Abstract
Peptides influence cardiac dysfunction; however, peptidergic modulation of contractile performance remains relatively uncharacterized. We identified a novel human peptide that modulates mammalian contractile performance. Members of the FMRFamide-related peptide (FaRP) family contain a C-terminal RFamide but structurally variant N-terminal extension. We report human RFamide-related peptide-1 (hRFRP-1) and rat RFRP-1 rapidly and reversibly decreased shortening and relaxation in isolated mammalian cardiac myocytes in a dose dependent manner. The mammalian FaRP, 26RFa, structurally related to RFRP-1 by only an RFamide did not influence myocyte contractile function. The protein kinase C (PKC) inhibitor bisindolylmaleimide-1 blocked hRFRP-1 activity. Pretreatment with pertussis toxin (PTX) did not diminish hRFRP-1 influence on contractile function. In addition, intravenous injection of hRFRP-1 in mice decreased heart rate, stroke volume, ejection fraction, and cardiac output. Collectively these findings are consistent with the conclusion RFRP-1 is an endogenous signaling molecule that activates PKC and acts through a PTX-insensitive pathway to modulate cardiac contractile function. Taken together these negative chronotropic, inotropic, and lusitropic effects of hRFRP-1 are significant; they suggest direct acute cellular and organ-level responses in mammalian heart. This is the first known study to identify a mammalian FaRP with cardio-depressant effects, opening a new area of research on peptidergic modulation of contractile performance. The high degree of RFRP structure conservation from amphibians to mammals, and similarity to invertebrate cardioinhibitory peptides suggests RFRP-1 is involved in important physiological functions. Elucidation of mechanisms involved in hRFRP-1 synthesis, release, and signaling may aid the development of strategies to prevent or attenuate cardiac dysfunction.
Keywords: 26RFa, brain, cardiovascular, Drosophila, FMRFamide, heart, myosuppressin, protein kinase C (PKC)
1. Introduction
Heart failure is the leading cause of death, yet the peptidergic mechanisms involved in cardiac dysfunction are not completely understood. Identifying small cardioregulatory peptides is significant because it may provide potential target molecules for drug development and therapeutic approaches to address cardiac dysfunction. The first RFamide-containing peptide discovered was the invertebrate tetrapeptide, FMRFamide [19]. Isolation of FMRFamide from clam ganglia as a cardioregulatory peptide led to the subsequent identification of structurally-related bio- and cardio-active peptides throughout the animal kingdom [7, 9, 10, 17]. The vast majority of FaRP-related cardiovascular research to date has been done in invertebrates. Mammalian FMRFamide-related peptides (FaRPs) are of interest in cardiovascular research because they are expressed in regions of the central nervous system involved in cardiac regulation [2, 7, 10, 13, 15, 24, 30]. However, relatively little is known about the influence of FaRPs on cardiovascular function in mammals.
The FaRP superfamily of FMRFamide-related peptides can be subdivided into smaller groups based on the XRFamide motif, where X defines the subgroup. The invertebrate myosuppressin peptides are members of the LRFamide subgroup. Myosuppressins have been extensively studied in invertebrates as cardioactive peptides which decrease heart rate and amplitude of ejection [1, 16, 18, 20-22, 26]. However, a cardioactive LRFamide peptide has not been reported in mammals.
The mammalian RFamide-related peptide (RFRP) gene encodes RFRP-1, which contains a C-terminal LRFamide [11, 13]. The human RFRP-1 peptide (hRFRP-1; MPHSFANLPLRFamide) was isolated from human hypothalamus [23]. Structurally similar peptides were isolated from frog brain (SLKPAANLPLRFamide) and bovine hypothalamus (MPPSFANLPLRFamide), and predicted from the rat RFRP gene (VPHSAANLPLRFamide) [5, 10, 11, 13]. Additionally, clusters of hRFRP-1 immunoreactive neurons and fibers are found in mammalian hypothalamus and nucleus tractus solitarius, an important site for integrative regulation of the cardiovascular system [10, 23, 24, 30]. However, the effects of RFRP-1 peptides on cardiovascular function are not reported. Based on peptide structure and cellular expression our hypothesis was RFRP-1 may be a cardioactive peptide in vertebrates. To test our prediction, the functional effects of hRFRP-1 were characterized in isolated mammalian cardiac myocytes and mouse hearts in vivo.
2. Material and methods
2.1. Myocyte isolation and measurement of sarcomere length shortening in single myocytes
Adult rat and rabbit ventricular cardiac myocytes were isolated as previously described [27-29]. Hearts from Sprague-Dawley rats and New Zealand white rabbits were perfused and enzymatically digested to isolate myocytes; the protocol was approved by The University of Michigan University Committee on Use and Care of Animals (UCUCA) in accordance with university and federal regulatory guidelines. Aliquots of isolated ventricular myocytes were plated on laminin-coated glass coverslips in serum-containing Dulbecco's Modified Eagle Media (Invitrogen, CA, US) supplemented with 5% fetal bovine serum, and 50 U/ml penicillin and 50 μg/ml streptomycin (pen/strep; Sigma-Aldrich, MO, US). Two hours later, media was replaced with serum-free M199 (Invitrogen) supplemented with 1.8 mM Ca2+, 10 mM HEPES, 10 mM glutathione, and pen/strep. Rat myocytes were transferred to a stimulation chamber and electrically paced the day after isolation. Media was changed daily for all myocyte preparations. Sarcomere shortening was detected using a video-based detection system (IonOptix, MA, USA) as described earlier. Rat myocytes were paced at 0.2 Hz and rabbit myocytes were paced at 0.5 Hz or 1 Hz for these studies. Recordings were made prior to and at 1, 3, 5, 10 and 15 minutes after application of each peptide concentration, the protein kinase C (PKC) inhibitor bisindolylmaleimide-1, (bis-1; CalBiochem/EMD, NJ, US) in dimethyl sulfoxide (DMSO, Sigma-Aldrich), 3 hours after treatment with pertussis toxin (PTX; Sigma-Aldrich), or media only (control). Signal averaged data were analyzed to determine resting sarcomere length, shortening amplitude (peak shortening), shortening rate (departure velocity), and re-lengthening rate (return velocity), as previously described [27-29], in 6-26 myocytes from 3-5 rat hearts for each peptide concentration, bis-1, PTX-pretreatment, and media only.
2.2. Peptide syntheses
Peptides were synthesized by standard Fmoc protocol. The following structures were confirmed by amino acid analysis and mass spectrometry: MPHSFANLPLRFamide, hRFRP-1, VPHSAANLPLRFamide, rat RFRP-1 (rRFRP-1), LAEELSSYSRRKGGFSFRFamide, 26RFa(8-26), and KGGFSFRFamide, 26RFa(19-26).
2.3. Echocardiography
Echocardiograms were performed as previously described [3] according to the recommendations of the American Society of Echocardiography. All echocardiography was performed by one registered echocardiographer. Female C57BL/6 mice were weighed to accurately calculate the amount of peptide delivered per kilogram body weight (kg bw). Animal use for echocardiography was approved by The University of Michigan UCUCA in accordance with university and federal regulatory guidelines. Physiological saline or peptide was intravenously delivered via tail-vein injections to a total maximum volume of 150 μl to achieve 5 μmols or 500 ηmols hRFRP-1/kg bw. Each animal was used only once for an injectant, either physiological saline or peptide (n = 4-5). Briefly, a mouse was placed in an induction chamber and lightly sedated with 4% isoflurane mixed with 100% oxygen, then placed in a supine position on a heated platform with electrocardiogram contact pads (VEVO™ mouse handling platform; VisualSonics, ON, CA), and its nose placed in a cone with 1% isoflurane in 100% oxygen. High-resolution, two-dimensionally guided recordings of amplitude and rate of motion (M-mode) were obtained with a real-time 30-MHz microvisualization scanhead, RMV™ 707B, interfaced to a Vevo 770™ in vivo micro-imaging system (VisualSonics). Heart rate along with left ventricular end-systolic and end-diastolic dimensions were measured from the two-dimensional sector scans obtained from the parasternal long axis and apical four chamber views using the conventions of the American Society of Echocardiography. For each M-mode measurement, at least three consecutive cardiac cycles were sampled. Left ventricular volumes were measured at end systole (Vols) and end diastole (Vold) and used to calculate stroke volume (SV = Vold − Vols) and ejection fraction (EF % = endocardial SV/endocardial Vold × 100). Cardiac output (CO = endocardial SV × heart rate) was calculated from stroke volume and heart rate.
2.4. Statistical analysis
All values reported are expressed as mean ± standard error of mean (SEM). Data were analyzed using a 1-way analysis of variance (ANOVA) and a Dunnett's Multiple Comparison Test was performed as a post hoc test; statistical significance was established at a p value < 0.05. The half-maximal effective concentration (EC50) values were calculated from best-fit curves using either Microsoft Excel XP or GraphPad Prism 3.0 statistics software (GraphPad, CA, USA).
3. Results
3.1. Human RFRP-1 produces dose-dependent effects on rat cardiac myocyte contractile function
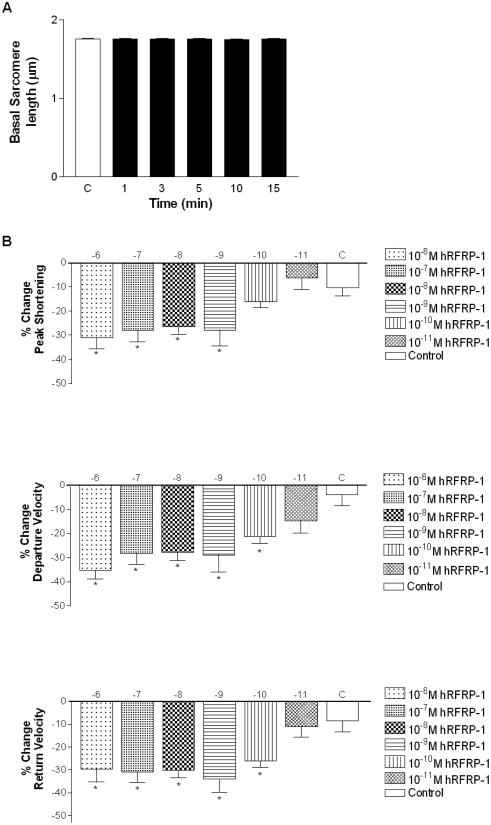
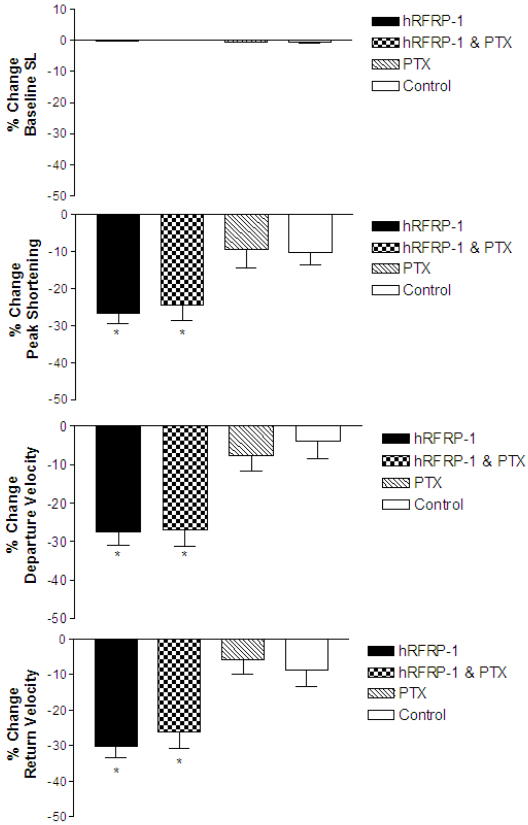
The influence of hRFRP-1 on cardiac function was measured in isolated adult rat cardiac myocytes to test our hypothesis that this vertebrate FaRP is a cardioactive peptide in mammals. Acute dose-dependent alterations in sarcomere shortening were measured over 15 minutes in response to 10−6 M to 10−11 M hRFRP-1 in isolated adult rat myocytes. There was no significant effect of 10−11 M hRFRP-1 compared to control, and resting sarcomere length remained unchanged at all peptide concentrations (Fig. 1A). Human RFRP-1 peptide dramatically decreased shortening amplitude, and shortening and re-lengthening rates in the isolated cardiac myocytes (Fig. 1B; Table 1). Significant reductions in the shortening and re-lengthening rates were detected in response to concentrations of 10−10 M hRFRP-1 and higher (Fig. 1B; Table 1). A ten-fold increase to 10−9 M hRFRP-1 and higher was required to detect significant reductions in shortening amplitude (Fig. 1B; Table 1). The best-fit EC50 values were 5×10−10 M, 5×10−11 M, and 5×10−11 M for shortening amplitude, and shortening and re-lengthening rates, respectively. These results demonstrate hRFRP-1 acutely modulates contractile function at the cellular level by directly acting on mammalian cardiac myocytes.
Fig. 1.
Basal sarcomere length and percent change in peak shortening, departure velocity, and return velocity in response to 15 minute perfusion with 10−6 M to 10−11 M hRFRP-1 at 37°C and paced at 0.2 Hz in isolated adult rat cardiac myocytes (Table 1). Fig. 1A. The resting sarcomere lengths were 1.76 ± 0.01 μm (baseline), 1.76 ± 0.01 μm (1 minute), 1.76 ± 0.01 μm (3 minutes), 1.76 ± 0.01 μm (5 minutes), 1.75 ± 0.01 μm (10 minutes), and 1.75 ± 0.01 μm (15 minutes). Fig. 1B. Percent change in peak shortening, departure velocity, and return velocity during 15 minutes perfusion with peptide. Values for each concentration (y-axis; hRFRP-1 (log [ ])) were compared to media control (C) with 1-way ANOVA followed by a Dunnett's Multiple Comparison Test with p < 0.05 considered statistically significant (*; Table 1). The best-fit EC50 values were calculated to be 5×10−10 M (peak shortening), 5×10−11 M (departure velocity), and 5×10−11M (return velocity). Recordings were made from n = 7-20, 1-day and 2-day myocytes isolated from n = 1-4 hearts.
Table 1.
The influence of hRFRP-1 on adult rat cardiac myocyte contractile function over 15 minutes (* denotes statistical significance from control, p < 0.05).
| % Change (mean ± SEM) | |||||
|---|---|---|---|---|---|
| hRFRP-1 (log []) | n | baseline SL (μm) | peak s (μm) | dep v (μm/sec) | ret v (μm/sec) |
| −6 | 10 | 0.3 ± 0.3 | −31.2 ± 4.4* | −35.2± 3.8* | −29.7 ± 5.4* |
| −7 | 18 | −0.3 ± 0.2 | −27.9 ± 4.9* | −28.2 ± 4.6* | −31.0 ± 4.6* |
| −8 | 14 | −0.01 ± 0.2 | −26.6 ± 2.9* | −27.7 ± 3.5* | −30.1 ±3.2* |
| −9 | 10 | 0.3 ± 0.1 | −26.8 ± 7.4* | −29.5 ± 7.9* | −31.7 ± 6.2* |
| −10 | 7 | −0.06 ± 0.2 | −9.6 ± 2.7 | −23.1 ± 4.3* | −21.8 ± 2.9* |
| −11 | 10 | −0.2 ± 0.2 | −6.3 ± 4.7 | −14.7 ± 5.2 | −11.2 ± 4.5 |
| Control | 20 | −0.1 ± 0.1 | −10.3 ± 3.4 | −4.0 ± 4.5 | −8.7 ± 4.6 |
3.2. Rat RFRP-1 mimics the influence of hRFRP-1 on rat cardiac myocyte contractile function
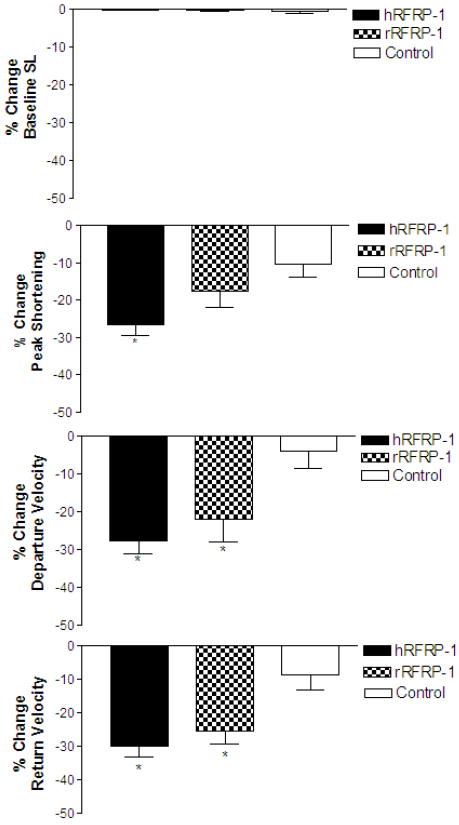
To begin to investigate the structure specificity of RFRP-1 on contractile function we compared the effects of two mammalian RFRP-1 peptides, rat and human, on isolated cardiac myocytes. Rat RFRP-1 (VPHSAANLPLRFamide) differs from hRFRP-1 (MPHSFANLPLRFamide) by two amino acids in the N-terminal amino acid extension, M1→V1 and F5→A5 (Table 2). The influence of 10−8 M rRFRP-1 was compared to 10−8 M hRFRP-1 and control (media only) in isolated adult rat cardiac myocytes (Fig. 2; Table 3). The rat RFRP-1 peptide decreased shortening amplitude, and shortening and re-lengthening rates (−17.7 ± 4.3%, −22.1 ± 6.0%, −25.5 ± 3.9%, respectively; n = 12) without significant changes in resting sarcomere length. Thus, similar changes were observed in the shortening and re-lengthening rates with 10−8 M rRFRP-1 and 10−8 M hRFRP-1. However, the greater effect of 10−8 M hRFRP-1 on shortening amplitude compared to 10−8 M rRFRP-1 suggests the structure differences of the N-terminal extensions of these two peptides may provide important clues into ligand binding and future characterization of an RFRP-1 receptor(s).
Table 2.
RFamide peptide structures compared; the amino acids and post-translational modification strictly conserved between the RFRP-1 peptides are in bold type.
| Human RFRP-1 | M | P | H | S | F | A | N | L | P | L | R | F | amide | |||||||
| Rat RFRP-1 | V | P | H | S | A | A | N | L | P | L | R | F | amide | |||||||
| Rat 26RFa (8-26) | L | A | E | E | L | S | S | Y | R | R | R | K | G | G | F | S | F | R | F | amide |
| Rat 26RFa (19-26) | K | G | G | F | S | F | R | F | amide |
Fig. 2.
Percent change in baseline sarcomere length (SL), peak shortening, departure velocity, and return velocity in response to 15 minutes of perfusion with 10−8 M rRFRP-1, 10−8 M hRFRP-1, and control (media, only) in isolated adult rat cardiac myocytes (Table 3). There were no significant changes in baseline sarcomere length. The influence of 10−8 M rRFRP-1 and 10−8 M hRFRP-1 on departure velocity and in return velocity were comparable and significantly different from control values; however, 10−8 M rRFRP-1 did not produce the significant decrease in peak shortening observed with 10−8 M hRFRP-1 and was not significantly different from the media only control response. Data were analyzed using 1-way ANOVA followed by a Dunnett's Multiple Comparison Test with p < 0.05 considered statistically significant (*; Table 3). Recordings were made from n = 12-14, 1-day and 2-day myocytes isolated from n = 2-4 hearts.
Table 3.
The influence of rRFRP-1 on adult rat cardiac myocyte contractile function over 15 minutes (* denotes statistical significance from control, p < 0.05).
| % Change (mean ± SEM) | |||||
|---|---|---|---|---|---|
| n | baseline SL (μm) | peak s (μm) | dep v (μm/sec) | ret v (μm/sec) | |
| 10−8M hRFRP-1 | 14 | −0.01 ± 0.2 | −26.6 ± 2.9* | −27.7 ± 3.5* | −30.1 ± 3.2* |
| 10−8M rRFRP-1 | 12 | −0.3 ± 0.1 | −17.7 ± 4.3 | −22.1 ± 6.0* | −25.5 ± 3.9* |
| Control | 20 | −0.1 ± 0.1 | −10.3 ± 3.4 | −4.0 ± 4.5 | −8.7 ± 4.6 |
3.3. The vertebrate FaRP 26RF, does not mimic RFRP-1 influence on rat cardiac myocyte contractile function
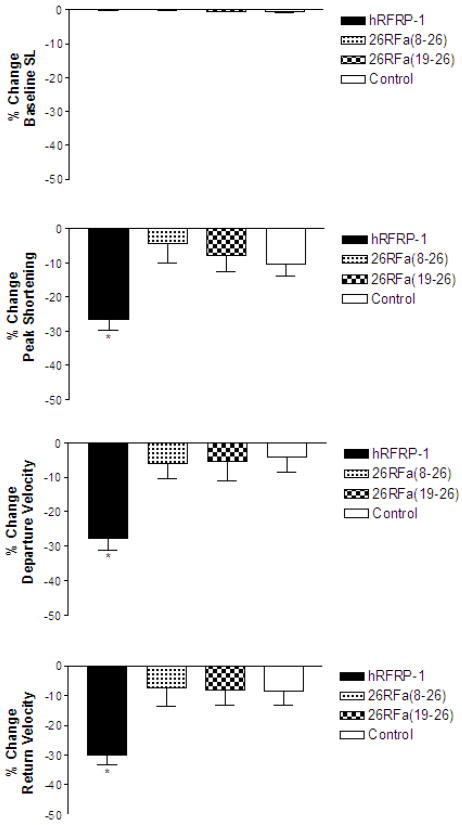
RFamide-related peptide-1 structure-activity requirements were further investigated using rat 26RFa [8], which is a FaRP and thus contains an RFamide C terminus. Although the RFamide C terminus is identical, the structure and length of the 26RFa N-terminal extension is different from RFRP-1. The effects of 10−8 M 26RFa(8-26) (LAEELSSYSRRKGGFSFRFamide) and 10−8 M 26RFa(19-26) (KGGFSFRFamide) were measured and compared to hRFRP-1 in isolated adult rat cardiac myocytes (Fig. 3; Table 4). The decreases in shortening amplitude, and shortening and re-lengthening rates in response to 10−8 M 26RFa(8-26) and 10−8 M 26RFa(19-26) were modest, not different from control (p > 0.05), and statistically less than the response to 10−8 M hRFRP-1 (Fig. 3; Table 4; p < 0.05). These results provide direct evidence for the conclusion that the RFRP-1 N-terminal extension is required for its influences on cardiac contractile function, and the strictly conserved C-terminal RFamide is not sufficient for the effect of RFRP-1 on mammalian cardiac myocytes. These results show 26RFa peptides do not induce a RFRP-1-like response in cardiac myocytes.
Fig. 3.
Percent change in baseline sarcomere length (SL), peak shortening, departure velocity, and return velocity in response to 15 minutes of perfusion with 10−8 M 26RFa(8-26), 10−8 M 26RFa(19-26), 10−8 M hRFRP-1, or control (media only) in isolated adult rat cardiac myocytes (Table 4). There were no significant changes in baseline sarcomere length. There were no significant effects on peak shortening, departure velocity, and return velocity in response to 10−8 M 26RFa(8-26) or 10−8 M 26RFa(19−26). Data were analyzed using 1-way ANOVA followed by a Dunnett's Multiple Comparison Test with p < 0.05 considered statistically significant (*; Table 4). Recordings were made from n = 17-20, 1-day and 2-day myocytes isolated from n = 2 hearts.
Table 4.
The influence of 26RFa peptides on adult rat cardiac myocyte contractile function over 15 minutes (* denotes statistical significance from control, p < 0.05).
| % Change (mean ± SEM) | |||||
|---|---|---|---|---|---|
| n | baseline SL (μm) | peak s (μm) | dep v (μm/sec) | ret v (μm/sec) | |
| 10−8M hRFRP-1 | 14 | −0.01 ± 0.2 | −26.6 ± 2.9* | −27.7 ± 3.5* | −30.1 ± 3.2* |
| 10−8M 26RFa(8-26) | 17 | −0.04 ± 0.1 | −4.3 ± 5.7 | −6.0 ± 4.4 | −7.3 ± 6.3 |
| 10−8M 26RFa(19-26) | 20 | −0.4 ± 0.2 | −7.7 ± 4.8 | −5.3 ± 5.6 | −8.1 ± 5.0 |
| Control | 20 | −0.1 ± 0.1 | −10.3 ± 3.4 | −4.0 ± 4.5 | −8.7 ± 4.6 |
3.4. Human RFRP-1 produces cardio-depressant effects in mouse
The influence of hRFRP-1 on cardiovascular function in vivo was then studied to determine whether integrated responses similar to the cellular effects were observed in mammals. Cardiac function was measured by echocardiography after peptide or saline (control) was delivered via intravenous tail-vein injections in mice [3]. Representative two-dimensional M-mode recordings (Fig. 4) demonstrate diminished cardiac function in response to 5 μmols/kg bw hRFRP-1 compared to control. At both concentrations (5μmols and 500 ηmols/kg bw), hRFRP-1 produced a peak effect at 5 minutes post-injection with partial recovery of cardiac function by 15 minutes. The higher dose of hRFRP-1 produced acute and dramatic effects on cardiovascular function (n = 5; Fig. 4A, pre-injection; Fig. 4B, 5 minutes post-injection; Table 5) compared to the modest variations observed in the saline control group (n = 4; Fig. 4C, pre-injection; Fig. 4D, 5 minutes post-injection; Table 5). Interestingly, the negative chronotropic effect of the lower hRFRP-1 dose (500 ηmols/kg bw) was absent. Although this dose significantly decreased stroke volume, ejection fraction, and cardiac output, the relative magnitude was attenuated compared to the higher dose (Table 5). These in vivo data are consistent with a direct dose- dependent effect of hRFRP-1 on myocardium which is in agreement with the influence of hRFRP-1 observed in isolated adult rat cardiac myocytes.
Fig. 4.
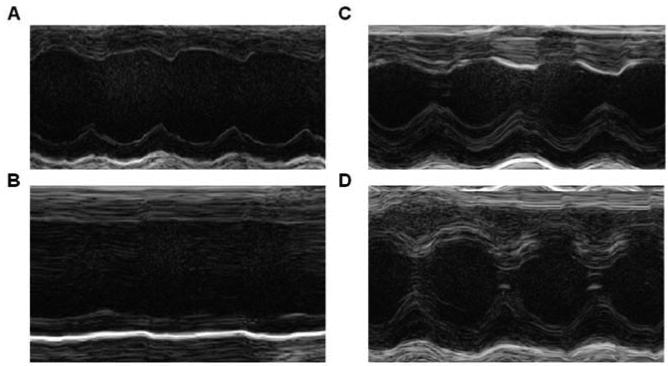
Representative M-mode images in response to hRFRP-1 and saline (control) on mouse heart. Intravenous administration of hRFRP-1 at 5 μmols/kg bw (n = 5) resulted in acute and dramatic effects on heart function (Fig. 4A = pre-injection; Fig. 4B = 5 minutes post-injection). Composite results for echocardiographic studies are shown in Table 5. However, saline (n = 4) did not result in dramatic changes in cardiac function (Fig. 4C = pre-injection; Fig. 4D = 5 minutes post-injection). Data were analyzed using 1-way ANOVA followed by a Dunnett's Multiple Comparison Test with p < 0.05 considered a statistically significant difference from control (*; Table 5).
Table 5.
Echocardiographic assessment of mouse cardiovascular function in response to intravenous hRFRP-1 at 5 minutes (* denotes statistical significance from control, p < 0.05).
| % Change (mean ± SEM) | ||||
|---|---|---|---|---|
| HR | SV | EF % | CO | |
| Saline | −17 ± 6% | 17 ± 3% | 32% ± 3% | −20 ± 2% |
| 500 ηmol/kg bw hRFRP-1 | −25 ± 2% | −28 ± 10%* | −33% ± 10%* | −44 ± 9%* |
| 5 μmol/kg bw hRFRP-1 | −54 ± 7%* | −57 ± 9%* | −49% ± 8%* | −79 ± 7%* |
3.5. Human RFRP-1 effects on rat cardiac myocyte contractile function in the presence of bis-1, a PKC inhibitor
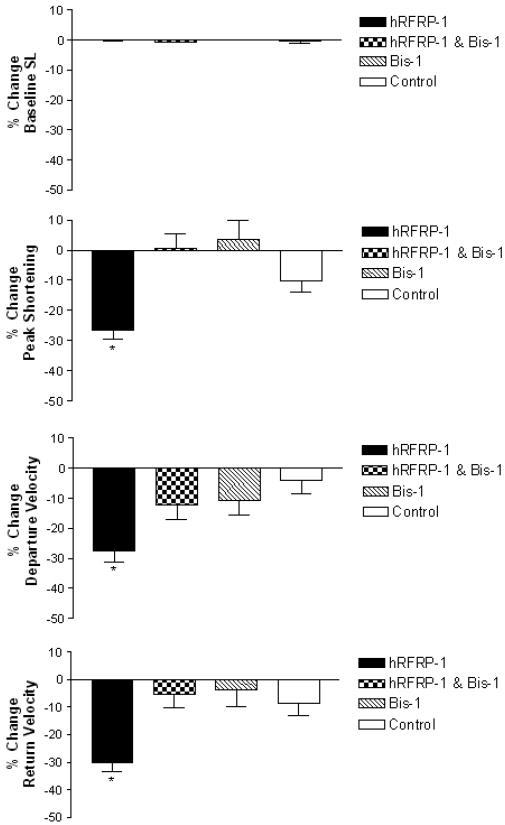
In order to initiate studies of the mechanisms involved in the influence of hRFRP-1 on cardiac function, the PKC inhibitor bis-1 was used as previously established [27]. The effect of PKC inhibitor bis-1 (500 ηM) on the influence of 10−8 M hRFRP-1 on shortening and relaxation was measured in isolated adult rat cardiac myocytes over 15 minutes (Fig. 5, Table 6). Bisindolylmaleimide-1 largely blocked the influence of 10−8 M hRFRP-1 on shortening amplitude, and shortening and re-lengthening rates (−0.58 ± 4.8%, −12.3 ± 4.6%, −5.6 ± 4.7%, respectively; n = 23) without significant changes in sarcomere resting length. The effect of bis-1 on hRFRP-1 activity was statistically different from peptide in the absence of the PKC inhibitor. Application of bis-1 dissolved in DMSO did not affect cardiac myocyte contractile function (Fig. 5; Table 6). These results provide direct evidence to support the conclusion that the influence of RFRP-1 on cardiac myocyte contractile function involves activation of a PKC signaling pathway.
Fig. 5.
Percent change in baseline sarcomere length (SL), peak shortening, departure velocity, and return velocity in response to 15 minute perfusion with 10−8 M hRFRP-1 in the presence of 5 × 10−7 M bis-1, or 10−8 M hRFRP-1 only, and controls of 5 × 10−7 M bis-1 or media only (Table 6). There were no significant changes in baseline sarcomere length. The influence of 10−8 M hRFRP-1 in the presence of 5 × 10−7 M bis-1 was blocked and was not significantly different from control on peak shortening, departure time, and return velocity. Application of bis-1 in DMSO was not significantly different from control. Data were analyzed using 1-way ANOVA followed by a Dunnett's Multiple Comparison Test with p < 0.05 considered statistically significant (*; Table 6). Recordings were made from n = 6-23, 1-day and 2-day myocytes isolated from n = 1-3 hearts.
Table 6.
The influence of hRFRP-1 in the presence of bis-1 on adult rat cardiac myocyte contractile function over 15 minutes (* denotes statistical significance from control, p < 0.05).
| % Change (mean ± SEM) | |||||
|---|---|---|---|---|---|
| n | baseline SL (μm) | peak s (μm) | dep v (μm/sec) | ret v (μm/sec) | |
| 10−8M hRFRP-1 | 14 | −0.01 ± 0.2 | −26.6 ± 2.9* | −27.7 ± 3.5* | −30.1 ± 3.2* |
| Bis-1; 10−8M hRFRP-1 | 23 | −0.52 ± 0.2 | 0.58 ± 4.8 | −12.3 ± 4.6 | −5.6 ± 4.7 |
| Bis-1 | 6 | 0.06 ± 0.1 | −10.5 ± 5.1 | −3.4 ± 6.3 | −3.9 5.9± |
| Control | 20 | −0.1 ± 0.1 | −10.3 ± 3.4 | −4.0 ± 4.5 | −8.7 ± 4.6 |
3.6. Human RFRP-1 effects on rat cardiac myocyte contractile function in the presence of PTX, a Gi protein inhibitor
Next we examined the involvement of PTX-sensitive guanine nucleotide binding proteins (G proteins) in the influence of hRFRP-1 on cardiac myocyte contractile function. Pertussis toxin treatment of isolated cells was performed according to established methods [12]. Isolated adult rat cardiac myocytes were pretreated with 100 ηg/ml PTX for 3 hours before cardiac myocyte contractility was measured in response to 10−8 M hRFRP-1 or media only. The pretreatment had no effect on the influence of hRFRP-1 on myocyte contractile function (Fig. 6; Table 7). The human RFRP-1 peptide decreased shortening amplitude, and shortening and re-lengthening rates (−24.4 ± 4.3%, −26.0 ± 4.4%, −26.1 ± 4.6%, respectively; n = 9) without significant changes in resting sarcomere length in PTX-treated myocytes. Thus, similar changes were observed in the influence of hRFRP-1 on shortening amplitude, and shortening and re-lengthening rates in the presence and absence of PTX pretreatment. PTX pretreatment also did not influence the application of media only on contractile function (Fig.6; Table 7). These results suggest hRFRP-1 does not act through a G inhibitory (Gi) protein-mediated signaling pathway to influence cardiac myocyte contractile function. This is consistent with a mechanism activating PKC, which is insensitive to PTX.
Fig. 6.
Percent change in baseline sarcomere length (SL), peak shortening, departure velocity, and return velocity in response to 15 minute perfusion with 10−8 M hRFRP-1 after the pretreatment of myocytes with 10−7 M PTX, 10−8 M hRFRP-1 only, and controls of media only in myocytes pretreated with 10−7 M PTX or media only in myocytes not pretreated with PTX (Table 7). There were no significant changes in baseline sarcomere length. The influence of 10−8 M rRFRP-1 in myocytes pretreated with 10−7 M PTX and 10−8 M hRFRP-1 only on departure time and in return velocity were comparable and significantly different from control values. Data were analyzed using 1-way ANOVA followed by a Dunnett's Multiple Comparison Test with p < 0.05 considered statistically significant (*; Table 7). Recordings were made from n = 7-9, 1-day and 2-day myocytes isolated from n = 2-3 hearts.
Table 7.
The influence of hRFRP-1 in the presence of PTX on adult rat cardiac myocyte contractile function over 15 minutes (* denotes statistical significance from control, p < 0.05).
| % Change (mean ± SEM) | |||||
|---|---|---|---|---|---|
| n | baseline SL (μm) | peak s (μm) | dep v (μm/sec) | ret v (μm/sec) | |
| 10−8M hRFRP-1 | 14 | −.01 ± 0.2 | −26.6 ± 2.9* | −27.7 ± 3.5* | −30.1 ± 3.2* |
| PTX; 10−8M hRFRP-1 | 9 | 0.2 ± 0.1 | −24.4 ± 4.3* | −26.9 ± 4.4* | −26.1 ± 4.6* |
| PTX | 7 | −0.4 ± 0.2 | −9.6 ± 4.9 | −7.7 ± 3.8 | −5.8 ± 4.1 |
| Control | 20 | −0.1 ± 0.1 | −10.3 ± 3.4 | −4.0 ± 4.5 | −8.7 ± 4.6 |
3.7. Human RFRP-1 attenuates rabbit cardiac myocyte contractile function
Our research turned to rabbit cardiac myocytes to further evaluate the effects and mechanism of the conserved RFRP-1 peptide in cardiac function. Human RFRP-1 was examined in isolated adult rabbit cardiac myocytes due to their similarity in heart rate compared to humans, relative to rat. At sub-nanomolar concentrations of hRFRP-1 the peptide significantly reduced mammalian cardiac function. Compared to media only (control), 10−10 M hRFRP-1 dramatically decreased shortening amplitude and re-lengthening rates in isolated adult rabbit cardiac myocytes (Fig. 7). The shortening rate also decreased in response to 10−10 M hRFRP-1; however, it was not significant in the myocytes studied. The recordings were made at 0.5 Hz (Fig. 7) and at 1 Hz (results not shown) to assess both shortening and the potential to initiate arrhythmic contractions. Arrhythmic beats and after-contractions were not observed in response to hRFRP-1 at either pacing frequency. These functional results establish that physiologically relevant concentrations of hRFRP-1 dramatically decreased contractile function in both rat and rabbit cardiac myocytes and suggest hRFRP-1 may play a direct role in modulating mammalian cardiac function.
Fig. 7.
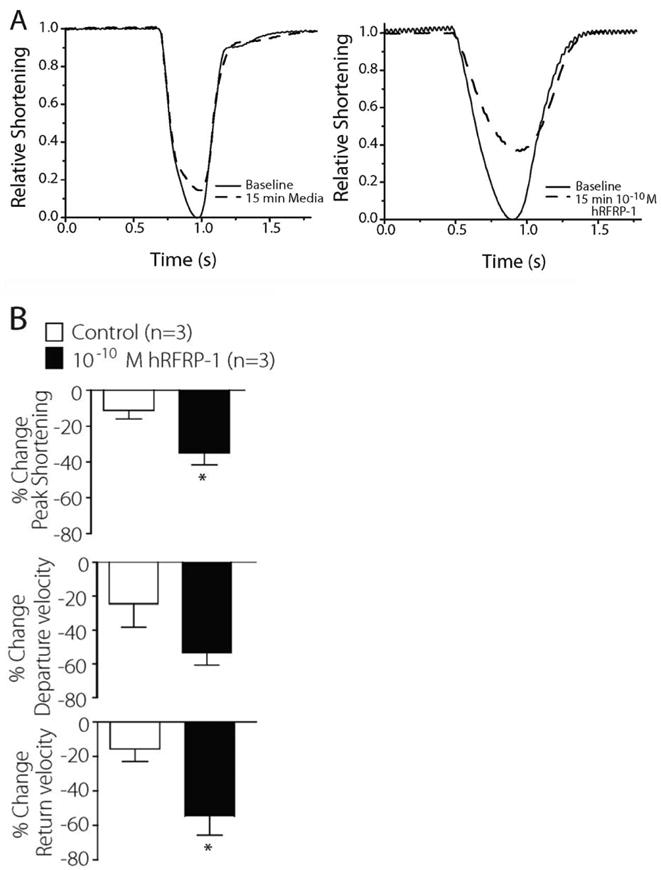
Representative sarcomere shortening traces in isolated adult rabbit cardiac myocytes paced at 0.5 Hz. Fig. 7A. A signal-averaged recording from 10 traces was made in myocytes 1 day after isolation (n = 3). Recordings show shortening before and 15 minutes after initiating perfusion with 10−10 M hRFRP-1 or media (control) at 37°C. Fig. 7B. Percent change in peak shortening, departure velocity, and return velocity.
4. Discussion
Our results are the first to describe the specific cellular and integrated cardiac actions of mammalian RFRP-1. Dose-dependent cardiac effects were found at the cellular level using two mammalian orthologs, hRFRP-1 and rRFRP-1 and two mammalian models, rat and rabbit (Figs. 1, 2, 7) and at the organ system level in mammals (Fig. 4). These cardiac responses were over a concentration range that would be expected if a peptide is released as a neuro-hormonal modulator of cardiovascular function. The consistency of the cardio-depressant effects in multiple mammalian models and with the two mammalian orthologs suggests RFRP-1 plays a role in modulating cardiac performance. Until now, there has been no published work about the effects of the highly conserved RFRP-1 peptides on mammalian cardiac function.
Members of a FaRP subgroup defined by XRFamide generally have similar functional activities, which may be different from other subgroups within the RFamide superfamily. The invertebrate LRFamide myosuppressin family of peptides has been studied extensively in invertebrates as cardioinhibitory peptides that decrease heart rate and amplitude of ejection [1, 20-22, 26]. In the present study we report the novel finding that RFRP-1, a mammalian LRFamide peptide, dramatically decreases cardiac function. This conservation of structure and activity indicates members of the LRFamide subgroup decrease cardiac function in invertebrates and mammals and may act through a common mechanism across phylogeny. Structural and functional similarity of neuropeptides and their receptors across the animal kingdom [25] suggests studies in invertebrates such as Drosophila melanogaster may shed light on how a conserved peptide acts in mammalian cardiovascular physiology.
Recently, another vertebrate FaRP, 26RFa was reported to increase heart rate and blood pressure in rat [8]. Our studies show no significant effects of this FaRP on isolated cardiac myocyte shortening or relaxation (Fig. 3). Although 26RFa shares a common C terminus with RFRP-1, our results show the RFRP-1 N-terminal sequence is required for cardiomyocyte-specific effects. This structure-specific, high affinity response observed with hRFRP-1 but not 26RFa is consistent with a novel peptidergic receptor, most likely linked to one or more cellular signaling pathways. In addition, the substantial functional response to nanomolar RFRP-1 (Figs. 1, 2, 7) is consistent with a pathway utilizing a high affinity receptor. Though the identity of the RFRP-1 cardiac receptor and molecular signaling mechanisms are not yet known, other FaRPs likely act through G-protein coupled receptors in the brain [11, 13]. The ability of bis-1 but not PTX pretreatment to block the influence of hRFRP-1 on cardiac function suggests the peptide activates a PKC pathway but does not signal through a Gi protein.
Results from the present study also demonstrate similar and different effects of RFRP on isolated mammalian cardiac myocytes compared to cardiac function in the intact animal (Figs. 1, 2, 7 versus Fig. 4). The profound decrease in heart rate in vivo was not reflected in detected rhythm disturbances in the isolated myocyte. This difference may be due to effects on neural targets present in the in vivo studies. Although rhythmic disturbances were not detected at pacing frequencies including 0.2, 0.5, and 1 Hz in the isolated myocyte studies, it remains possible this aspect of the response may not be evident at the lower pacing frequencies used for the functional studies in isolated myocytes. Human RFRP-1 induced a decrease in systolic function observed in vivo that was consistent with the cellular response, an indication that the in vivo effect is due at least in part to a direct suppression of cardiac myocyte contractile function. However, the slowing of relaxation observed in isolated adult myocytes was not detected in vivo. This slowing of in vitro re-lengthening and lack of change in in vivo diastolic performance may reflect variability in the non-invasive assessment of diastolic performance, attenuated detection due to rate-related changes in function [6], and/or the influence of factors such as load and compensatory responses within a whole animal model.
Neurohormones play a critical role in modulating heart function under physiological as well as acute and chronic pathophysiological conditions. The study of β-adrenergic signaling has been investigated under physiological and pathophysiological conditions, [4, 14], yet other neurally-mediated signaling pathways may also play significant roles in the regulation of heart function. Of particular interest are small peptidergic signaling molecules with cardioregulatory properties. The present work describes the substantial and consistent cardiac response to a conserved mammalian FaRP dodecamer, which may prove to be a target for future drug development, and diagnostic and/or therapeutic treatments.
Acknowledgments
National Institutes of Health funding (R21HL093627 to RN) is acknowledged for support of this research. The Nathan Shock Center is acknowledged for funding which supported, in part, the technical assistance to perform tail-vein injections, and re-charge fees on echocardiography equipment. Gail Romanchuk is acknowledged for expert technical assistance in myocyte preparation, and Janet Hoff is acknowledged for expert technical assistance in tail-vein injections. We thank Professor Rick Neubig for the generous gift of PTX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angioy AM, Muroni P, Barbarossa IT, McCormick J, Nichols R. Evidence dromyosuppressin acts at posterior and anterior pacemakers to decrease the fast and the slow cardiac activity in the blowfly Protophormia terraenovae. Peptides. 2007;28:585–93. doi: 10.1016/j.peptides.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard CS, Dockray GJ. Increases in arterial blood pressure in the rat in response to a new vertebrate neuropeptide, LPLRFamide, and a related molluscan peptide, FMRFamide. Regul Pept. 1984;8:209–15. doi: 10.1016/0167-0115(84)90062-4. [DOI] [PubMed] [Google Scholar]
- 3.Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96:822–8. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 4.Brodde OE. Beta-adrenergic receptors in failing human myocardium. Basic Res Cardiol. 1996;91(2):35–40. doi: 10.1007/BF00795360. [DOI] [PubMed] [Google Scholar]
- 5.Chartrel N, Dujardin C, Leprince J, Desrues L, Tonon MC, Cellier E, Cosette P, Jouenne T, Simonnet G, Vaudry H. Isolation, characterization, and distribution of a novel neuropeptide, Rana RFamide (R-RFa), in the brain of the European green frog Rana esculenta. J Comp Neurol. 2002;448:111–27. doi: 10.1002/cne.10253. [DOI] [PubMed] [Google Scholar]
- 6.Dias FA, Walker LA, Arteaga GM, Walker JS, Vijayan K, Peña JR, Ke Y, Fogaca RTH, Sanbe A, Robbins J, Wolska BM. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J Mol Cell Cardiol. 2006;41:330–9. doi: 10.1016/j.yjmcc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Dockray GJ, Reeve JR, Jr, Shively J, Gayton RJ, Barnard CS. A novel active pentapeptide from chicken brain identified by antibodies to FMRFamide. Nature. 1983;305:328–30. doi: 10.1038/305328a0. [DOI] [PubMed] [Google Scholar]
- 8.Fang Q, Liu Q, Li N, Jiang TN, Li YL, Yan X, Wang R. Cardiovascular effects of intravenous administered 26RFa, a novel RFamide peptide ligand for GPR103, in anaesthetised rats. Eur J Pharmacol. 2009;621:61–6. doi: 10.1016/j.ejphar.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 9.Fukusumi S, Fujii R, Hinuma S. Recent advances in mammalian RFamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides. 2006;27:1073–86. doi: 10.1016/j.peptides.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Fukusumi S, Habata Y, Yoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim Biophys Acta. 2001;1540:221–32. doi: 10.1016/s0167-4889(01)00135-5. [DOI] [PubMed] [Google Scholar]
- 11.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2:703–8. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 12.Kelly RA, Eid H, Krämer BK, O'Neill M, Liang BT, Reers M, Smith TW. Endothelin enhances the contractile responsiveness of adult rat ventricular myocytes to calcium by a pertussis toxin-sensitive pathway. J Clin Invest. 1990;86:1164–71. doi: 10.1172/JCI114822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, Zeng Z, Jacobson M, Williams DL, Jr, Yu H, Bomford D, Figueroa D, Mallee J, Wang R, Evans J, Gould R, Austin CP. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem. 2001;276:36961–9. doi: 10.1074/jbc.M105308200. [DOI] [PubMed] [Google Scholar]
- 14.Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: fine-tuning cardiac stimulation. Trends Mol Med. 2007;13:503–11. doi: 10.1016/j.molmed.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Mues G, Fuchs I, Wei ET, Weber E, Evans CJ, Barchas JD, Chang, JK Blood pressure elevation in rats by peripheral administration of Tyr-Gly-Gly-Phe-Met-Arg-Phe and the invertebrate neuropeptide, Phe-Met-Arg-Phe-NH2. Life Sci. 1982;31:2555–61. doi: 10.1016/0024-3205(82)90728-7. [DOI] [PubMed] [Google Scholar]
- 16.Nichols R. Isolation and structural characterization of Drosophila TDVDHVFLRFamide and FMRFamide-containing neural peptides. J Mol Neurosci. 1992;3:213–8. doi: 10.1007/BF03380141. [DOI] [PubMed] [Google Scholar]
- 17.Nichols R. Signaling pathways and physiological functions of Drosophila melanogaster FMRFamide-related peptides. Annu Rev Entomol. 2003;48:485–503. doi: 10.1146/annurev.ento.48.091801.112525. [DOI] [PubMed] [Google Scholar]
- 18.Nichols R, McCormick J, Cohen M, Howe E, Jean C, Paisley K, Rosario C. Differential processing of neuropeptides influences Drosophila heart rate. J Neurogenet. 1999;13:89–104. doi: 10.3109/01677069909083468. [DOI] [PubMed] [Google Scholar]
- 19.Price DA, Greenberg MJ. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977;197:670–1. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- 20.Robb S, Evans P. The modulatory effect of SchistoFLRFamide on heart and skeletal muscle in the locust Schistocerca gregaria. J Exp Biol. 1994;197:437–42. doi: 10.1242/jeb.197.1.437. [DOI] [PubMed] [Google Scholar]
- 21.Robb S, Packman LC, Evans PD. Isolation, primary structure and bioactivity of schistoflrf-amide, a FMRF-amide-like neuropeptide from the locust, Schistocerca gregaria. Biochem Biophys Res Commun. 1989;160:850–6. doi: 10.1016/0006-291x(89)92512-6. [DOI] [PubMed] [Google Scholar]
- 22.Stevens JS, Cashman CR, Smith CM, Beale KM, Towle DW, Christie AE, Dickinson PS. The peptide hormone pQDLDHVFLRFamide (crustacean myosuppressin) modulates the Homarus americanus cardiac neuromuscular system at multiple sites. J Exp Biol. 2009;212:3961–76. doi: 10.1242/jeb.035741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One. 2009;4:e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ukena K, Tsutsui K. Distribution of novel RFamide-related peptide-like immunoreactivity in the mouse central nervous system. Neurosci Lett. 2001;300:153–6. doi: 10.1016/s0304-3940(01)01583-x. [DOI] [PubMed] [Google Scholar]
- 25.Vanden Broeck J. Neuropeptides and their precursors in the fruit fly, Drosophila melanogaster. Peptides. 2001;22:241–54. doi: 10.1016/s0196-9781(00)00376-4. [DOI] [PubMed] [Google Scholar]
- 26.Wasielewski O, Skonieczna M. Pleiotropic effects of the neuropeptides CCAP and myosuppressin in the beetle, Tenebrio molitor L. J Comp Physiol B. 2008;178:877–85. doi: 10.1007/s00360-008-0276-6. [DOI] [PubMed] [Google Scholar]
- 27.Westfall MV, Borton AR. Role of troponin I phosphorylation in protein kinase C-mediated enhanced contractile performance of rat myocytes. J Biol Chem. 2003;278:33694–700. doi: 10.1074/jbc.M305404200. [DOI] [PubMed] [Google Scholar]
- 28.Westfall MV, Lee AM, Robinson DA. Differential contribution of troponin I phosphorylation sites to the endothelin-modulated contractile response. J Biol Chem. 2005;280:41324–31. doi: 10.1074/jbc.M506043200. [DOI] [PubMed] [Google Scholar]
- 29.Westfall MV, Rust EM, Albayya F, Metzger JM. Adenovirus-mediated myofilament gene transfer into adult cardiac myocytes. Methods Cell Biol. 1997;52:307–22. [PubMed] [Google Scholar]
- 30.Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, Ibata Y. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res. 2003;982:156–67. doi: 10.1016/s0006-8993(03)02877-4. [DOI] [PubMed] [Google Scholar]