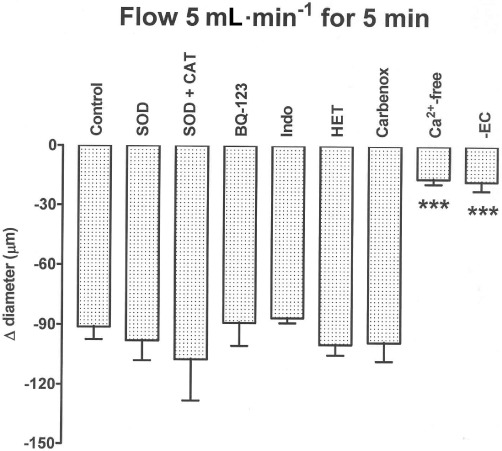
Bar graph showing constriction induced by flow at 5 mL·min−1 for 5 min in carotid artery segments at a low level of U46619-induced tone. This flow-induced constriction was unaffected by superoxide dismutase (SOD, 100 u·mL−1), either alone or in combination with catalase (CAT, 1200 u·mL−1), by BQ-123 (1 µM) alone, by indomethacin (Indo, 3 µM) alone, by HET0016 (HET, 3 µM) alone, or by carbenoxolone (Carbenox, 100 µM) alone, but was powerfully inhibited in Ca2+-free solution, or by endothelial denudation (-EC). Data are presented as the mean ± SEM of 4–10 observations. ***P < 0.001 indicates a significant difference from control.
While originally set at ∼10% maximal U46619-induced tone (at 100 mmHg), the effects of flow at 5 mL·min−1 for 5 min were examined over a range of distending pressures (25–125 mmHg). Vessel diameter increased with increasing pressure throughout this range (Figure 3). The magnitude of the flow-mediated constriction was small at 25 and 50 mmHg, rose steeply at 75 mmHg, was optimal at 100 mmHg and fell back at 125 mmHg.
Figure 3.
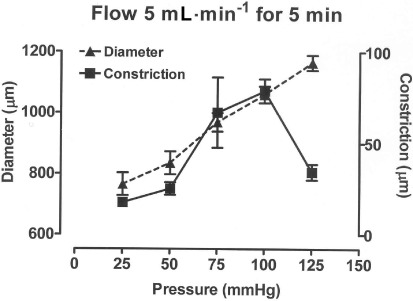
Graph showing how the external diameter and the constriction induced by flow at 5 mL·min−1 for 5 min varied over a range of distending pressures (25–125 mmHg) in carotid artery segments. All vessels were initially set at a low level of U46619-induced tone while at a pressure of 100 mmHg. Data are presented at the mean ± SEM of 5 observations.
Effects of varying flow rate and duration of flow on flow-mediated responses
In the absence of any blocking agents, and at an intermediate level of tone (105 ± 23 µm constriction) induced using U46619 (5–30 nM), low rates of flow elicited a biphasic response, comprising an initial dilatation that was interrupted by the onset of a more slowly developing constriction that was sustained until the termination of flow; higher rates of flow elicited mainly constriction (Figure 4). Figure 5 gives fuller details of how the magnitude of these dilatations and constrictions varied with flow rate (0.5–10 mL·min−1) and duration of flow (2–10 min).
Figure 4.
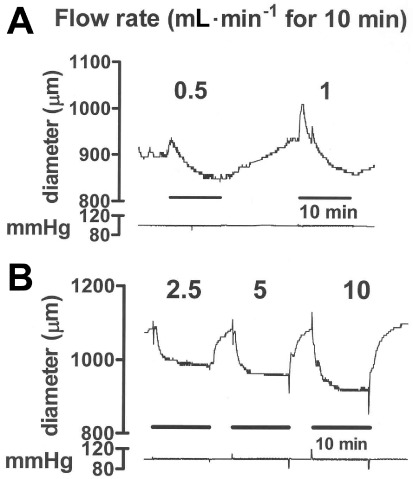
Experimental traces showing the effects of inducing flow for 10 min periods at (A) 0.5 and 1.0 mL·min−1 and (B) 2.5, 5.0 and 10 mL·min−1 in carotid artery segments at an intermediate level of U46619-induced tone. Traces (A) and (B) were from different vessels. Low rates of flow induced a biphasic response, comprising an initial dilatation, followed by a more slowly developing constriction that was sustained until the termination of flow. Higher rates of flow induced mostly constriction. The 10 min periods of flow are indicated by the time bars. The numbers on the trace indicate the flow rate in mL·min−1. The pressure traces (in mmHg) show that flow was induced while maintaining constant pressure.
Figure 5.
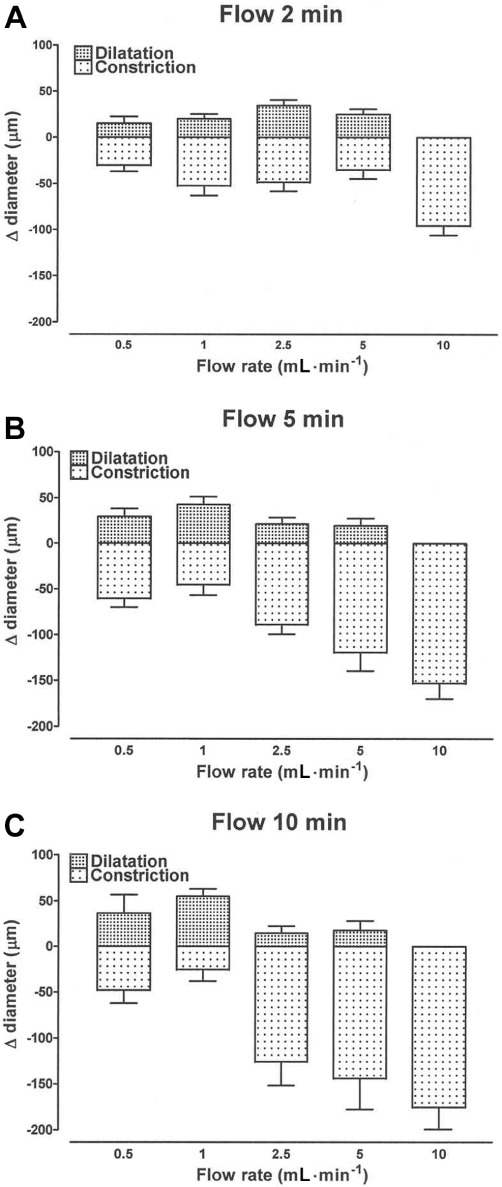
Bar graphs showing how flow-induced dilatation and constriction varied with flow rate (0.5–10 mL·min−1) and with the duration of flow for 2 min (A), 5 min (B) and 10 min (C) in carotid artery segments at an intermediate level of U46619-induced tone. Data are presented as the mean ± SEM of 5–11 observations.
When the duration of flow was 2 min, the magnitude of the dilatation increased with increasing flow rate up to a maximum of 34 ± 6 µm (n= 11) at 2.5 mL·min−1 and declined thereafter (Figure 5); at the optimum flow rate, the latency for onset of dilatation and the time to peak were 17 ± 2 s and 32 ± 2 s, respectively. With flow for longer periods (5 and 10 min), the maximum dilatation occurred at 1 mL·min−1. No dilatation was seen at a flow rate of 10 mL·min−1, regardless of the duration of flow.
Constriction was clearly the dominant response of the tissue to flow, and its magnitude increased with increasing flow rate and duration of flow, reaching 176 ± 25 µm (n= 5) at 10 mL·min−1 for 10 min (Figure 5); at this flow rate, the latency for onset of constriction and the time to peak were 16 ± 2 s and 407 ± 96 s, respectively.
In the combined presence of L-NAME (100 µM), indomethacin (3 µM), apamin (0.5 µM) and TRAM-34 (10 µM), the initial dilatation induced by flow (0.5–5 mL·min−1 for 2 min) was inhibited, but the magnitude of the ensuing constriction was unaffected (Figure 6).
Figure 6.
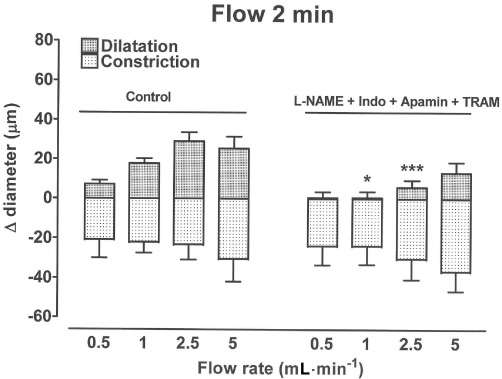
Bar graph showing how flow for 2 min periods at a range of flow rates (0.5–5 mL·min−1) induced dilatation and constriction in carotid artery segments at an intermediate level of U46619-induced tone. The combined presence of L-NAME (100 µM), indomethacin (Indo, 3 µM), apamin (0.5 µM) and TRAM-34 (10 µM) inhibited the dilatation but had no effect on the magnitude of the flow-induced constriction. Data are presented as the mean ± SEM of five to nine observations. *P < 0.05 and ***P < 0.01 indicate significant differences from respective controls.
Effects of L-NAME, indomethacin, apamin and TRAM-34, and endothelial denudation on flow-mediated dilatation
In the presence of ∼75% maximal tone (289 ± 27 µm constriction; n= 5) induced using U46619 (30–150 nM), flow-mediated dilatation could be observed against a stable baseline without flow-mediated constriction obscuring the response (Figure 7A). Under these conditions, induction of flow at 0.5–5 mL·min−1 for 2 min elicited graded relaxations that peaked at 2.5 mL·min−1 (Figure 7A,B). The COX blocker, indomethacin (3 µM), had no effect by itself on flow-mediated dilatation (data not shown) and neither did its combination with the NO synthase blocker, L-NAME (100 µM; Figure 7B). The EDHF blockers, apamin (0.5 µM) and TRAM-34 (10 µM), when combined with indomethacin, did inhibit flow-mediated dilatation, and the magnitude of this blockade was enhanced further by L-NAME. Like control tissues, endothelium-denuded segments of carotid artery constricted in response to U46619, but they did not dilate in response to flow (data not shown).
Figure 7.
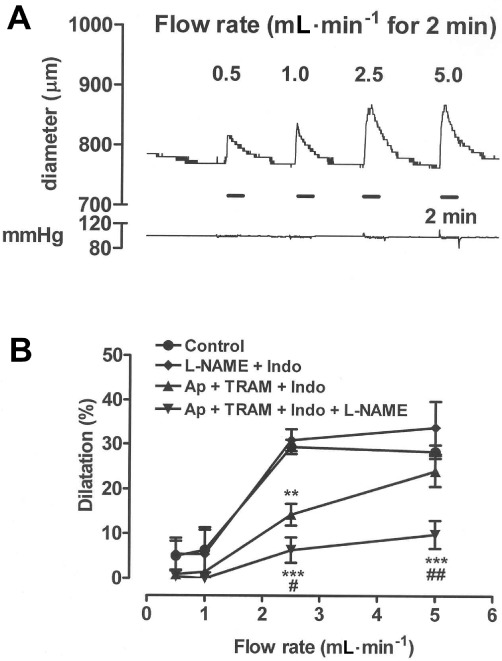
An experimental trace (A) and the results from several experiments (B) showing how flow-induced dilatation varied with flow rate (0.5–5 mL·min−1 for 2 min) in carotid artery segments at ∼75% of maximal U46619-induced tone. Dilatation was unaffected by combined treatment with L-NAME (100 µM) and indomethacin (Indo, 3 µM), but was inhibited by the combination of apamin (Ap, 0.5 µM) and TRAM-34 (10 µM) with indomethacin. Inhibition by the latter combination was enhanced following additional inclusion of L-NAME. The 2 min periods of flow are indicated by the time bars. The pressure trace (in mmHg) shows that flow was induced while maintaining constant pressure. Data are presented as the mean ± SEM of four or five experiments. **P < 0.01 and ***P < 0.001 indicate significant differences from respective controls. #P < 0.05 and ##P < 0.01 indicate a significant difference from tissues treated with the combination of apamin, TRAM-34 and indomethacin.
Discussion
The major new finding presented in this study is that while a weak, transient flow-mediated dilatation can be seen in the rat carotid artery, the dominant response of the vessel to flow at a constant pressure of 100 mmHg is a profound and sustained vasoconstriction. The magnitude of the constrictor response is proportional to the rate of flow and the response is reversible, decaying rapidly upon cessation of flow.
In most systemic arteries and arterial beds, the shearing forces generated by flow elicit vasodilatation, which is uniformly found to be endothelium-dependent, and results from the release of either one or a combination of the endothelium-derived vasodilators, NO, EDHF or prostacyclin (Sun et al., 1999; Dube and Canty, 2001; Huang et al., 2001; Fujioka et al., 2004; Bellien et al., 2006). These dilator responses are explained by the shearing forces acting on the endothelium, elevating the level of intracellular calcium (Hoyer et al., 1998), which promotes the activation of the calcium-dependent potassium channels necessary for the action of EDHF (Busse et al., 2002), calcium-sensitive phospholipase A2 needed for the synthesis of prostacyclin (Chang et al., 1987), and calcium-calmodulin-sensitive NO synthase (Salter et al., 1991). Sustained release of NO by flow, however, occurs through the continuous activation of the phosphatidylinositol 3-kinase and subsequent kinase PKB (Akt) and PKA pathways, which activate endothelial NO synthase by phosphorylation at Ser1177 (Dimmeler et al., 1999). In the rat carotid artery examined in this study, the initiation of flow (0.5–5 mL·min−1) at constant pressure elicited dilator responses, but these were transient, peaking at around 30 s, and their magnitude was almost certainly curtailed by the onset of the more slowly developing but sustained constrictor response. The dilatations were best observed using short (2 min) durations of flow, which reduced the magnitude of the opposing constrictor response, and at relatively high levels of vessel tone (∼75% of max induced by U46619), which helped maintain a stable baseline. Under these conditions, the magnitude of the dilator response increased with increasing flow rate over the range 0.5–2.5 mL·min−1 and declined at higher rates of flow. At 10 mL·min−1 no dilatation was seen, presumably because the magnitude of the concomitant flow-mediated constriction was so intense. In keeping with previous reports, flow-mediated dilatation was abolished following endothelial denudation (Sun et al., 1999; Dube and Canty, 2001; Fujioka et al., 2004). Unlike in rat and mouse skeletal muscle arterioles (Koller et al., 1994; Sun et al., 1999), the COX product, prostacyclin, appeared to play no role in the flow-mediated dilator response in the rat carotid artery, because indomethacin was without effect. Although the NO synthase blocker, L-NAME, had no effect by itself on flow-mediated dilatation, it did enhance the blockade induced by the combination of apamin and TRAM-34 (Waldron and Garland, 1994; Crane et al., 2003). It is therefore likely that flow-mediated dilatation in the rat carotid artery is the result of the combined effects of EDHF and NO, as is also the case in the human radial artery and coronary arterioles (Miura et al., 2001; Bellien et al., 2006). We acknowledge that our use of the term ‘EDHF’ is far from ideal because it can equally apply to situations where dilatation occurs by electrotonic spread of hyperpolarization and thus not through the action of a chemical mediator per se, or when it is not blocked by the combination of apamin and TRAM-34, such as when it is mediated by epoxyeicosatrienoic acids. It is therefore appropriate to use this term with caution when the precise mechanisms underlying vasodilatation are unknown (Busse et al., 2002; Félétou and Vanhoutte, 2006).
Although the onset of the flow-mediated constrictor response clearly suppresses the flow-mediated dilatation, blocking the dilatation with the combination of L-NAME, indomethacin, apamin and TRAM-34 has no effect on the magnitude of the constriction. It is therefore likely that the magnitude of the flow-mediated dilatation is too weak to produce effective physiological antagonism of the more powerful concomitant constriction. Although the majority of experiments were conducted at a constant pressure of 100 mmHg, the magnitude of the flow-induced constriction was seen to increase with increasing pressure from 25 to 100 mmHg and declined at 125 mmHg. The magnitude of the flow-mediated constriction was also related to the flow rate (0.5–10 mL·min−1) and to the duration of flow (2–10 min), and was thus proportional to the shear stress acting on the vessel wall. Shear stress is inversely related to the third power of the internal radius (Sun et al., 1999), and this probably explains why flow-mediated constriction was absent at basal tone but was uncovered following low-level constriction with either U46619 or phenylephrine. Flow-mediated constriction has previously been found in a number of other arteries and arterial beds, but its precise origins are still a matter of debate. For example, in cat and pig pulmonary arteries and piglet cerebral arteries (Shimoda et al., 1996; 1997; Liu et al., 1998), flow-mediated constriction is reported to be endothelium dependent, whereas in rat and cat cerebral arteries (Madden and Christman, 1999; Bryan et al., 2001b; New et al., 2003) it appears to be independent of the endothelium. Why different vascular sites appear to differ so radically in this respect is presently unknown, but in the rat carotid artery flow-mediated constriction was virtually abolished by endothelial denudation, suggesting it occurs by activation of an endothelium-derived process.
At vascular sites where the process of flow-mediated constriction is endothelium dependent, it could occur by a variety of mechanisms, for example, by electrotonic spread of a signal through myoendothelial gap junctions, or by release from the endothelium of either a vasoconstrictor agent or, alternatively, a substance that removes the influence of a tonically active vasodilator. Although inhibitors of gap-junctional communication, such as carbenoxolone, are known to impair the vasodilatation induced by the putative EDHF (Chaytor et al., 2000; Kenny et al., 2002), there are no reports of this agent inhibiting flow-mediated constriction. In fact, we found that carbenoxolone had no effect on flow-mediated constriction in the rat carotid artery, ruling out involvement of gap-junctional communication. In pig pulmonary arteries and rat skeletal muscle arterioles (Liu et al., 1998; Bagi et al., 2002), flow-induced constriction is impaired by treatment either with a NO synthase inhibitor or with SOD, either alone or in combination with catalase, suggesting it occurs indirectly through the production of superoxide anion, which destroys NO thereby removing a background vasodilator activity. This is clearly not the mechanism by which flow elicits constriction in the rat carotid artery, because we found that neither L-NAME nor SOD, either alone or with catalase, has any effect in this tissue. It is therefore more likely that flow elicits release of a directly acting vasoconstrictor from the endothelial cells in the rat carotid artery. Three potential candidates that seemed worthy of consideration are endothelin-1, thromboxane A2, and 20-HETE, as flow-mediated constriction is inhibited by the ETA-receptor blocker, BQ-123, in the cat pulmonary artery (Shimoda et al., 1997), by the TP receptor or COX blockers, SQ 29,548 and indomethacin, respectively, in rat skeletal muscle venules and arterioles (Bagi et al., 2002; Racz et al., 2010), and by SQ 29,548, indomethacin, or the 20-HETE synthesis inhibitor, HET0016, in rat and human cerebral arteries (Toth et al., 2011). In the rat carotid artery, however, we found that BQ-123, indomethacin and HET0016 each had no effect on flow-mediated constriction, ruling out the involvement of endothelin-1, a COX product or 20-HETE. Thus, in rat carotid artery, flow-mediated constriction appears to involve the release of an as yet unidentified endothelium-derived mediator. Experiments in Ca2+-free solution suggest, however, that Ca2+ ions are involved in the release of this agent.
Blood flow through the carotid artery in anaesthetized rats in vivo varies with age (Kohler and Jawien, 1992; Miyashiro et al., 1997), and is around 2.6 mL·min−1 for the 150–200 g rats used in our study. As this falls within the rates of flow 0.5–10 mL·min−1 we used in our experiments, it is possible that flow-mediated constriction may be of physiological importance in the rat carotid artery, and could potentially function to limit cerebral blood flow. This is suggested because in the cerebral circulation, large proximal arteries such as the middle cerebral artery are important determinants of vascular resistance (Bohlen, 1987; Faraci and Heistad, 1990; 1998). As a consequence, it has been proposed that flow-mediated constriction in these large feeder arteries may protect the more delicate, thin-walled intracranial vessels from high pressures that could provoke haemorrhagic stroke (Faraci and Heistad, 1998; New et al., 2003). Thus, flow-mediated constriction in the rat carotid artery could, by limiting cerebral blood flow, function as an extension of this intracranial protective mechanism.
The myogenic response, by which increases in transmural pressure elicit increases in vascular tone (Bayliss, 1902), is an important characteristic of cerebral vessels (Harder, 1984; Harder et al., 2011), and acts in concert with the flow-mediated constrictor responses discussed above to hold brain blood flow constant over a wide range of pressures. We found, however, that the rat carotid artery displays very little myogenic tone, as revealed by the smooth muscle relaxant, papaverine, even when pressurized to 100 mmHg. Therefore, this important component of the autoregulatory function of cerebral arteries is absent in the carotid artery.
In conclusion, while a weak, transient flow-mediated dilatation resulting from the combined actions of NO and EDHF occurs in the rat carotid artery, the dominant response of the vessel to flow is a profound and sustained vasoconstriction. This flow-mediated vasoconstriction is endothelium-dependent, but is not explained by release of superoxide anion, endothelin-1, a COX product, or 20-HETE from the endothelium, or by electrotonic spread of excitability through myoendothelial gap junctions. As flow-mediated constriction in the rat carotid artery occurs at rates of flow found in vivo, the possibility that it may function as an extracranial mechanism to limit cerebral blood flow warrants further investigation.
Acknowledgments
We are grateful to the Wellcome Trust for funding including that for the pressure myograph (Grant No. 075682MA).
Glossary
- BQ-123
cyclo[D-Trp-D-Asp-Pro-D-Val-Leu]
- DMSO
dimethylsulfoxide
- EDHF
endothelium-derived hyperpolarizing factor
- HET0016
N-(4-butyl-2-methylphenyl)-N’-hydroxy-methanimidamide
- L-NAME
NG-nitro-L-arginine methyl ester
- SOD
superoxide dismutase
- TRAM-34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- U46619
9,11-dideoxy-11α,9α-epoxy-methanoprostaglandin F2α
Conflicts of interest
No conflicts of interest are declared by the authors.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagi Z, Ungvari Z, Koller A. Xanthine oxidase-derived reactive oxygen species convert flow-induced arteriolar dilation to constriction in hyperhomocysteinemia: possible role of peroxynitrite. Arterioscler Thromb Vasc Biol. 2002;22:28–33. doi: 10.1161/hq0102.101127. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellien J, Iacob M, Gutierrez L, Isabelle M, Lahary A, Thuillez C, et al. Crucial role of NO and endothelium-derived hyperpolarizing factor in human sustained conduit artery flow-mediated dilatation. Hypertension. 2006;48:1088–1094. doi: 10.1161/01.HYP.0000246672.72188.bd. [DOI] [PubMed] [Google Scholar]
- Bohlen HG. Enhanced cerebral vascular regulation occurs by age 4 to 5 weeks in spontaneously hypertensive rats. Hypertension. 1987;9:325–331. doi: 10.1161/01.hyp.9.4.325. [DOI] [PubMed] [Google Scholar]
- Bongrazio M, Baumann C, Zakrzewicz A, Pries AR, Gaehtgens P. Evidence for modulation of genes involved in vascular adaptation by prolonged exposure of endothelial cells to shear stress. Cardiovasc Res. 2000;47:384–393. doi: 10.1016/s0008-6363(00)00111-5. [DOI] [PubMed] [Google Scholar]
- Bryan RM, Marrelli SP, Steenberg ML, Schildmeyer LA, Johnson TD. Effects of luminal shear stress on cerebral arteries and arterioles. Am J Physiol Heart Circ Physiol. 2001a;280:H2011–H2022. doi: 10.1152/ajpheart.2001.280.5.H2011. [DOI] [PubMed] [Google Scholar]
- Bryan RM, Steenberg ML, Marrelli SP. Role of endothelium in shear stress-induced constrictions in rat middle cerebral artery. Stroke. 2001b;32:1394–1400. doi: 10.1161/01.str.32.6.1394. [DOI] [PubMed] [Google Scholar]
- Busse R, Fleming I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci. 2003;24:24–29. doi: 10.1016/s0165-6147(02)00005-6. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte P, Weston A. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Chang J, Musser JH, Mcgregor H. Phospholipase-A2 – function and pharmacological regulation. Biochem Pharmacol. 1987;36:2429–2436. doi: 10.1016/0006-2952(87)90512-0. [DOI] [PubMed] [Google Scholar]
- Chaytor AT, Marsh WL, Hutcheson IR, Griffith TM. Comparison of glycyrrhetinic acid isoforms and carbenoxolone as inhibitors of EDHF-type relaxations mediated via gap junctions. Endothelium. 2000;7:265–278. doi: 10.3109/10623320009072213. [DOI] [PubMed] [Google Scholar]
- Chen BPC, Li YS, Zhao YH, Chen KD, Li S, Lao JM, et al. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Madden JA, Hamann JJ, Buckwalter JB, Valic Z. Absence of flow-mediated vasodilation in the rabbit femoral artery. Physiol Res. 2010;59:331–338. doi: 10.33549/physiolres.931672. [DOI] [PubMed] [Google Scholar]
- Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Dube S, Canty JM. Shear stress-induced vasodilation in porcine coronary conduit arteries is independent of nitric oxide release. Am J Physiol Heart Circ Physiol. 2001;280:H2581–H2590. doi: 10.1152/ajpheart.2001.280.6.H2581. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Fujii K, Heistad DD, Faraci FM. Flow-mediated dilatation of the basilar artery in vivo. Circ Res. 1991;69:697–705. doi: 10.1161/01.res.69.3.697. [DOI] [PubMed] [Google Scholar]
- Fujioka H, Ayajiki K, Shinozaki K, Okamura T. Mechanisms underlying endothelium-dependent flow increase in perfused rat mesenteric vascular bed. Eur J Pharmacol. 2004;485:219–225. doi: 10.1016/j.ejphar.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Gaw AJ, Bevan JA. Flow-induced relaxation of the rabbit middle cerebral artery is composed of both endothelium-dependent and endothelium-independent components. Stroke. 1993;24:105–110. doi: 10.1161/01.str.24.1.105. [DOI] [PubMed] [Google Scholar]
- Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- Harder DR, Narayanan J, Gebremedhin D. Pressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood flow. Am J Physiol Heart Circ Physiol. 2011;300:H1557–H1565. doi: 10.1152/ajpheart.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerwerf N, Zijlstra E, Vanderlinden PJW, Westerhof N, Sipkema P. Endothelium function is protected by albumin and flow-induced constriction is independent of endothelium and tone in isolated rabbit femoral artery. J Vasc Res. 1992;29:367–375. doi: 10.1159/000158953. [DOI] [PubMed] [Google Scholar]
- Hoyer J, Kohler R, Distler A. Mechanosensitive Ca2+ oscillations and STOC activation in endothelial cells. FASEB J. 1998;12:359–366. doi: 10.1096/fasebj.12.3.359. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, et al. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2001;280:H2462–H2469. doi: 10.1152/ajpheart.2001.280.6.H2462. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. The role of gap junctions in mediating endothelium-dependent responses to bradykinin in myometrial small arteries isolated from pregnant women. Br J Pharmacol. 2002;136:1085–1088. doi: 10.1038/sj.bjp.0704817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler TR, Jawien A. Flow affects development of intimal hyperplasia after arterial injury in rats. Arterioscler Thromb Vasc Biol. 1992;12:963–971. doi: 10.1161/01.atv.12.8.963. [DOI] [PubMed] [Google Scholar]
- Koller A, Sun D, Huang A, Kaley G. Corelease of nitric oxide and prostaglandins mediates flow-dependent dilation of rat gracilis muscle arterioles. Am J Physiol Heart Circ Physiol. 1994;267:H326–H332. doi: 10.1152/ajpheart.1994.267.1.H326. [DOI] [PubMed] [Google Scholar]
- Lamack JA, Friedman MH. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am J Physiol Heart Circ Physiol. 2007;293:H2853–H2859. doi: 10.1152/ajpheart.00244.2007. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wiener CM, Flavahan NA. Superoxide and endothelium-dependent constriction to flow in porcine small pulmonary arteries. Br J Pharmacol. 1998;124:331–336. doi: 10.1038/sj.bjp.0701846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden JA, Christman NJT. Integrin signaling, free radicals, and tyrosine kinase mediate flow constriction in isolated cerebral arteries. Am J Physiol Heart Circ Physiol. 1999;277:H2264–H2271. doi: 10.1152/ajpheart.1999.277.6.H2264. [DOI] [PubMed] [Google Scholar]
- Miura H, Wachtel RE, Liu YP, Loberiza FR, Saito T, Miura M, et al. Flow-induced dilation of human coronary arterioles – important role of Ca2+-activated K+ channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- Miyashiro JK, Poppa V, Berk BC. Flow-induced vascular remodeling in the rat carotid artery diminishes with age. Circ Res. 1997;81:311–319. doi: 10.1161/01.res.81.3.311. [DOI] [PubMed] [Google Scholar]
- New DI, Chesser AMS, Thuraisingham RC, Yaqoob MM. Cerebral artery responses to pressure and flow in uremic hypertensive and spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2003;284:H1212–H1216. doi: 10.1152/ajpheart.00644.2002. [DOI] [PubMed] [Google Scholar]
- Racz A, Veresh Z, Lotz G, Bagi Z, Koller A. COX-2 derived thromboxane A(2) and reactive oxygen species mediate flow-induced constrictions of venules in hyperhomocysteinemia. Atherosclerosis. 2010;208:43–49. doi: 10.1016/j.atherosclerosis.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Salter M, Knowles RG, Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca2+-dependent and Ca2+-independent nitric oxide synthases. FEBS Lett. 1991;291:145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Norins NA, Jeutter DC, Madden JA. Flow-induced responses in piglet isolated cerebral arteries. Pediatr Res. 1996;39:574–583. doi: 10.1203/00006450-199604000-00002. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Norins NA, Madden JA. Flow-induced responses in cat isolated pulmonary arteries. J Appl Physiol. 1997;83:1617–1622. doi: 10.1152/jappl.1997.83.5.1617. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, et al. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288–293. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab. 2011;31:2096–2105. doi: 10.1038/jcbfm.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron GJ, Garland CJ. Effect of potassium channel blockers on L-NAME insensitive relaxations in rat small mesenteric artery. Can J Physiol Pharmacol. 1994;72:11. [Google Scholar]
- Wasserman SM, Mehraban F, Komuves LG, Yang RB, Tomlinson JE, Zhang Y, et al. Gene expression profile of human endothelial cells exposed to sustained fluid shear stress. Physiol Genomics. 2002;12:13–23. doi: 10.1152/physiolgenomics.00102.2002. [DOI] [PubMed] [Google Scholar]


