Abstract
BACKGROUND AND PURPOSE
Decoctions of the Chinese herb houpu contain honokiol and are used to treat a variety of mental disorders, including depression. Depression commonly presents alongside sleep disorders and sleep disturbances, which appear to be a major risk factor for depression. Here, we have evaluated the somnogenic effect of honokiol and the mechanisms involved.
EXPERIMENTAL APPROACH
Honokiol was administered i.p. at 20:00 h in mice. Flumazenil, an antagonist at the benzodiazepine site of the GABAA receptor, was administered i.p. 15 min before honokiol. The effects of honokiol were measured by EEG and electromyogram (EMG), c-Fos expression and in vitro electrophysiology.
KEY RESULTS
Honokiol (10 and 20 mg·kg−1) significantly shortened the sleep latency to non-rapid eye movement (non-REM, NREM) sleep and increased the amount of NREM sleep. Honokiol increased the number of state transitions from wakefulness to NREM sleep and, subsequently, from NREM sleep to wakefulness. However, honokiol had no effect on either the amount of REM sleep or EEG power density of both NREM and REM sleep. Honokiol increased c-Fos expression in ventrolateral preoptic area (VLPO) neurons, as examined by immunostaining, and excited sleep-promoting neurons in the VLPO by whole-cell patch clamping in the brain slice. Pretreatment with flumazenil abolished the somnogenic effects and activation of the VLPO neurons by honokiol.
CONCLUSION AND IMPLICATIONS
Honokiol promoted NREM sleep by modulating the benzodiazepine site of the GABAA receptor, suggesting potential applications in the treatment of insomnia, especially for patients who experience difficulty in falling and staying asleep.
Keywords: c-Fos, electrophysiology, honokiol, receptor, rodents, sleep
Introduction
Insomnia is characterized by difficulties in sleep initiation and sleep maintenance and by poor sleep quality, resulting in impairment of daytime function. It is the most common subjective sleep difficulty in anxious and depressed patients (Winokur et al., 2001; Buckner et al., 2008). Approximately 80% of hospitalized depressed patients also have objective alterations in sleep EEG patterns (Winokur et al., 2001). Diazepam and zolpidem are used to treat insomnia in humans. These compounds significantly increase total non-rapid eye movement (non-REM, NREM) sleep time, but they also cause a remarkable decrease in delta activity during NREM sleep (van Lier et al., 2004). The discrepancy between the increase in sleep continuity and the reduction of power in the lower EEG frequencies caused by benzodiazepines in humans is consistent with the findings in mice (Tobler et al., 2001). It is well known that benzodiazepines have many untoward reactions, such as drug dependence, tolerance, rebound insomnia, amnesia and muscle relaxation (Vgontzas et al., 1995; Aragona, 2000). Therefore, we need sleep-promoting compounds that induce physiological sleep without significant adverse effects.
Honokiol (5,3′-diallyl-2,4′-dihydroxybiphenyl, C18H18O2; for chemical structure, see Figure 1) is an active component of the Chinese herb houpo (Teng et al., 1988). Decoctions of houpu, a traditional Chinese medicinal formula containing honokiol, is used to treat depression effectively in China (Ding, 1992; Li and Kong, 2001). Honokiol extends the time spent in the open arms of the maze in mice, and this anxiolytic effect is inhibited by flumazenil, an antagonist at the benzodiazepine site of the GABAA receptor (Kuribara et al., 1998; 2000; receptor nomenclature follows Alexander et al., 2011). A mixture of honokiol and magnolol, an isomer of honokiol, possesses an antidepressant-like effect in the forced swimming test and chronic mild stress in rodents (Xu et al., 2008). Because attenuation of depression and anxiety can improve sleep quality, these results can be interpreted to mean that honokiol may promote sleep.
Figure 1.
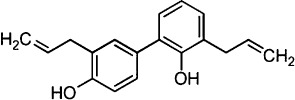
The chemical structure of honokiol.
In the present study, we administered honokiol, by a single i.p. injection, to mice during the active period and characterized their sleep–wake behaviour by recording EEGs and electromyograms (EMG). We also examined the effects of flumazenil on the honokiol-induced sleep. In addition, c-Fos immunostaining and whole-cell patch clamping in brain slices were used to explore the mechanisms involved in the sleep-promoting effects of honokiol. Our findings indicate that honokiol promoted NREM sleep via the benzodiazepine site of the GABAA receptor, suggesting that it may have applications in the treatment of insomnia.
Methods
Animals
All animal care and experimental protocols were approved by the Medical Experimental Animal Administrative Committee of Shanghai. Every effort was made to minimize the number of animals used and any pain and discomfort that they might experience. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). Male C57BL/6 J mice (20–26 g; 11–13 weeks old), and post-natal Sprague-Dawley rats (14–21 days old) were obtained from the Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). The animals were housed in an insulated, sound-proof recording room maintained at an ambient temperature of 22 ± 0.5°C, with a relative humidity (60 ± 2%) on an automatically controlled 12 h light/12 h dark cycle (lights on at 8:00). They had free access to food and water. A total of 84 mice and 23 rats were used in the experiments reported here.
Pharmacological treatments
Honokiol (Wako Pure Chemicals, Osaka, Japan) was dissolved in sterile saline containing 10% DMSO immediately before use and administered i.p. at 20:00 on the experimental day at doses of 5, 10 or 20 mg·kg−1. Diazepam (Tianjin Pharmaceutical Co., Ltd. Tianjin, China) at 6 mg·kg−1 was diluted with sterile saline immediately before use and administered i.p. at 20:00 on the experimental day. Flumazenil (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile saline and administered i.p. 15 min before the injection of honokiol. For baseline data, mice were injected i.p. with vehicle (saline containing 10% DMSO) at 19:45 and 20:00. (–)-Noradrenaline (Sigma-Aldrich) was diluted in fresh artificial cerebrospinal fluid (ACSF; composition see below) to the final concentration immediately before the experiment of whole-cell patch-clamp studies.
Polygraphic recordings and vigilance state analysis
Under pentobarbital anaesthesia (50 mg·kg−1, i.p.), the mice were chronically implanted with EEG and EMG electrodes for polysomnographic recordings. The implant consisted of two stainless steel screws (1 mm diameter) inserted through the skull of the cortex (anteroposterior, +1.0 mm and left-right, −1.5 mm from bregma or lambda) according to the atlas of Franklin and Paxinos (1997). These served as EEG electrodes. Two insulated, stainless steel, Teflon-coated wires were placed bilaterally into both trapezius muscles. These served as EMG electrodes. All electrodes were attached to a micro-connector and fixed to the skull with dental cement.
The EEG and EMG recordings were carried out by means of a slip ring designed so that the behavioural movement of the mice would not be restricted. After a 10 day recovery period, the mice were housed individually in transparent barrels and habituated to the recording cable for 3–4 days prior to polygraphic recording. Sleep–wakefulness states were monitored for a period of 48 h, which comprised baseline and experimental days. Baseline recordings were taken in each animal for 24 h, beginning at 08:00 h. These served as controls for the same animal.
Cortical EEG and EMG signals were amplified and filtered (EEG, 0.5–30 Hz; EMG, 20–200 Hz) and then digitized at a sampling rate of 128 Hz and recorded by using SleepSign (Kissei Comtec, Nagano, Japan) as described previously (Qu et al., 2006; 2008; Qiu et al., 2009). When complete, polygraphic recordings were automatically scored off-line by 10 s epochs as wakefulness, REM and NREM sleep by SleepSign according to the standard criteria (Huang et al., 2006; Oishi et al., 2008; Qu et al., 2010; Yan et al., 2011). As a final step, defined sleep–wake stages were examined visually and corrected if necessary.
c-Fos immunohistochemistry
Six groups of mice were used. One group was treated with vehicle and the others were injected i.p. with honokiol at doses of 5, 10 or 20 mg·kg−1. To test the receptor mechanisms, two groups of mice were used. Flumazenil (1 mg·kg−1) was given 15 min before the honokiol (20 mg·kg−1) or vehicle injection. Two hours after receiving a test dose of honokiol, the animals received deep anaesthesia with chloral hydrate (350 mg·kg−1, i.p.) and were immediately perfused transcardially with 200 mL of saline, followed by 200 mL of 4% formalin. The brain was removed, post-fixed for 24 h at 4°C in 4% formalin and then equilibrated in phosphate buffer containing 30% sucrose for 24 h at 4°C. Coronal sections (30 µm) were cut serially on a cryostat.
Immunohistochemistry was performed in accordance with the free-floating method described earlier (Qiu et al., 2003; 2009; Chen et al., 2011). Sections were washed with PBS (three changes) and incubated in PBS containing 0.25% Triton X-100 at room temperature and then incubated at 4°C for 24 h with a rabbit polyclonal antibody against c-Fos (1:20 000, Calbiochem, Merck KGaA, Darmstadt, Germany) diluted in PBS containing 0.25% Triton X-100. Sections were then washed and incubated in biotinylated anti-rabbit IgG antibody (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), washed again with PBS and incubated in avidin–biotin complex reagents at room temperature for 1 h (1:200; Santa Cruz Biotechnology, Inc.). Sections were then washed again and incubated in a solution of 0.05% 3,3-diaminobenzidine (Sigma) plus 0.03% H2O2. All sections were mounted onto gelatin-coated slides, dehydrated in graded ethanol, placed into xylenes, and coverslipped. As controls, adjacent sections were incubated without primary antibody to confirm that no non-specific staining had occurred.
The sections were examined under bright-field illumination with a Leica DMLB microscope (Leica Microsystems, Wetzlar, Germany). Images were captured with a CoolSNAP-Procf digital camera (RTKE Diagnostic, Spot Instruments, Sterling Heights, MI, USA). Using light microscopy, neurons positive for c-Fos were identified by their characteristic dense brown nuclear staining. Locations in the brain were confirmed by staining and reference to the primary literature and a mouse brain atlas. Four sections through the middle of each structure per animal were counted (by technicians unaware of the treatments) and averaged.
Electrophysiological experiments
Preparation of brain slices
Electrophysiological experiments were performed on slices retrieved from post-natal Sprague-Dawley rats that were 14–21 days old. The hypothalamic slices were prepared as described previously (Gallopin et al., 2000). Briefly, Sprague-Dawley rats were killed by decapitation. The ventrolateral preoptic area (VLPO) was identified according to the stereotaxic coordinates (Paxinos and Watson, 1997). Coronal midbrain slices (300 µm thick) containing VLPO were cut using a vibratome (Leica VT 1000 S) in ice-cold glycerol-based artificial cerebrospinal fluid (GACSF) containing (in mM): glycerol (260), KCl (5), KH2PO4 (1.25), MgSO4 (1.3), CaCl2 (0.5), NaHCO3 (20) and glucose (10), and saturated with 95% O2-5% CO2 (carbogen). Slices were allowed to recover for at least 1 h in a holding chamber at room temperature (32°C) in a carbogen-saturated normal ACSF (composition (in mM): NaCl (125), KCl (5), KH2PO4 (1.25), MgSO4 (1.3), CaCl2 (2.4), NaHCO3 (20) and glucose (10). Note this has the same composition as GACSF, except that glycerol was replaced by 125 mM NaCl, and that the concentration of CaCl2 was adjusted to 2.4 mM.
Extracellular recordings in loosely cell-attached configuration
This recording configuration was used to study the effects of multiple drug applications on a single VLPO cell. In contrast to intracellular and whole-cell patch-clamp configurations, this mode allows stable extracellular recordings of healthy neurons for the long periods of time necessary to complete pharmacological experiments, including synaptic uncoupling conditions (low Ca2+/high Mg2+). Infrared differential interference contrast videomicroscopy was used to locate the sleep neurons in the VLPO according to their triangular and multipolar shape as reported previously (Gallopin et al., 2000). Recordings of the numbers of loosely attached cells in the soma were made using patch micropipettes (5–8 MÙ) pulled from borosilicate glass capillaries (1.5 mm o.d., 0.86 mm i.d.; Harvard Apparatus, Les Ulis Cedex, France) on a Brown-Flaming micropipette puller (Model P-97, Sutter Instrument, Novato, CA, USA). The micropipettes, filled with ACSF, were attached to an electric microdrive (Luigs and Neumann, Ratingen, Germany) and placed under visual control in contact with the soma of the selected cell. During extracellular recordings of the loosely cell-attached configuration, a seal resistance of 10–15 MÙ was maintained to avoid damage or mechanical stimulation to the cell.
In whole-cell and cell-attached configurations, electrical signals were obtained using an Axon 200B amplifier, a Digidata 1440A A/D converter and pCLAMP 10.2 software (Molecular Devices Co., Union City, CA, USA). Data were filtered at 1 kHz and sampled at 5 kHz.
Data analysis
All results are expressed as means ± SEM. For the time-course data, the different numbers of the different sleep–wake states were analysed using the paired t-test, with each animal serving as its own control. For the total numbers of each vigilance stage during the 4 h immediately following drug treatment and the number of c-Fos immunoreactive neurons, one-way repeated measures anova was performed, followed by Fisher's probable least-squares difference (PLSD) test to determine whether the difference among groups was statistically significant. In all cases, P < 0.05 was taken as the level of significance.
Results
Effects of honokiol on NREM sleep in mice
To determine the effects of honokiol on sleep–wake profiles, honokiol was injected i.p. into C57BL/6 mice at 20:00 h at doses of 5, 10 or 20 mg·kg−1, and diazepam was given at 6 mg·kg−1 as a positive control. A greater difference in the sleep–wake cycle was observed between the injection of vehicle and honokiol (20 mg·kg−1). Typical examples of polygraphic recordings and corresponding hypnograms illustrated the effects of honokiol on sleep–wake profiles from an individual mouse (Figure 2A,B). During the period from 20:00 h to 0:00 h, this mouse spent more time in wakefulness when under vehicle control than when given honokiol (Figure 2A). When honokiol was injected on the experimental day, however, the animal spent more time asleep than it had while its control values were being recorded (Figure 2B). The latency to NREM sleep, which is defined as the time from injection to the appearance of the first NREM sleep episode lasting for at least 20 s, was 26.8 min in the mice treated with honokiol at 20 mg·kg−1. This is significantly shorter than 63.3 min for the latency in mice after vehicle injection (Figure 2C). The short sleep latency observed in honokiol-injected mice indicates that honokiol accelerates the initiation of NREM sleep.
Figure 2.
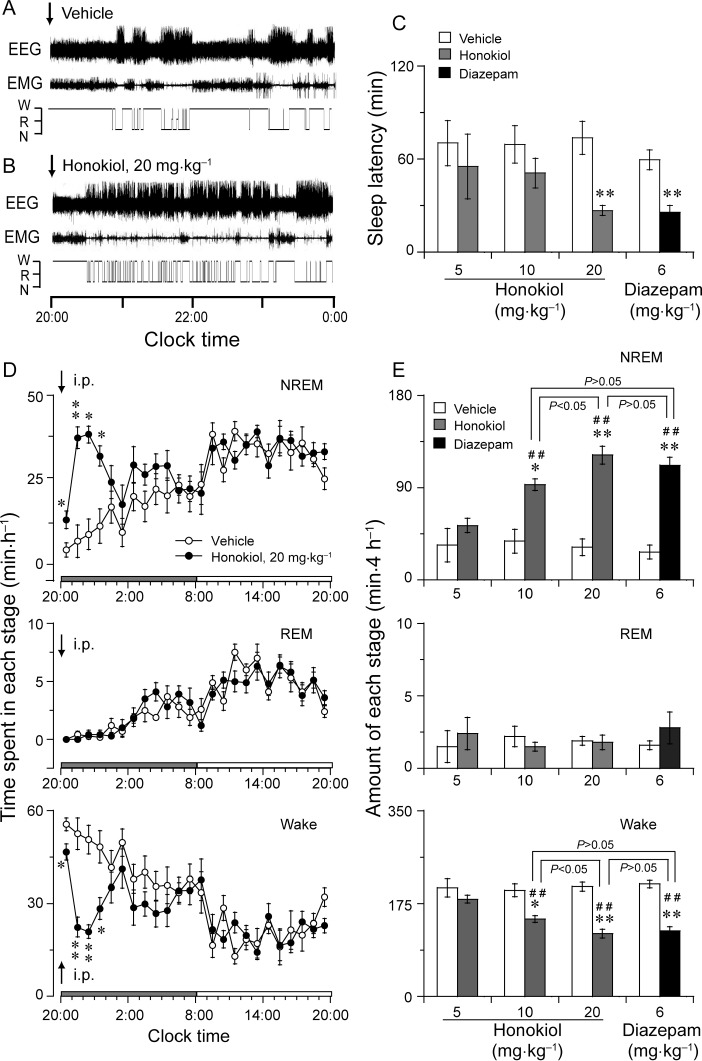
Effect of honokiol on sleep–wake profiles in mice. Typical examples of polygraphic recordings and corresponding hypnograms illustrating the effects of injection with vehicle (A) or honokiol (B) given to a mouse at 20:00 h. (C) Sleep latency after administration of honokiol and diazepam. (D) Time-course changes in NREM and REM sleep and wakefulness after administration of honokiol (20 mg·kg−1, i.p.) to mice. The horizontal filled and open bars on the X-axis (Clock time) indicate the 12 h dark and 12 h light periods, respectively. (E) Total time spent in each stage for 4 h after administration of vehicle and honokiol or diazepam. Data shown are the means ± SEM (n = 5–6). *P < 0.05, **P < 0.01, significantly different from their vehicle controls, ##P < 0.01, significantly different from honokiol at 5 mg·kg−1; one-way anova, with the PLSD test.
Time-course changes in NREM sleep showed that the sleep-promoting effects of honokiol at 20 mg·kg−1 lasted for 4 h. Honokiol is compared with the vehicle control in Figure 2D, it increased the amount of NREM sleep during the first, second, third and fourth hours by 3-, 5.4-, 4.3- and 2.8-fold, respectively. This enhancement of NREM sleep was accompanied by a decrease in wakefulness. However, REM sleep did not change after the administration of honokiol. There was no further disruption of the sleep architecture during the subsequent period. Similar time-course profiles were observed at the lower dose of 10 mg·kg−1, but the effect on sleep was slighter, lasting about 2 h after the injection. Honokiol at 5 mg·kg−1 did not affect the sleep profiles (data not shown).
We calculated the total amount of NREM and REM sleep and wakefulness during the 4 h immediately following administration. Honokiol at doses of 10 and 20 mg·kg−1 was found to increase NREM sleep by 2.5- and 3.8-fold and to decrease the total amount of wakefulness by 27% and 43%, respectively, as compared with the baseline values (Figure 2E). Honokiol given at 5 mg·kg−1 did not affect the amounts of NREM sleep and wakefulness for 4 h post-injection. anova revealed that honokiol increased NREM sleep [F(3,20) = 15.5, P < 0.01]. The effect of honokiol at 20 mg·kg−1 was stronger than those of honokiol at 5 and 10 mg·kg−1 but not significantly different from that of diazepam at 6 mg·kg−1 (P > 0.05, PLSD). There was no essentially significant difference in REM sleep after the administration of honokiol at the three dosage levels tested. These results clearly revealed that honokiol increased NREM sleep with concomitant reduction in wakefulness in a dose-dependent manner, but did not alter REM sleep in mice.
Diazepam at 6 mg·kg−1 increased the total amounts of NREM sleep by 4.1-fold and decreased wakefulness by 41%, during that 4 h period, relative to the vehicle control (Figure 2E). However, there was no significant difference in REM sleep for 4 h after injection between diazepam and its vehicle control.
Effects of flumazenil on the sleep-promoting effects of honokiol
To determine whether the GABAergic system might be involved in the somnogenic effects of honokiol, honokiol-treated mice were pretreated with flumazenil, a selective antagonist at the benzodiazepine site of GABAA receptor. Flumazenil (1 mg·kg−1) alone showed no significant effects on sleep latency or total amounts of sleep (data not shown). Honokiol increased the amount of NREM sleep by 2.4-fold during the first 4 h after administration. In contrast, pretreatment with flumazenil completely abolished the somnogenic effect of honokiol (Figure 3A,B). These results indicate that the induction of sleep caused by honokiol is modulated by the benzodiazepine site of the GABAA receptor.
Figure 3.
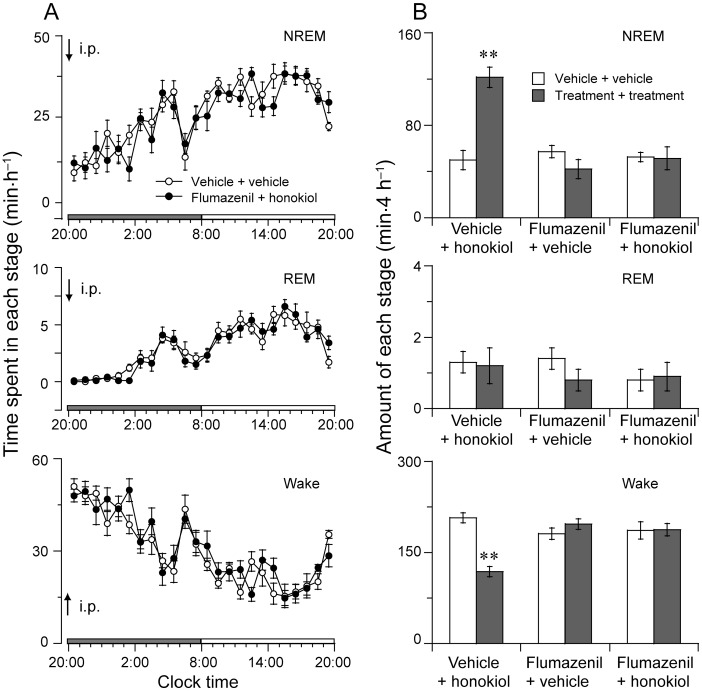
Effect of flumazenil on honokiol-induced sleep. (A) Time-course changes in NREM and REM sleep and wakefulness after pretreatment with flumazenil (1 mg·kg−1, i.p. at 19:45 h) and injection of honokiol (20 mg·kg−1, i.p. at 20:00 h) or vehicle in mice.. The horizontal filled and open bars on the X-axis (Clock time) indicate the 12 h dark and 12 h light periods, respectively. (B) Total time spent in each stage for 4 h after administration of vehicle, flumazenil and honokiol. Data shown are the means ± SEM (n = 8). **P < 0.01, significantly different from vehicle; two-tailed paired t-test.
Effects of honokiol on characteristics of sleep–wake episodes and power density
To better understand the sleep–wake profile caused by honokiol, we determined the total number and mean duration of NREM and REM sleep, and wake episodes. As shown in Figure 4A, honokiol at 20 mg·kg−1 increased the number of NREM bouts by 4.4-fold and wake episodes by 4.2-fold, but the mean duration of NREM and REM sleep was not altered during the 4 h period after the injection. The mean duration of episodes of wakefulness was significantly decreased by 83% after honokiol injection.
Figure 4.
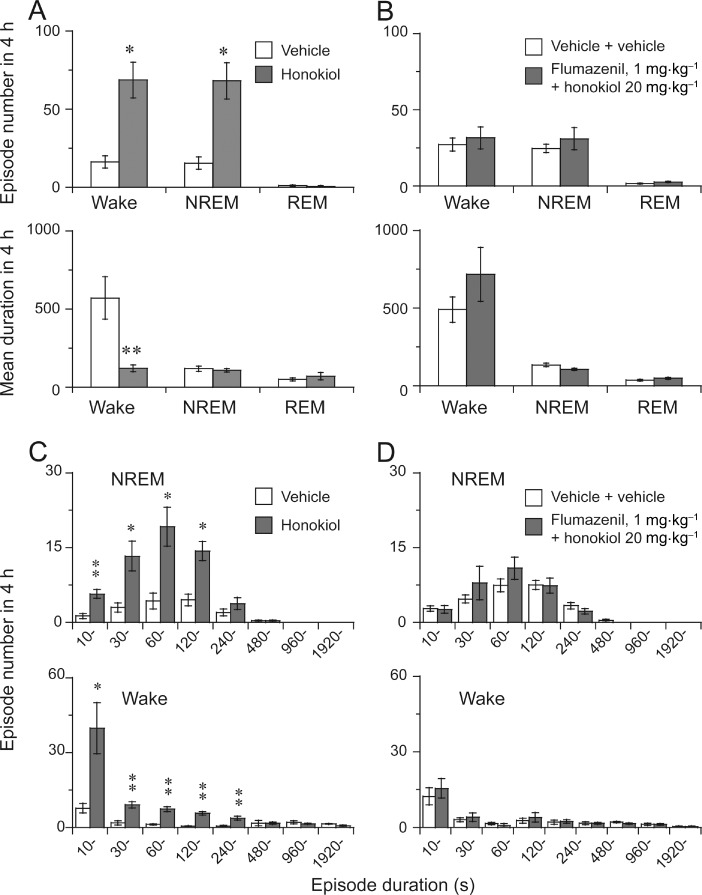
Characteristics of sleep–wake episodes caused by honokiol and flumazenil. Total number and mean duration of wake, NREM and REM bouts in a 4 h period after the administration of honokiol (A) or flumazenil + honokiol (B). Changes in the numbers of NREM and REM sleep bouts for different ranges of episode duration during the 4 h following the administration of honokiol (C) or flumazenil + honokiol (D). Data shown are means ± SEM (n = 5–6). *P < 0.05, **P < 0.01, significantly different from corresponding vehicle; two-tailed paired t-test.
In addition, honokiol at 20 mg·kg−1 increased NREM sleep and wake bouts relative to the vehicle control. These bouts ranged from 0 to 240 s and from 10 to 480 s, respectively (Figure 4C), whereas the longer duration of NREM sleep and wakefulness was not altered after honokiol injection. In contrast, REM sleep bouts did not change after administration of honokiol (data not shown).
As a result, the numbers of state transitions from wakefulness (W) to NREM sleep (N) and from N to W were increased after the injection with honokiol (Figure 5A). However, no change in either the number of transitions from N to REM (R) or that from R to W was observed.
Figure 5.
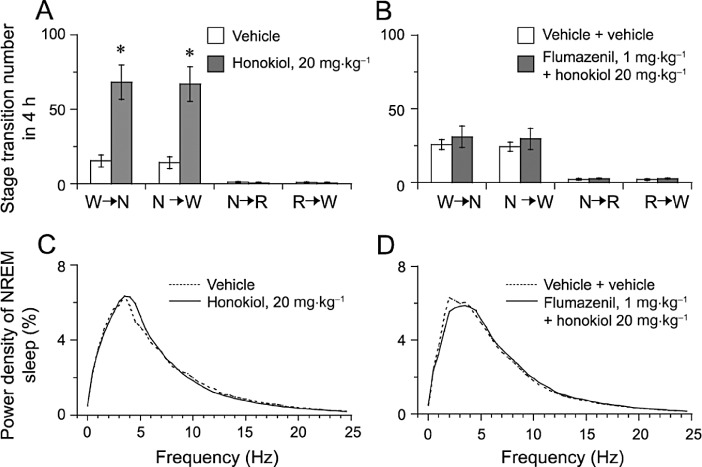
Sleep–wake state transitions and EEG power density produced by honokiol. Sleep–wake state transitions during the 4 h following the administration of honokiol (A) or flumazenil + honokiol (B).. W, N and R represent the stages for wakefulness, NREM and REM sleep respectively. Data shown are means ± SEM (n = 5–6). *P < 0.05, significantly different from vehicle: two-tailed paired t-test. EEG power density of NREM sleep after the administration of honokiol (C) or flumazenil + honokiol (D).
Honokiol changed the number and durations of episodes of wakefulness and NREM sleep and the transitions between these two states. These alterations were completely abolished by flumazenil (Figures 4B,D and 5B,D), supporting the conclusion that honokiol promotes sleep via the benzodiazepine site of the GABAA receptor.
Seeing that honokiol increased NREM sleep, we determined the EEG power spectra of NREM sleep in mice. As shown in Figure 5C, there were no significant differences in the EEG power density of NREM sleep between honokiol treatment and vehicle control, indicating that honokiol did not affect the EEG power density of NREM sleep. These results suggest that honokiol induces NREM sleep very similar to physiological NREM sleep.
Effects of honokiol on c-Fos expression in the VLPO and tuberomammillary nucleus (TMN)
To study the effects of honokiol on the VLPO sleep centre and the histaminergic TMN arousal centre, we investigated the number of c-Fos immunoreactive neurons in these areas after honokiol treatment. The VLPO consists of the VLPO cluster, just lateral to the optic chiasma, and the extended VLPO that extends dorsally and medially from this cluster (Lu et al., 2000). Figure 6A–D shows representative photomicrographs of immunostaining for c-Fos in the VLPO and TMN of mice treated with honokiol at 20 mg·kg−1 or with vehicle. Honokiol at 10 and 20 mg·kg−1 increased the expression of c-Fos in the VLPO cluster by 3.6- and 6.3-fold [Figure 6I, F(3,19) = 16.944, P < 0.01], respectively, relative to the vehicle control. Fos-positive neurons in the extended VLPO were also increased from 7.1 ± 1.4 to 16.8 ± 1.7 (P < 0.01) and 35.5 ± 2.6 (P < 0.01) after treatment of honokiol at 10 and 20 mg·kg−1[F(3,19) = 39.568, P < 0.01] respectively.
Figure 6.
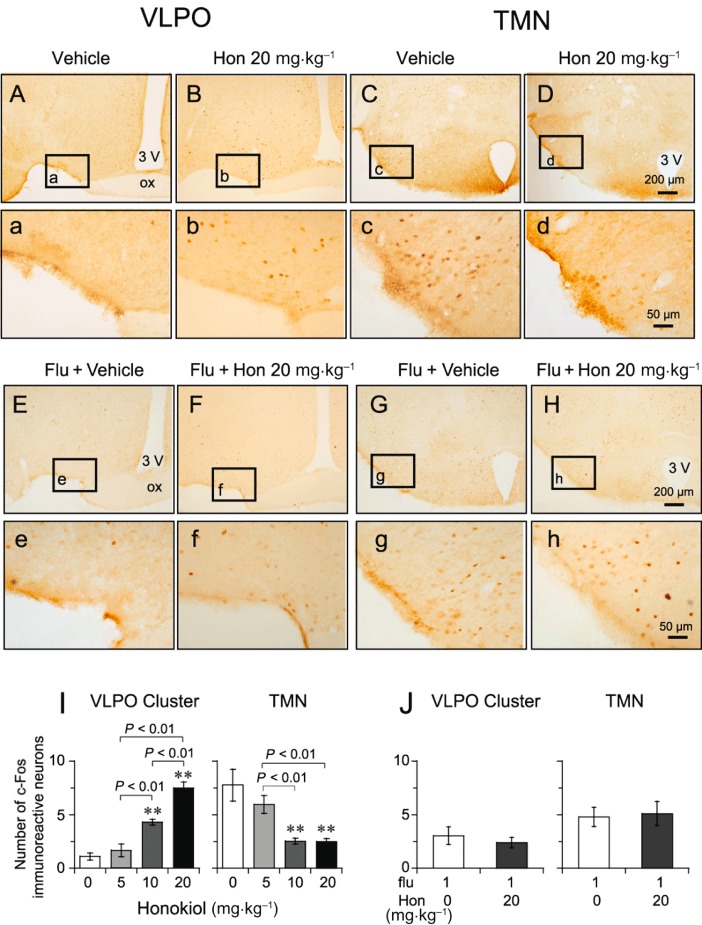
Effect of honokiol and flumazenil on c-Fos expression in the VLPO and the TMN. (A–D) Low-power and (a–d) high-power photomicrographs representative of the VLPO and TMN in mice treated with vehicle and honokiol (Hon; 20 mg·kg−1). (I) Mean numbers of Fos-positive cells in VLPO cluster and TMN after honokiol treatment. Pretreatment with flumazenil (Flu) at 1 mg·kg−1 antagonized Fos expression in VLPO and TMN induced by honokiol (20 mg·kg−1). (E–H) Low-power and (e–h) high-power photomicrographs representative of the VLPO and TMN in mice pretreated with flumazenil (1 mg·kg−1), then given vehicle or honokiol (20 mg·kg−1) 15 min later. (J) Flumazenil (1 mg·kg−1) blocked the change in the number of Fos-positive cells in the VLPO cluster and TMN caused by honokiol (Hon; 20 mg·kg−1). Data shown are the means ± SEM (n = 5–6). **P < 0.01, significantly different from the vehicle group; one-way anova, with Tukey's test.
Analysis of the numbers of c-Fos immunoreactive nuclei showed that honokiol at 10 and 20 mg·kg−1 decreased the expression of c-Fos in the TMN by 67% and 69% [Figure 6I, F(3,31) = 7.188, P < 0.01], respectively, relative to the vehicle control. These findings indicate that honokiol activates the VLPO sleep centre and inhibits the activity of the TMN arousal system.
Effects of flumazenil on increased c-Fos immunoreactivity induced by honokiol in the VLPO
Figure 6E–H shows composite drawings of representative photomicrographs of c-Fos expression in the VLPO and TMN in mice pretreated with flumazenil at 1 mg·kg−1, followed by honokiol at 20 mg·kg−1 or by vehicle. Flumazenil alone did not affect c-Fos expression compared with vehicle. Pretreatment with flumazenil (1 mg·kg−1) completely antagonized the change in the number of Fos-positive cells caused by honokiol given at 20 mg·kg−1 in VLPO cluster [F(1,6) = 0.321, P > 0.05], extended VLPO [F(1,6) = 0.659, P > 0.05] and TMN [F(1,6) = 0.082, P > 0.05] (Figure 6J).
Effects of honokiol on the firing rate of VLPO sleep-promoting neurons in in vitro brain slices
Electrophysiological experiments were conducted in acute brain slices. As shown in Figure 7A, the majority of cells within the VLPO were triangular and multipolar in shape. In the cell-attached configuration, the sleep-promoting neurons were inhibited by a brief application (50 s) of noradrenaline (10 µM) (Figure 7B), as shown earlier by Gallopin et al., (2000).
Figure 7.
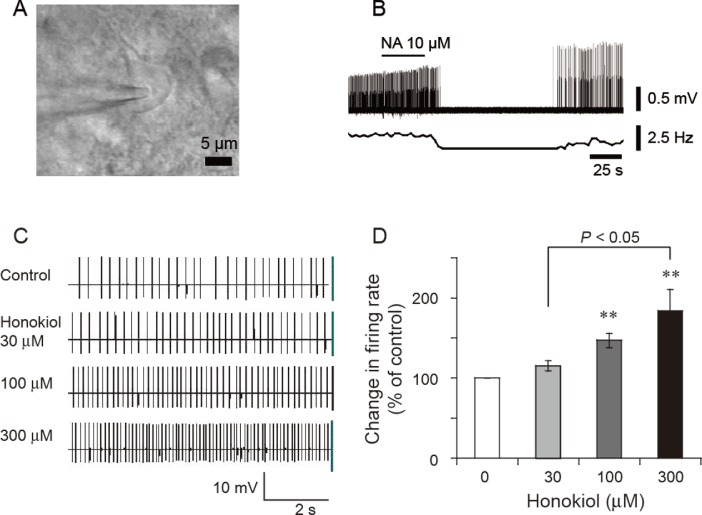
Effects of honokiol on the firing rate of VLPO sleep-promoting neurons in in vitro brain slices. (A) A multipolar and triangular neuron located in the VLPO with an extracellular recording electrode in place. (B) In the loosely cell-attached configuration, the sleep-promoting neuron was identified by noradrenaline (10 µM) inhibiting their firing. (C) Typical traces of the spontaneous firing in noradrenaline-inhibited VLPO neurons, in the presence of honokiol at a concentration of 30, 100 or 300 µM. (D) Relative changes in firing rate of the noradrenaline-inhibited VLPO neurons (n = 8–14) induced by various concentrations of honokiol. **P < 0.01, significantly different from baseline firing rate.
To determine the effects of honokiol on the activity of VLPO sleep-promoting neurons in acute brain slice of rat, loosely attached cells were employed under current-clamp mode. The sleep-promoting neurons were identified according to the inhibitory effect of noradrenaline on the firing rate. Bath application of honokiol to acute brain slices excited the majority of VLPO neurons, which were inhibited by noradrenaline. Honokiol at concentrations of 100 and 300 µM increased the firing rate of VLPO sleep-promoting neurons [F(2,27) = 5.215, P < 0.05] by 47% and 84% respectively (Figure 7C,D). These results suggest that honokiol can increase the firing rate of sleep-promoting neurons.
Discussion
In the present study, we found that honokiol increased the total duration of NREM sleep but did not change the EEG power density of NREM sleep. EEG delta activity is an indicator of the depth of NREM sleep (Tobler et al., 2001). In humans and rodents, benzodiazepines significantly decrease the total duration of wakefulness and increase the total duration of NREM sleep, but typically reduce EEG delta activity in NREM sleep (Feinberg et al., 2000; Tobler et al., 2001; Kopp et al., 2004). These effects are common for agonists acting at the benzodiazepine site, irrespective of whether they are benzodiazepines or non-benzodiazepine compounds, such as zolpidem or zopiclone (Aeschbach et al., 1994; Landolt et al., 2000). Unlike benzodiazepines, honokiol induced a type of NREM sleep very similar to physiological NREM sleep, suggesting that honokiol is suitable for the treatment of insomnia.
The GABAA receptor forms a supramolecular complex with the benzodiazepine receptor and flumazenil is a specific antagonist of the benzodiazepine site on the GABAA receptor. Consistent with previous reports, we found that flumazenil caused no obvious effect on sleep when used alone under baseline conditions (Chu et al., 2007). Honokiol-induced increases in NREM sleep were antagonized by flumazenil, suggesting that the benzodiazepine recognition site is one of the targets for the hypnotic action of honokiol.
Both diazepam and honokiol promote sleep, but diazepam suppresses the EEG delta activity of NREM sleep, whereas honokiol does not. Why the effects of honokiol on sleep are different from those of benzodiazepines still remains to be clarified. Probably, they act at different GABAA receptor subunits. Benzodiazepines have a similar affinity for α1, α2 and α3 subunits of GABAA receptors (Atack et al., 1999). Mouse models carrying knock-in point mutations in GABAA receptor subunits have shown that suppression of delta activity by diazepam, is related to the α2 subunits (Kopp et al., 2004), whereas the hypnotic action of diazepam is mediated by GABAA receptors containing α1 and/or α3 subtypes (Tobler et al., 2001; Kopp et al., 2003). Honokiol enhanced chloride currents through GABAA receptors of seven different subunit compositions and was most effective at those containing α3β2 > α2β2 > α1β2 > α1β1 subunits (Taferner et al., 2011), indicating that honokiol was more effective on α3 than α2-containing receptors, and thus may have less influence on EEG delta activity of NREM sleep. Taken together, these findings suggest that sleep promotion by benzodiazepines and honokiol is mediated by different GABAA receptor subunits.
The immediate early gene, c-Fos, is rapidly induced by many extracellular stimuli. Therefore, c-Fos expression has been proposed as a cellular marker for neuronal activity. Sleep-associated increases in the number of Fos-immunoreactive cells occur in the VLPO and the median preoptic nucleus (Sherin et al., 1996; Gong et al., 2000). Activation of sleep-active neurons in the VLPO and median preoptic nucleus (data not shown) by the administration of honokiol supports our conclusion. Our patch clamping study showed that honokiol increased the firing rate of sleep-promoting neurons in the VLPO. A previous in vitro study has revealed two major types of neurons in the VLPO: the majority (69%) were inhibited by noradrenaline and a few were excited by noradrenaline (Gallopin et al., 2000). The noradrenaline-inhibited neurons containing GABA are sleep-promoting neurons and project to major arousal systems, including the TMN (Sherin et al., 1996; 1998; Szymusiak et al., 1998; Gallopin et al., 2000). The noradrenaline-excited VLPO neurons exhibit inhibitory postsynaptic currents mediated by GABAA receptors, indicating that they contain functional GABAA receptors (Liu et al., 2010). There has been no report of the presence of non-GABA neurons in the VLPO. We therefore propose that the noradrenaline-excited VLPO neurons are GABA-containing neurons. When the organism is awake, the noradrenaline-inhibited VLPO neurons are probably under the inhibitory control of the noradrenaline-excited neurons. When honokiol acted upon the noradrenaline-excited neurons, this inhibitory control was removed, and firing rates of these noradrenaline-inhibited VLPO neurons were significantly increased. When excited, these VLPO neurons release GABA into the TMN, thus inhibiting the activity of this arousal-producing nucleus, inducing the onset of sleep. In this way, honokiol increases firing rates of sleep-promoting neurons in the VLPO, probably via removal of inhibitory control by noradrenaline-excited neurons.
Clinical and epidemiological studies have shown that sleep disturbance is tightly linked to major depression. Epidemiological studies showed a comparable prevalence of insomnia symptoms in patients with depression (Breslau et al., 1996) and about 41% of depressed patients have reported symptoms of insomnia (Stewart et al., 2006). Sleep problems have also been identified as a prodromal feature of recurrent depressive episodes (Perlis et al., 1997). In clinical samples, about three-quarters of all depressed patients complain of difficulty either in initiation or in maintaining sleep (Tsuno et al., 2005). Honokiol can treat anxiety and depression models in mice (Kuribara et al., 2000; Xu et al., 2008)and we have found that honokiol increased NREM sleep, suggesting that honokiol may be useful as a sleep-promoting substance, especially for the insomnia that accompanies anxiety and depression.
In conclusion, honokiol increased the amount of NREM sleep and shortened sleep latency in mice. Unlike more commonly used sleeping pills, such as those containing benzodiazepines, honokiol did not alter the EEG power density, indicating that honokiol induces a type of NREM sleep similar to physiological NREM sleep and may be suitable for the treatment of insomnia.
Acknowledgments
We thank Dr. Ji-Jiang Wang of the State Key Laboratory of Medical Neurobiology, Fudan University, for his helpful discussion. This study was supported, in part, by Grants-in-Aid for Scientific Research from the National Natural Science Foundation of China (30970955, 30901797, 31070957, 31171010 and 31121061), the Shanghai Committee of Science and Technology (09JC1402500, 10XD1400400, 10ZR1402100 and 10441901600), National Basic Research Program of China Grants (2009CB5220004 and 2011CB711000), Shanghai Leading Academic Discipline Project (B119), PhD Programs Foundation of Ministry of Education of China (20110071110033), China National Science and Technology Major Project for Drug Discovery (2009ZX09303-006), the Japan Society for the Promotion of Science, and the Program of Basic and Applied Researches for Innovations in Bio-oriented Industry of Japan.
Glossary
- ACSF
artificial cerebrospinal fluid
- EMG
electromyogram
- NREM
non-rapid eye movement
- PLSD
probable least-squares difference
- REM
rapid eye movement
- TMN
tuberomammillary nucleus
- VLPO
ventrolateral preoptic area
Conflicts of interest
All authors declare no conflict of interest.
References
- Aeschbach D, Dijk DJ, Trachsel L, Brunner DP, Borbely AA. Dynamics of slow-wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacology. 1994;11:237–244. doi: 10.1038/sj.npp.1380110. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M. Abuse, dependence, and epileptic seizures after zolpidem withdrawal: review and case report. Clin Neuropharmacol. 2000;23:281–283. doi: 10.1097/00002826-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Atack JR, Smith AJ, Emms F, McKernan RM. Regional differences in the inhibition of mouse in vivo [3H]Ro 15-1788 binding reflect selectivity for alpha 1 versus alpha 2 and alpha 3 subunit-containing GABAA receptors. Neuropsychopharmacology. 1999;20:255–262. doi: 10.1016/S0893-133X(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Bernert RA, Cromer KR, Joiner TE, Schmidt NB. Social anxiety and insomnia: the mediating role of depressive symptoms. Depress Anxiety. 2008;25:124–130. doi: 10.1002/da.20282. [DOI] [PubMed] [Google Scholar]
- Chen C, Tan R, Qu W, Wu Z, Wang Y, Urade Y, et al. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, exerts anti-epileptic effects via GABA-benzodiazepine receptor complex in mice. Br J Pharmacol. 2011;164:1534–1546. doi: 10.1111/j.1476-5381.2011.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu QP, Wang LE, Cui XY, Fu HZ, Lin ZB, Lin SQ, et al. Extract of Ganoderma lucidum potentiates pentobarbital-induced sleep via a GABAergic mechanism. Pharmacol Biochem Behav. 2007;86:693–698. doi: 10.1016/j.pbb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Ding DZ. Application of Banxia houpu decoction in psychiatric disorder. Shaanxi J Tradit Chin Med. 1992;13:412–413. [Google Scholar]
- Feinberg I, Maloney T, Campbell IG. Effects of hypnotics on the sleep EEG of healthy young adults: new data and psychopharmacologic implications. J Psychiatr Res. 2000;34:423–438. doi: 10.1016/s0022-3956(00)00038-8. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2079–R2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Mochizuki T, Qu WM, Hong ZY, Watanabe T, Urade Y, et al. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc Natl Acad Sci U S A. 2006;103:4687–4692. doi: 10.1073/pnas.0600451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Rudolph U, Keist R, Tobler I. Diazepam-induced changes on sleep and the EEG spectrum in mice: role of the alpha3-GABA(A) receptor subtype. Eur J Neurosci. 2003;17:2226–2230. doi: 10.1046/j.1460-9568.2003.02651.x. [DOI] [PubMed] [Google Scholar]
- Kopp C, Rudolph U, Low K, Tobler I. Modulation of rhythmic brain activity by diazepam: GABA(A) receptor subtype and state specificity. Proc Natl Acad Sci U S A. 2004;101:3674–3679. doi: 10.1073/pnas.0306975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H, Stavinoha WB, Maruyama Y. Behavioural pharmacological characteristics of honokiol, an anxiolytic agent present in extracts of Magnolia bark, evaluated by an elevated plus-maze test in mice. J Pharm Pharmacol. 1998;50:819–826. doi: 10.1111/j.2042-7158.1998.tb07146.x. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Kishi E, Kimura M, Weintraub ST, Maruyama Y. Comparative assessment of the anxiolytic-like activities of honokiol and derivatives. Pharmacol Biochem Behav. 2000;67:597–601. doi: 10.1016/s0091-3057(00)00401-9. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Finelli LA, Roth C, Buck A, Achermann P, Borbely AA. Zolpidem and sleep deprivation: different effect on EEG power spectra. J Sleep Res. 2000;9:175–183. doi: 10.1046/j.1365-2869.2000.00192.x. [DOI] [PubMed] [Google Scholar]
- Li JM, Kong LD. Advances in the study on depressive and anxiety disorders treated with traditional Chinese medicine and herbal drugs. Zhongguo Zhong Yao Za Zhi. 2001;26:805–807. [PubMed] [Google Scholar]
- van Lier H, Drinkenburg WH, van Eeten YJ, Coenen AM. Effects of diazepam and zolpidem on EEG beta frequencies are behavior-specific in rats. Neuropharmacology. 2004;47:163–174. doi: 10.1016/j.neuropharm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Liu YW, Li J, Ye JH. Histamine regulates activities of neurons in the ventrolateral preoptic nucleus. J Physiol. 2010;588:4103–4116. doi: 10.1113/jphysiol.2010.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42:209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Zhang R, Sun FY. Enhancement of ischemia-induced tyrosine phosphorylation of Kv1.2 by vascular endothelial growth factor via activation of phosphatidylinositol 3-kinase. J Neurochem. 2003;87:1509–1517. doi: 10.1046/j.1471-4159.2003.02110.x. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Qu WM, Xu XH, Yan MM, Urade Y, Huang ZL. D(1)/D(2) receptor-targeting L-stepholidine, an active ingredient of the Chinese herb Stephonia, induces non-rapid eye movement sleep in mice. Pharmacol Biochem Behav. 2009;94:16–23. doi: 10.1016/j.pbb.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi N, Nambu F, et al. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci U S A. 2006;103:17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Xu XH, Yan MM, Wang YQ, Urade Y, Huang ZL. Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J Neurosci. 2010;30:4382–4389. doi: 10.1523/JNEUROSCI.4936-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Besset A, Bebbington P, Brugha T, Lindesay J, Jenkins R, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–1397. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Taferner B, Schuehly W, Huefner A, Baburin I, Wiesner K, Ecker GF, et al. Modulation of GABAA-receptors by honokiol and derivatives: subtype selectivity and structure-activity relationship. J Med Chem. 2011;54:5349–5361. doi: 10.1021/jm200186n. [DOI] [PubMed] [Google Scholar]
- Teng CM, Chen CC, Ko FN, Lee LG, Huang TF, Chen YP, et al. Two antiplatelet agents from Magnolia officinalis. Thromb Res. 1988;50:757–765. doi: 10.1016/0049-3848(88)90336-2. [DOI] [PubMed] [Google Scholar]
- Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc Natl Acad Sci U S A. 2001;98:6464–6469. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Kales A, Bixler EO. Benzodiazepine side effects: role of pharmacokinetics and pharmacodynamics. Pharmacology. 1995;51:205–223. doi: 10.1159/000139363. [DOI] [PubMed] [Google Scholar]
- Winokur A, Gary KA, Rodner S, Rae-Red C, Fernando AT, Szuba MP. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19–28. doi: 10.1002/da.1043. [DOI] [PubMed] [Google Scholar]
- Xu Q, Yi LT, Pan Y, Wang X, Li YC, Li JM, et al. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:715–725. doi: 10.1016/j.pnpbp.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Yan MM, Xu XH, Huang ZL, Yao MH, Urade Y, Qu WM. Selection of optimal epoch duration in assessment of rodent sleep-wake profiles. Sleep Biol Rhythms. 2011;9:46–55. [Google Scholar]


