Abstract
Ascorbic acid is well known to acutely stimulate norepinephrine synthesis in neurosecretory cells, but it has also been shown over several days to increase tyrosine hydroxylase mRNA and norepinephrine synthesis in cultured neurons. Since tyrosine hydroxylase is the rate-limiting step in catecholamine synthesis, an effect of ascorbate to increase tyrosine hydroxylase protein could contribute to its ability to increase or sustain catecholamine synthesis. Therefore, we evaluated whether tyrosine hydroxylase protein expression and function is increased in SH-SY5Y neuroblastoma cells by physiologically relevant intracellular ascorbate concentrations. SH-SY5Y neuroblastoma cells did not contain ascorbate and had only very low levels of norepinephrine in culture with L-tyrosine, the substrate for tyrosine hydroxylase. However, treatment of cells with ascorbate for 6 hours or more markedly increased norepinephrine synthesis, such that intracellular ascorbate and norepinephrine increased in parallel with half maximal intracellular concentrations of about 1 mM ascorbate and 150 µM norepinephrine. This increase was enhanced by supplementing tetrahydrobiopterin, but was not mimicked by several antioxidants or by catalase or superoxide dismutase. Tyrosine hydroxylase protein expression increased at intracellular ascorbate concentrations above 1.5 mM. This contributed to norepinephrine generation, which was decreased 50–60% by inhibition of protein synthesis with cycloheximide at high intracellular ascorbate. These results suggest that expected physiologic neuronal ascorbate concentrations enhance norepinephrine synthesis both by maintaining tetrahydrobiopterin and increasing tyrosine hydroxylase expression.
Keywords: catecholamines, tyrosine hydroxylase, norepinephrine, dopamine, SH-SY5Y neuroblastoma cells
1. Introduction
Neuronal catecholamine synthesis is acutely enhanced by ascorbic acid at two steps in the pathway [1]. First, ascorbate helps to maintain the activity of tyrosine hydroxylase by recycling its essential co-factor, tetrahydrobiopterin. Ascorbate does this in a one-electron reduction of the trihydrobiopterin radical that is generated on hydroxylation of L-tyrosine by tyrosine hydroxylase. Ascorbate is much more efficient in this recycling than cellular thiol antioxidants, including reduced glutathione and cysteine [2]. Second, ascorbate directly contributes an electron to dopamine β-hydroxylase in neurosecretory vesicles to allow it to hydroxylate dopamine to form norepinephrine. Without ascorbate, the activity of dopamine β-hydroxylase is low, possibly maintained by an electron from dopamine itself [3,4]. Both of these reactions are acute and depend on existing levels of the enzymes and co-factors involved.
A third effect of ascorbate to stimulate catecholamine synthesis may also be to enhance the transcription of tyrosine hydroxylase. Seitz, et al. [5] reported that culture of SK-N-SH neuroblastoma cells with 200 µM ascorbate for 5 days more than doubled both intracellular norepinephrine levels and tyrosine hydroxylase mRNA, although tyrosine hydroxylase protein was not measured. In that work, a 2 h treatment with ascorbate increased dopamine and norepinephrine synthesis from L-tyrosine, but did not affect tyrosine hydroxylase mRNA.
In this work we examined the mechanism by which ascorbate stimulates norepinephrine generation from L-tyrosine in SH-SY5Y neuroblastoma cells, finding that optimal stimulation by ascorbate required at least 6 h, occurred at what are likely physiologic intracellular ascorbate concentrations, and was associated with increased tyrosine hydroxylase protein expression.
2. Materials and methods
2.1. Materials
Sigma/Aldrich Chemical Co. (St. Louis, MO) supplied the analytical reagents, including Lascorbic acid, bovine liver catalase, L-3,4-dihydroxyphenylalanine (L-DOPA), dopamine, norepinephrine, N-2-hydroxyethylpiperazine N’-2-ethanesulfonic acid (Hepes), sepiapterin, bovine liver superoxide dismutase, Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), and Trolox (6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid).
2.2. Cell Culture
SH-SY5Y neuroblastoma cells were obtained from the American Type Culture Collection) and were cultured in Dulbecco’s minimal essential medium containing 10% (v/v) fetal bovine serum, which was prepared by the Cell Culture Core of the Vanderbilt Diabetes Research and Training Center. This culture medium contained 100 µM L-tyrosine. Cells were cultured to confluence at 37 °C in humidified air containing 5% CO2. All experiments were performed in culture medium, with rinsing of the cells after the experiment 2 times in 2 ml of Krebs-Ringer-Hepes buffer (KRH) at 37 °C. KRH buffer consisted of 20 mM Hepes, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2, pH 7.4.
2.3 Assay of catecholamines
The catecholamines norepinephrine, dopamine, and L-DOPA were measured in cell extracts by high performance liquid chromatography as previously described [6] with minor modifications. After treatments as noted for cells at confluence in 6-well culture plates, culture medium was removed followed by 2 rinses of cells with 2 ml of KRH. The cells were lysed in 5 0.5 ml of the HPLC mobile phase buffer, scraped from the plate and centrifuged at 13, 000 ×g for 1 min. Aliquots (0.1 ml) of the supernatant were taken for duplicate assays. The mobile phase consisted of 100 mM trichloroacetic acid, 10 mM sodium acetate, 0.1 mM EDTA, and 10.5% methanol. The isocratic flow rate was 1 ml/minute, pumped by an ECS Model 582 pump. The column was an Absorbosphere C18, 5 µ (4.6 × 150 mm). Samples were injected on a Rheodyne model 7725i injector and catecholamines were detected using an ESA Model 5011 analytic cell set at 0.5 volts on an ESA Model 5100A detector. Peak heights were quantified on a Shimadzu C-R5A integrator. Under these conditions, the void volume was 1.8 ml, ascorbate eluted at 2.2 min, norepinephrine eluted at 4.0 min, L-DOPA at 6.6 min, and dopamine eluted at 11 min. Sensitivity for norepinephrine was 50 nM. Intracellular concentrations of ascorbate and norepinephrine were calculated based on the intracellular space of 3-O-[3H]methylglucose in SH-SY5Y cells, which was 10.7 ± 3.4 µl/mg protein (N=24, ± SD) [7]. This space was measured as described previously for endothelial cells in culture [8]. Protein was measured by the Bradford method as described by the manufacturer (Bio-Rad Laboratories, Hercules, CA).
2.4 Western blotting
Western blotting was performed as previously described [9]. Confluent cells were lysed with Radio-Immunoprecipitation Assay (RIPA) buffer (#R0278, Sigma-Aldrich, St. Louis, MO). Solubilized protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis according to the method of Laemmli [10]. Tyrosine hydroxylase was probed with an affinitypurified rabbit polyclonal antibody (1:400) (sc-14007, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Protein levels were expressed relative to β-actin (1:400) to account for possible differences in protein loading (sc-1616, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Following appropriate secondary incubations, bands were illuminated using ECL Plus Western 6 blotting reagents (RPN 2132, Amersham Biosciences, Piscataway, NJ). Densitometry was determined using ImageJ and tyrosine hydroxylase expression was normalized to that of β-actin.
2.5 Data Analysis
Results are shown as mean + standard error. Statistical comparisons were made using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA). Differences between treatments were assessed by one- or two-way ANOVA as noted in the figure legends. Parametric or non-parametric tests were used, depending on whether the data appeared have a normal distribution and equal variances.
3. Results
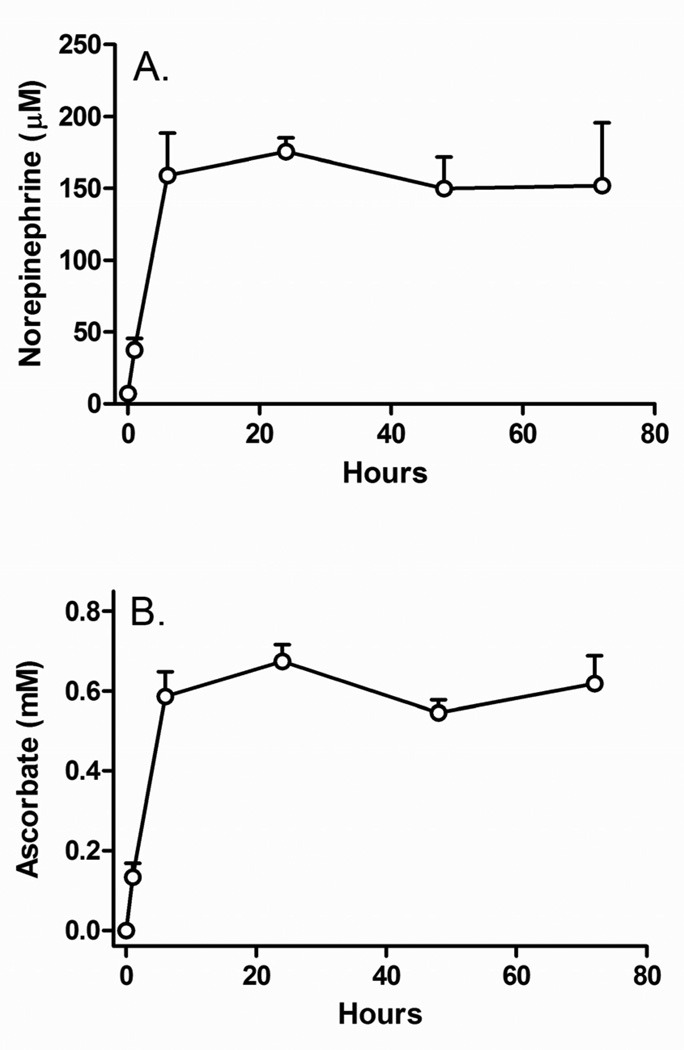
In the absence of added ascorbate, SH-SY5Y cells in culture contained very low amounts of norepinephrine (7.0 ± 3.1 µM, Fig. 1A, circle at zero hours) and no ascorbate (Fig. 1B, circle at zero hours). When cells were incubated with 100 µM ascorbate for various times, the norepinephrine content (Fig. 1A) of the cells increased in concert with intracellular ascorbate (Fig. 1B), both requiring 6 h to become maximal. Dopamine and L-DOPA levels were very low in these cells (results not shown), so norepinephrine was used to measure catecholamine generation.
Figure 1. Time course of stimulation of norepinephrine generation by ascorbate.
Panel A: Time-dependent accumulation of intracellular norepinephrine when SH-SY5Y cells were treated every for the times indicated in culture with 100 µM ascorbate. For incubations beyond 24 h, fresh ascorbate was added again at 24 h intervals. Panel B: Intracellular concentrations of ascorbate measured in the same cells as in Panel A. Results are shown from 4 separate experiments.
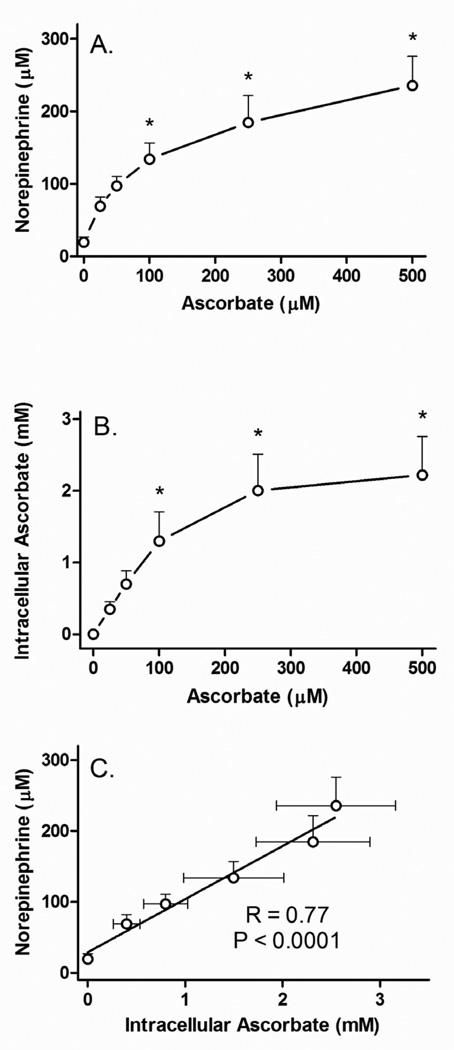
When cells were treated for 24 h in culture with a single addition of increasing concentrations of ascorbate, there was a progressive increase in norepinephrine (Fig. 2A) that again mirrored the intracellular ascorbate content (Fig. 2B). Increases in both ascorbate and norepinephrine tended to plateau above about 100 µM loading ascorbate. Ascorbate was concentrated up to 10-fold compared to the initial loading concentrations (Fig. 2B). Plotting norepinephrine as a function of the measured ascorbate concentration resulted in a significant linear dependence of intracellular norepinephrine on intracellular ascorbate (Fig. 2C).
Figure 2. Concentration dependence of ascorbate stimulation of norepinephrine synthesis.
Panel A: SH-SY5Y ells in culture were treated for 24 h with the indicated concentration of ascorbate, rinsed twice in in KRH and taken for assay of norepinephrine (Panel A) and ascorbate (Panel B). In Panel C, intracellular norepinephrine concentrations are plotted as a function of the measured intracellular ascorbate concentrations. The solid line shows the linear regression fit. Results are shown from 7 experiments, with an “*” indicating p < 0.05 compared to cells not treated with ascorbate by one-way ANOVA using the Kruskal-Wallis test followed by Dunn’s multiple comparison test.
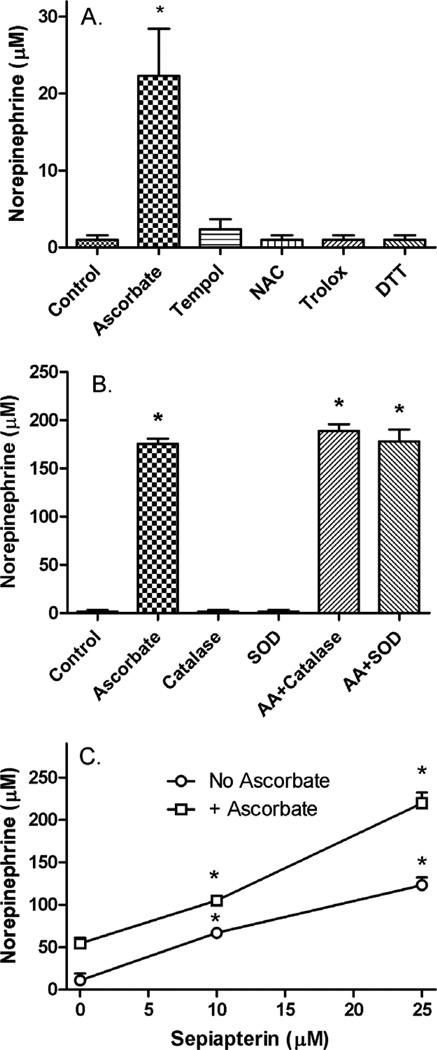
Treatment of cells for 24 h with concentrations of several antioxidants known to be effective in preventing oxidative stress in cultured cells did not enhance norepinephrine synthesis compared to a low concentration of ascorbate (Fig. 3A). To further test whether a pro-oxidant effect of ascorbate could account for its ability to increase norepinephrine, cells were cultured for 24 h with a relatively high concentration of ascorbate (500 µM) in the presence of catalase or superoxide dismutase (Fig. 3B). Neither enzyme alone increased norepinephrine, nor did either enzyme blunt the effects of ascorbate to increase norepinephrine levels. On the other hand, a 24 h treatment of cells with sepiapterin, which is a precursor to tetrahydrobiopterin through the salvage pathway [11], did progressively increase norepinephrine synthesis (Fig. 3C, circles), an 8 effect that was enhanced by ascorbate with a modest synergistic effect on the absolute norepinephrine levels at 25 µM sepiapterin.
Figure 3. Stimulation of norepinephrine synthesis by ascorbate compared to other agents.
Panel A: Cells were treated for 24 h in culture with the following agents: No additions (Control), ascorbate (50 µM), Tempol (250 µM), N-acetylcysteine (NAC, 250 µM), Trolox (250 µM), and dithiothreitol (DTT, 250 µM). After 2 rinses in KRH, cells were taken for assay of norepinephrine. Results are shown from 5 experiments, with an “*” indicating p < 0.05 compared to Control by one-way ANOVA using the Kruskal-Wallis test followed by Dunn’s multiple comparison test. Panel B: Cells in culture were treated for 24 h with no additions (Control), 500 µM ascorbate (AA), 750 units/ml catalase (CAT), 140 units/ml superoxide dismutase (SOD), or combinations thereof. After 24 h cells were taken for assay of norepinephrine. Results are shown from 3 experiments, with “*” indicating p < 0.01 compared to Control, catalase alone, or superoxide dismutase alone by repeated measures ANOVA and Tukey’s test. Panel C: Cells were treated in culture with the indicated concentrations of sepiapterin, without (circles) or with (squares) 50 µM ascorbate. At 24 h cells were rinsed twice and taken for assay of intracellular norepinephrine. Results are shown from 4 experiments, with “*” indicating p < 0.05 compared to zero sepiapterin with or without ascorbate by repeated measures ANOVA and Tukey’s test. The two curves were different at each point (p < 0.001) by two-way ANOVA and the Bonferroni test.
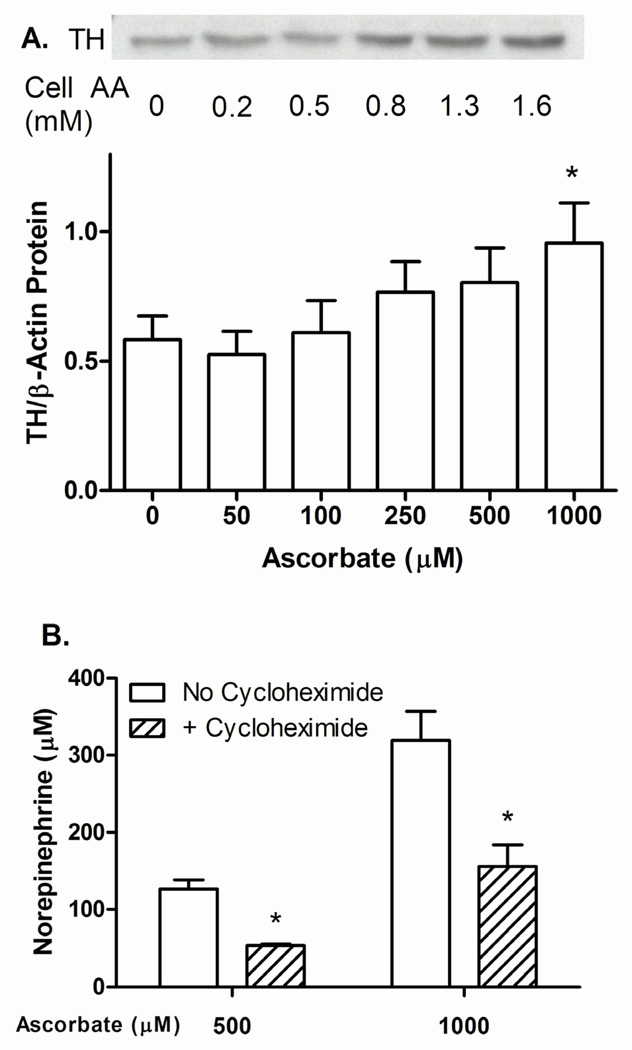
To test whether ascorbate might increase tyrosine hydroxylase protein, cells were treated for 24 h with increasing ascorbate concentrations and the cells taken for immunoblotting of tyrosine hydroxylase. Tyrosine hydroxylase was detected as a 60 kilodalton band, the intensity of which progressively increased above an intracellular ascorbate concentration of 0.5 mM in the example shown in Fig. 4A. Data from 4 separate experiments (Fig. 4B) also showed a progressive increase above an ascorbate loading concentration of 100 µM, although statistical significance was not reached until the loading concentration was 1000 µM, or between 1.6 and 2.5 mM intracellular ascorbate. The maximal increase was 170% of control
Figure 4. Stimulation of tyrosine hydroxylase expression and norepinephrine generation by ascorbate.
Panels A and B: SH-SY5Y cells were treated in culture with the indicated concentration of ascorbate for 24 h, rinsed twice in KRH, and removed from the plate for Western blotting as described in Methods. Panel A shows a representative Western blot with measured intracellular ascorbate concentrations in a parallel cell incubation. Panel B shows Western blots from 4 separate experiments, normalized for gel protein loading to β-actin. “TH” = tyrosine hydroxylase. An “*” indicates p < 0.05 compared to zero ascorbate by repeated measures one-way ANOVA and Dunn’s test. Panel C: Cells were treated for 24 h in culture with the indicated concentration of ascorbate in the absence or presence of 5 µg/ml cycloheximide, as indicated. Results are shown from 4 experiments with an asterisk (*) indicating p < 0.05 compared to cells not treated with cycloheximide by two-way ANOVA and the Bonferroni test.
To assess the extent to which ascorbate-stimulated tyrosine hydroxylase protein expression contributed to its ability to stimulate norepinephrine synthesis, SH-SY5Y cells were incubated for 24 h in the presence of relatively high concentrations of ascorbate with or without 5 µg/ml cycloheximide, an inhibitor of protein synthesis. As shown in Fig. 4C, cycloheximide decreased ascorbate-stimulated norepinephrine levels by 60% and 50%, at 500 µM and 1000 µM ascorbate, respectively. Intracellular ascorbate concentrations in these cells were 1.9 ± 0.13 mM at 500 µM loading ascorbate, and 2.2 ± 0.04 at 1000 µM loading ascorbate. Intracellular ascorbate concentrations were not significantly affected by co-treatment with cycloheximide (500 µ ascorbate = 1.75 ± 0.04 mM; 1000 µM ascorbate = 2.0 ± 0.04 mM).
4. Discussion
SH-SY5Y cells contained very little norepinephrine and no detectable ascorbate in culture with 100 µM L-tyrosine, the substrate for tyrosine hydroxylase. However, treatment of the cells for 6 hours with ascorbate increased norepinephrine to maximal levels. This effect was not mimicked by several other cell-penetrant antioxidants, including the one-electron-donor Tempol, a water-soluble vitamin E analog (Trolox), and two different thiol reagents (N-acetyl cysteine and dithiothreitol). It is also possible that ascorbate-stimulated norepinephrine synthesis could be due to a pro-oxidant effect of the vitamin, which is known to be caused by generation of H2O2 and other products by ascorbate in Dulbecco’s minimal essential medium [12,13]. However, culture of cells with either catalase or superoxide dismutase did not diminish the increase in norepinephrine generation due even to a relatively high concentration of ascorbate. This, and the finding that thiols, which also can generate H2O2 in culture media [14], failed to increase norepinephrine levels, suggest that stimulation of norepinephrine generation is specific for ascorbate and that it is not due to a pro-oxidant effect.
Seitz, et al. [5] previously reported stimulation of catecholamine synthesis after 2 h of ascorbate treatment in SK-N-SH cells, although ascorbate-stimulated levels of L-DOPA and dopamine were 20–30-fold greater than those of norepinephrine in that study. Very little LDOPA or dopamine were generated in response to ascorbate in SH-SY5Y cells, which suggests that they convert dopamine to norepinephrine more efficiently than the cells from which they were derived. The response to ascorbate in both neuronal-derived cell types differs from that observed in extracts from rat brain corpus striatum and rat pheochromocytoma PC-12 cells, in which ascorbate inhibited the activity of tyrosine hydroxylase [15]. However, in situ catecholamine levels were not measured in that study and measurement of TH enzyme activity in extracts may not reflect behavior of the enzyme in situ.
A major finding of this work is that a 24 h treatment of SH-SY5Y cells increased tyrosine hydroxylase protein in a concentration-dependent manner to about 170% of basal when the intracellular ascorbate concentration was 1.6 mM and higher. This result shows that the 2–3-fold increase in tyrosine hydroxylase mRNA after 5 days of every other day treatment with 200 µM ascorbate observed by Seitz, et al. [5] extends to tyrosine hydroxylase protein expression, although at a more modest level. Such an ascorbate effect on protein expression is novel and likely contributes to the marked increase in norepinephrine in 24 h cultures observed in the present study at higher intracellular concentrations of the vitamin. This is also supported by the finding that a 24 h treatment of cells with cycloheximide to inhibit new protein synthesis partially prevented the ability of ascorbate to stimulate norepinephrine generation. The mechanism of this ascorbate-induced increase in tyrosine hydroxylase expression remains to be determined, but it has been attributed to activation of a cyclic AMP response element on the tyrosine hydroxylase promoter [5]. This is based on findings that increases in intracellular cyclic AMP [16] or protein kinase A [17] increased tyrosine hydroxylase mRNA levels in PC-12 cells, and also on the observation that ascorbate increased cyclic AMP in hypothalamic neuronal cultures [18].
It is clear from the present study that SH-SY5Y cells are “deficient” in tetrahydrobiopterin, since supplementation of the cells with the tetrahydrobiopterin precursor sepiapterin alone caused a modest increase in cellular norepinephrine levels. This response was significantly increased by ascorbate supplements at 24 h, either because of the ascorbate effect on tyrosine hydroxylase expression, or because ascorbate continued to recycle tetrahydrobiopterin near the enzyme active site, or both. Our data and that of Seitz, et al. [5] favor a combined mechanism in which tetrahydrobiopterin recycling accounts for stimulation of norepinephrine generation during the first 1–2 hours after ascorbate addition, whereas increases in tyrosine hydroxylase expression contribute to greater increases in norepinephrine levels after at least 6 hours, especially at higher intracellular ascorbate concentrations.
It is thus relevant to consider whether intracellular ascorbate levels that increase norepinephrine levels and tyrosine hydroxylase expression are in the physiologic range. We found in the present and in a previous study that treatment of SH-SY5Y cells with 100–250 µM ascorbate for 24 h results in intracellular ascorbate levels of 1.5 to 2 mM, which significantly increased tyrosine hydroxylase protein expression. Since ascorbate concentrations measured in CSF are also 100–300 µM [19,20], it seems likely that intracellular ascorbate in vivo will be in the range required for increased tyrosine hydroxylase expression. Indeed, ascorbate concentrations in neurons may be even higher, since whole cortex ascorbate levels are 4 µmol/g in mice [21], which would translate into even higher levels when expressed as a function of the intracellular cytoplasmic space.
In conclusion, loading SH-SY5Y cells with ascorbate for 24 h markedly increased intracellular levels of norepinephrine, an effect due in part to enhanced tyrosine hydroxylase expression as well as to the well-known effect of ascorbate to recycle tetrahydrobiopterin required for enzyme function. It will be important to assess the mechanism of this effect of ascorbate on tyrosine hydroxylase expression, as well as whether it is affected by ascorbate deficiency or excess in vivo.
Highlights.
Ascorbic acid enhances norepinephrine synthesis in SH-SY5Y neuroblastoma cells
Norepinephrine synthesis increases linearly with intracellular ascorbate
Ascorbate enhances tetrahydrobiopterin-stimulated norepinephrine synthesis
Ascorbate increases protein expression of tyrosine hydroxylase
Ascorbate increases tyrosine hydroxylase expression at physiologic concentrations
Acknowledgments
This work was supported by NIH grant NS 057674 and by the Vanderbilt Diabetes Research and Training Center (DK 020593).
Abbreviations used
- Hepes
N-2-hydroxyethylpiperazine-NN-2-ethanesulfonic acid
- KRH
Krebs-Ringer Hepes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diliberto EJ, Jr, Daniels AJ, Viveros OH. Multicompartmental secretion of ascorbate and its dual role in dopamine beta-hydroxylation. Am. J. Clin. Nutr. 1991;54:1163S–1172S. doi: 10.1093/ajcn/54.6.1163s. [DOI] [PubMed] [Google Scholar]
- 2.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols - Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 3.Levin EY, Kaufman S. Studies on the enzyme catalyzing the conversion of 3,4- dihydroxyphenylethylamine to norepinephrine. J. Biol. Chem. 1961;236:2043–2049. [PubMed] [Google Scholar]
- 4.Stewart LC, Klinman JP. Characterization of alternate reductant binding and electron transfer in the dopamine beta-monooxygenase reaction. Biochemistry. 1987;26:5302–5309. doi: 10.1021/bi00391a013. [DOI] [PubMed] [Google Scholar]
- 5.Seitz G, Gebhardt S, Beck JF, Bohm W, Lode HN, Niethammer D, Bruchelt G. Ascorbic acid stimulates DOPA synthesis and tyrosine hydroxylase gene expression in the human neuroblastoma cell line SK-N-SH. Neurosci. Lett. 1998;244:33–36. doi: 10.1016/s0304-3940(98)00129-3. [DOI] [PubMed] [Google Scholar]
- 6.Lindsey JW, Jung AE, Narayanan TK, Ritchie GD. Acute effects of a bicyclophosphate neuroconvulsant on monoamine neurotransmitter and metabolite levels in the rat brain. J Toxicol. Environ. Health A. 1998;54:421–429. doi: 10.1080/009841098158827. [DOI] [PubMed] [Google Scholar]
- 7.May JM, Li L, Hayslett K, Qu ZC. Ascorbate transport and recycling by SH-SY5Y neuroblastoma cells: Response to glutamate toxicity. Neurochem. Res. 2006;31:785–794. doi: 10.1007/s11064-006-9077-z. [DOI] [PubMed] [Google Scholar]
- 8.Jones W, Li X, Perriott LM, Whitesell RR, May JM. Uptake, recycling, and antioxidant functions of α-lipoic acid in endothelial cells. Free Radic. Biol. Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 9.Meredith ME, Harrison FE, May JM. Differential regulation of the ascorbic acid transporter SVCT2 during development and in response to ascorbic acid depletion. Biochem. Biophys. Res. Commun. 2011;414:737–742. doi: 10.1016/j.bbrc.2011.09.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Sheng JZ, Wang D, Braun AP. DAF-FM (4-Amino-5-methylamino-2',7'- difluorofluorescein) diacetate detects impairment of agonist-stimulated nitric oxide synthesis by elevated glucose in human vascular endothelial cells: Reversal by vitamin C and L-sepiapterin. J. Pharmacol. Exp. Ther. 2005;315:931–940. doi: 10.1124/jpet.105.087932. [DOI] [PubMed] [Google Scholar]
- 12.Clement MV, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid. Redox. Signal. 2001;3:157–163. doi: 10.1089/152308601750100687. [DOI] [PubMed] [Google Scholar]
- 13.Long LH, Halliwell B. Artefacts in cell culture: pyruvate as a scavenger of hydrogen peroxide generated by ascorbate or epigallocatechin gallate in cell culture media. Biochem. Biophys. Res. Commun. 2009;388:700–704. doi: 10.1016/j.bbrc.2009.08.069. [DOI] [PubMed] [Google Scholar]
- 14.Long LH, Halliwell B. Oxidation and generation of hydrogen peroxide by thiol compounds in commonly used cell culture media. Biochem. Biophys. Res. Commun. 2001;286:991–994. doi: 10.1006/bbrc.2001.5514. [DOI] [PubMed] [Google Scholar]
- 15.Wilgus H, Roskoski R., Jr Inactivation of tyrosine hydroxylase activity by ascorbate in vitro and in rat PC12 cells. J Neurochem. 1988;51:1232–1239. doi: 10.1111/j.1471-4159.1988.tb03092.x. [DOI] [PubMed] [Google Scholar]
- 16.Lewis EJ, Harrington CA, Chikaraishi DM. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc. Natl. Acad. Sci. U. S. A. 1987;84:3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KS, Park DH, Wessel TC, Song B, Wagner JA, Joh TH. A dual role for the cAMP-dependent protein kinase in tyrosine hydroxylase gene expression. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3471–3475. doi: 10.1073/pnas.90.8.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W, Yang Z, Lee D, Copolov DL, Lim AT. Ascorbic acid enhances forskolininduced cyclic AMP production and pro-ANF mRNA expression of hypothalamic neurons in culture. Endocrinology. 1993;132:2271–2273. doi: 10.1210/endo.132.5.8386616. [DOI] [PubMed] [Google Scholar]
- 19.Reiber H, Ruff M, Uhr M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin. Chim. Acta. 1993;217:163–173. doi: 10.1016/0009-8981(93)90162-w. [DOI] [PubMed] [Google Scholar]
- 20.Miele M, Fillenz M. In vivo determination of extracellular brain ascorbate. J. Neurosci. Methods. 1996;70:15–19. doi: 10.1016/S0165-0270(96)00094-5. [DOI] [PubMed] [Google Scholar]
- 21.Harrison FE, Best JL, Meredith ME, Gamlin CR, Borza DB, May JM. Increased expression of SVCT2 in a new mouse model raises ascorbic acid in tissues and protects against paraquat-induced oxidative damage in lung. PLoS. One. 2012;7:e35623. doi: 10.1371/journal.pone.0035623. [DOI] [PMC free article] [PubMed] [Google Scholar]