Abstract
The culture of human embryonic stem cells (hESCs) is limited, both technically and with respect to clinical potential, by the use of mouse embryonic fibroblasts (MEFs) as a feeder layer. The concern over xenogeneic contaminants from the mouse feeder cells may restrict transplantation to humans and the variability in MEFs from batch-to-batch and laboratory-to-laboratory may contribute to some of the variability in experimental results. Finally, use of any feeder layer increases the work load and subsequently limits the large-scale culture of human ES cells. Thus, the development of feeder-free cultures will allow more reproducible culture conditions, facilitate scale-up and potentiate the clinical use of cells differentiated from hESC cultures. In this review, we describe various methods tested to culture cells in the absence of MEF feeder layers and other advances in eliminating xenogeneic products from the culture system.
Keywords: Human embryonic stem cells, MEF, Feeder-free cell culture, Matrigel
1. Introduction
Human embryonic stem cell (hESC) lines are derived from the inner cell mass of 3–5-day-old blastocysts and were originally described byThomson et al. (1998) and Reubinoff, Pera, Fong, Trounson, and Bongso (2000). A full description of the characterization of these cells is beyond the scope of this review, but has been reviewed elsewhere (Carpenter, Rosler, & Rao, 2003; Draper, Moorer, Ruban, Gokhale, & Andrews, 2004). However, the basic characteristics of these cells may be described as a high nucleus to cytoplasm ratio, prominent nucleoli and distinct colony morphology. Human ES cells also possess high levels of telomerase activity and express the surface markers, SSEA4, Tra-1–60 and Tra-1–81, but, unlike mouse ES cells, not SSEA1 (Reubinoff et al., 2000; Thomson et al., 1998). In addition, they also show high expression of the POU transcription factor, Oct-4 (Reubinoff et al., 2000) and Nanog (Chambers et al., 2003; Mitsui et al., 2003). They proliferate continuously when maintained in appropriate culture conditions and differentiate into representatives of all three germ layers, i.e. endoderm, mesoderm and ectoderm, both in vitro and in vivo. This differentiation is defined by the formation of embryoid bodies in vitro and teratomas in vivo (Thomson et al., 1998). Teratomas form when embryonic stem cells are injected into severe combined immunodeficient (SCID) mice and tissue types found include gut epithelium, cartilage, bone and neural epithelium among others (Thomson et al., 1998). The potential to generate and expand specific cell types in vitro has great implications for drug discovery, toxicology and regenerative medicine.
The original growth conditions for the derivation and culture of hESC lines were very similar to those for mouse ES cell lines. It was soon discovered that, although mouse ES cells could proliferate and remain undifferentiated in the absence of a fibroblast feeder layer when leukemia inhibitory factor (LIF) was included in the culture medium, this was not the case for hESCs (Thomson et al., 1998). LIF activates the STAT3/gp130 pathway but, despite activation of this pathway in hESC by the addition of exogenous LIF, it is insufficient to maintain the self-renewal of these cells (Daheron et al., 2004). Thus, hESCs have been mostly derived and cultured on a layer of mouse embryonic fibroblasts (MEFs). The therapeutic potential of ES cells lies in the transplantation of differentiated cell types for disorders such as Parkinson’s Disease and diabetes which arise from loss, or non-function, of a single cell type. With a view to future transplantation of hESC derivatives, it is important, therefore, to eliminate or, at least reduce, the potential for contamination by pathogens, etc., from the mouse feeder cells. Despite a recent publication suggesting no evidence for infection of hESCs by feeder-derived viruses (Amit et al., 2005), there is still some concern over xenogeneic contaminants regarding future clinical use of these cells. This is highlighted by a report of contamination of hESCs with Neu5Gc, a non-human sialic acid capable of inducing an immune response in humans (Martin, Muotri, Gage, & Varki, 2005). Apart from restrictions concerning transplantation studies, the feeder layer may be also an unwitting source of variability in experimental conditions (Heng, Liu, & Cao, 2004). In this review, we discuss the different approaches to culturing cells or deriving newlines on substrates other than MEFs. Cell lines on the US federal register are referred to by both the supplier and NIH designated nomenclature throughout.
2. Alternative feeder cells
Several alternative cell lines have been investigated for their ability to support existing hESC lines as well as being used to derive new hESC lines (Table 1). The mouse embryonic fibroblast cell line, STO, has been used to establish nine cell lines from frozen blastocysts and zygotes (Park et al., 2004). The advantage of STO cells over primary cultures of MEFs is that, being immortalized, they are easy to maintain and propagate. Human ES cells cultured on this feeder layer exhibited a similar doubling time to those on MEFs and expressed surface markers as expected. In addition, prolonged culture did not lead to abnormal karyotypes. However, Xu et al. (2001) have found increased differentiation when conditioned medium (CM) from these cells was used in feeder-free conditions. Thus, the STO line is not a direct substitute for MEFs but can reduce some of the work load regarding MEF isolation and culture.
Table 1.
Summary of recent advances toward xeno-free culture of hESCs
| Cell lines | Substrate | Key medium components | Longest time in culture |
Characterization |
Reference | ||
|---|---|---|---|---|---|---|---|
| Mkr | Plur | Kary | |||||
| Novel | STO cells | 20% FBS | >30 passages | Yes | EB | Yes | Park et al. (2004) |
| Novel | Foreskin fibroblasts | 20% FBS + LIF | 9 months | Yes | Ter | Yes | Hovatta et al. (2003) |
| HES-3 | FM fibroblasts | MEF-CM | >20 passages | Yes | Ter | Yes | Richards et al. (2002) |
| HES-4 | FS fibroblasts | ||||||
| Novel | AFT epithelial cells | ||||||
| HES-3 | AS fibroblasts | 20% FBS | >30 passages | Yes | Ter | Yes | Richards et al. (2003) |
| HES-4 | 20% KSR | ||||||
| H1 | Human marrow stromal cells |
20% KSR | 13 passages | Yes | EB | Yes | Cheng et al. (2003) |
| Novel | hES-df | 20% KSR | 44 passages | Yes | Ter, M | Yes | Stojkovic et al. (2005a) |
| Matrigel | hES-df-CM | 14 passages | Yes | N/D | N/D | ||
| H1 | hES-df | 20% KSR | 18 passages | Yes | Ter, M | Yes | |
| Matrigel | hES-df-CM | 12 passages | Yes | N/D | N/D | ||
| H1 | Matrigel | MEF-CM | 6 months | Yes | EB | Yes | Xu et al. (2001) |
| H7 | Laminin | Ter | Rosler et al. (2004) | ||||
| H9 | 2 years | ||||||
| H14 | |||||||
| BG01 | Matrigel | MEF-CM | >24 passages | Yes | EB | Yes | Brimble et al. (2004) |
| BG02 | Fibronectin | ||||||
| BG03 | |||||||
| H1 | Matrigel | HEF-TERT-CM | 14 passages | Yes | EB | Yes | Xu et al. (2004) |
| H7 | |||||||
| H9 | |||||||
| H7 | Matrigel | 40 ng/ml bFGF ± other GFs | 15 passages | Yes | EB | Yes | Xu, Rosler, et al. (2005) |
| H9 | Ter | ||||||
| H1 | Matrigel | 40 ng/ml bFGF + 500 ng/ml Noggin | 33 passages | Yes | EB | Yes | Xu, Peck, et al. (2005) |
| H9 | Ter | ||||||
| H14 | |||||||
| H1 | Matrigel | NIH/3T3-Nog-CM, 40 ng/ml | 7 passages | Yes | M | Yes | Wang et al. (2005) |
| bFGF + 500 ng/ml Noggin | |||||||
| I3 | Human fibronectin | TGFβ1 ± LIF + bFGF | >50 passages | Yes | EB | Yes a | Amit et al. (2004) |
| I6 | Ter | ||||||
| H9 | |||||||
| HSF6 | Laminin | 50 ng/ml activin A, 50 ng/ml KGF, | >20 passages | Yes | Ter | Yes | Beattie et al. (2005) |
| 10 mM NIC | |||||||
| H1 | Matrigel | 25 ng/ml activin A | Yes | EB | N/D | James et al. (2005) | |
| BGN1 | |||||||
| BGN2 | |||||||
| H9 | FBS | CDM + 10 ng/ml activin + 12 ng/ml | 10 passages | Yes | EB | Yesa | Vallier et al. (2005) |
| bFGF | |||||||
| H1 | Matrigel Laminin | X-VIVO 10 + 80 ng/ml bFGF | >240 days | Yes | Ter | Yes | Li et al. (2005) |
| H1 | MEF-ECM | 8% KSR + 8% plasmanate + 16 ng/ml | 20 passages | Yes | EB | Yes | Klimanskaya et al. (2005) |
| H7 | bFGF + 20 ng/ml LIF | ||||||
| H9 | |||||||
| 4 others | |||||||
| Novel | 6 months | ||||||
| H1 | Human serum | hES-df-CM | 27 passages | Yes | M | Yes | Stojkovic et al. (2005b) |
| Novel | |||||||
Abbreviations AFT, adult fallopian tube; AS, adult skin; CDM, chemically defined medium (1:1 IMDM:F12 supplemented with insulin, transferrin, monothioglycerol and bovine serum albumin fraction V); FBS, fetal bovine serum; FM, fetal muscle; FS, fetal skin; HEF-TERT-CM, conditioned medium from human ES cell-derived fibroblasts, stably transfected with TERT; hES-df, human ES cell-derived fibroblasts; hES-df-CM, human ES cell-derived fibroblast conditioned medium; KGF, keratinocyte growth factor; KSR, knockout serum replacement; LIF, leukemia inhibitory factor; MEF-CM, mouse embryonic fibroblast conditioned medium; MEF-ECM, extracellular matrix of MEFs; NIC, nicotinamide.
Characterization key: Mkr, normal undifferentiated marker expression; Plur, pluripotency determined by embryoid body formation in vitro (EB), teratoma formation in vivo (Ter) or by monolayer differentiation in vitro (M); Kary, normal karyotype; N/D, not described.
Authors describe some abnormalities at late passage consistent with previous observations for cells grown on MEF feeders.
To eliminate the murine element from feeder cells, Hovatta et al. (2003) have derived novel hESC lines on neonatal human foreskin fibroblasts. The hESC lines were grown on these fibroblasts for up to 9 months, at which time they still expressed appropriate undifferentiated hESC markers, had a normal karyotype and exhibited teratoma formation in SCID mice. These cells are capable of 61 population doublings and have an advantage over the STO cell line in terms of their human origination.
Various human cell lines have been examined and compared by Richards, Fong, Chan, Wong, and Bongso (2002) and Richards et al. (2003) for the ability to support thehESClines, HES-3 and HES-4 (ES03 and ES04). Initially they compared fetal muscle, fetal skin and adult fallopian tube epithelial cells, all of which were found to support undifferentiated hESC culture through 20 passages (Richards et al., 2002). At this time, human serum was substituted for fetal bovine serum (FBS) in the fetal fibroblast culture medium to further reduce potential contamination by animal products. However, it was later found that increasing differentiation of hESCs was observed beyond 10 passages when fibroblasts were maintained in human serum (Richards et al., 2003). The growth medium was, therefore, switched to standard growth medium supplemented with 20% knockout serum replacer (KSR) or FBS. In this study, the comparison of human feeders extended to include several cell lines derived from other adult tissues but it was determined that the best lines were still those of fetal origin. The hESCs were grown for more than 30 passages and maintained normal undifferentiated hESC marker expression and karyotype.
Adult bone marrow is the source of many different types of stem cells. Known as the primary site of synthesis of the hematopoietic system, it has the ability to produce a microenvironment that can maintain hESCs in an undifferentiated state. Human marrow-derived stromal cells (hMSCs) were expanded in culture and used as a feeder layer for H1 cells (WA01) (Cheng, Hammond, Ye, Zhan, & Dravid, 2003). They were found to support undifferentiated growth of karyotypically normal hESC for 9 passages and were still being cultured at 13 passages. The use of human-sourced cell lines carries its own risks, particularly with respect to pathogens or potential immunoreaction. The advantage of these cells lies in the fact that unrelated hMSCs do not generate alloreactive T lymphocytes in culture or in large animals. This would suggest that they may not induce an immune response if they were to be transplanted to humans along with differentiated hESCs. However, the authors stated that the proliferation rate of the hMSCs dropped off after six passages thus requiring continuous isolation from human marrow for prolonged culture of hESCs. This provides little gain, in terms of work load, over the use of MEFs.
Recently, Stojkovic et al. (2005a) have derived fibroblast-like cells from hESC cultures, denoted hESC-df, and used them as a feeder layer for H1 cells (WA01). These hESC-df cells were shown to express markers similar to fibroblasts and were passaged weekly for up to 12 weeks. They were capable of supporting undifferentiated growth of the hESCs for at least 44 passages, and medium conditioned by the hESC-df allowed hESC growth on Matrigel for 12–14 passages. Apart from being of human origin, this method is advantageous in that the feeder layer introduces no particles which are foreign to the original culture. In addition, hESC-df may be derived from the hESCs at any time, unlike MEFs which are best used between passages 4 and 6 and thus require continuous isolation from fetal mice.
The use of mouse cell lines, such as STO, as feeders eliminates the need for repeated generation of new MEF cultures with the intrinsic potential for variability in quality and ability to maintain hESCs. This approach does not address the xenogeneic problems which may be overcome by using human feeders, especially those derived from the original cell line. However, use of feeder cells in a co-culture system will always exhibit inherent variability and so feeder-free culture would be preferable.
3. Feeder-free culture
The contribution of MEFs to the culture of hESCs is not fully understood but efforts to eliminate their use must account for ECM molecules, i.e. provide a suitable substrate, as well as for soluble growth factors and other trophic factors. In terms of substrate, Matrigel was the initial matrix of choice in the feeder-free culture of hESCs. Matrigel is the manufacturer’s Trademark for extracellular matrix extracted from the Engelbreth–Holm–Swarm tumor originally described byKleinman et al. (1982). As it is mostly comprised of laminin and collagen, these molecules have also been used, in purified form, to avoid lot-to-lot variations in the Matrigel extract. Soluble factors may be provided by using normal hESC medium conditioned by overnight incubation with MEFs or other cell types described in the previous section.
3.1. Conditioned medium
Several different sources of conditioned medium have been tested for their ability to sustain undifferentiated hESC culture and are summarized in Table 1. In 2001, Xu et al. compared the culture of various WiCell lines on Matrigel, laminin, fibronectin and collagen IV in the presence of MEF-CM. They found that the cells survived poorly and differentiated rapidly when cultured on gelatin, but both laminin and Matrigel, which contains a significant proportion of laminin, were able to support undifferentiated growth of the hESCs. The authors also found that hESCs express both integrin α6 and β1, suggesting they may be capable of expressing a laminin-specific receptor. The expression of these integrins was not affected by the culture conditions. They further tested CM from other cell lines, including the mouse fibroblast STO line, and found that they did not support undifferentiated hESC growth as well as MEF-CM. H9 cells (WA09) have been cultured in this feeder-free environment for at least 2 years and remain undifferentiated (Rosler et al., 2004).
Bresagen hESC lines have also been cultured in feeder-free conditions using MEF-CM (Brimble et al., 2004). Using Matrigel or fibronectin-coated plates, BG03 was shown to remain undifferentiated and karyotypically normal after 6 passages on Matrigel followed by 10 passages on fibronectin. A comparison of mRNA expression between cells cultured on MEFs and in feeder-free conditions was performed using focused arrays and the expression profile was found to be similar. This suggests that there are no additional stresses on the cells in feeder-free conditions leading to activation of alternative signaling pathways.
Since the initial studies ofXu et al. (2001), there has been further investigation of the use of human cells to generate CM capable of sustaining undifferentiated growth of hESCs in feeder-free conditions. Fibroblast-like cells have been derived from H1 cells (WA01), denoted HEF1, and immortalized by infection with a retrovirus expressing human telomerase reverse transcriptase (TERT) (Xu et al., 2004). It was found that conditioned medium from these cells supported the growth of both H7 and H9 cells (WA07 and WA09) on Matrigel. After 13 passages these cells expressed the relevant surface markers and were karyotypically normal. This approach addresses two issues: the xeno component of MEF-CM and ease of use of an immortalized cell line versus primary culture. As described in the previous section, conditioned medium from hES-df supported growth of H1 cells (WA01) on Matrigel for 12–14 passages (Stojkovic et al., 2005a).
The use of human feeders versus mouse feeders in the generation of conditioned medium is preferable with respect to xeno-contamination concerns and, if derived from the cell line itself, eliminates any other cross-contamination issues. However, by its nature CM will contain levels of growth factors and other molecules which are undefined and, hence, will be subject to variability.
3.2. Defined medium
The use of CM has obviously been of great value in establishing the feeder-free culture of hESCs but several novel methods have been developed recently to use more defined media to support culture of hESCs without feeders and, in some cases, without Matrigel (Table 1).
Human ES cells have been found to express receptors for various growth factors including basic fibroblast growth factor (bFGF), stem cell factor (SCF) and fetal liver tyrosine kinase-3 ligand (Flt3L). Xu, Rosler, et al. (2005) tested the ability of combinations of these, and other growth factors, to support undifferentiated growth of H7 and H9 cells (WA07 and WA09) on Matrigel. They found that 40 ng/ml bFGF was sufficient to maintain cells for up to 15 passages with appropriate surface marker expression. Cells cultured in this manner were found to exhibit a normal karyotype and were able to form both EBs and teratomas.
Xu, Peck, et al. (2005) carried out a similar study in which they stated that, in unconditioned medium, hESCs are subjected to high levels of BMP signaling, which are reduced in MEF-CM. They found that a combination of the BMP antagonist, Noggin (500 ng/ml), and bFGF (40 ng/ml) was sufficient to sustain undifferentiated growth of several WiCell lines on Matrigel. It was proposed that Noggin synergizes with bFGF to repress BMP signaling, thereby sustaining this undifferentiated proliferation. In this study, the authors also examined the use of laminin as a substrate and showed that, although it could also be used in this system, there was much variability. These culture conditions were found to be incapable of supporting clonal growth of hESCs which is important in the absolute determination of pluripotency or in gene manipulation experiments. Cultures were maintained for 33 passages at which time the hESCs stained with expected surface markers and exhibited a normal karyotype. Pluripotency was demonstrated by the formation of EBs in vitro and teratomas in vivo.
A different approach has also led to the use of Noggin in feeder-free culture. NIH/3T3 cells are a mouse fibroblast cell line but, unlike MEF-CM, conditioned medium from these cells does not support feeder-free culture of hESCs. Wang et al. (2005) compared MEFs and NIH/3T3 cells for the expression of various signaling molecules and found that several factors, including Noggin, were expressed only by MEFs. Using a retroviral vector, they transduced NOG, which codes for Noggin, into the NIH/3T3 cells and showed that conditioned medium from the transductants was able to sustain undifferentiated culture of H1 cells (WA01). However, they found that Noggin alone was unable to sustain prolonged, undifferentiated culture at 500 ng/ml and that 40 ng/ml or 80 ng/ml of bFGF alone could support undifferentiated growth only up to about 15 days. This appears to disagree with the data of Xu, Rosler, et al. (2005) which could, perhaps, be due to differences in local culture conditions or the cell lines used. A combination of both 500 ng/ml Noggin and 40 ng/ml bFGF was able to maintain growth of the H1 cells up to at least seven passages. These cells expressed high levels of Tra-1–60, exhibited a normal karyotype and were able to differentiate in vitro.
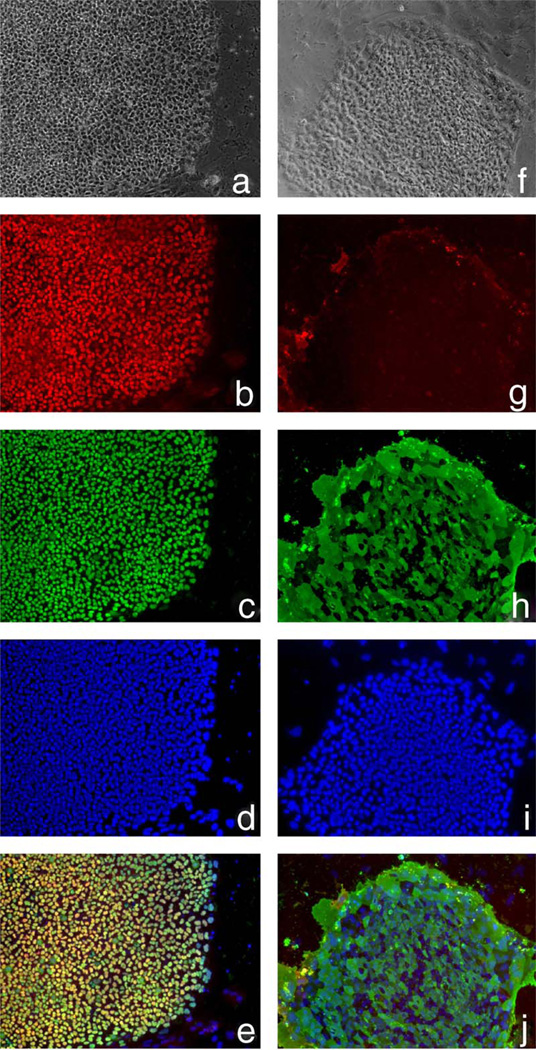
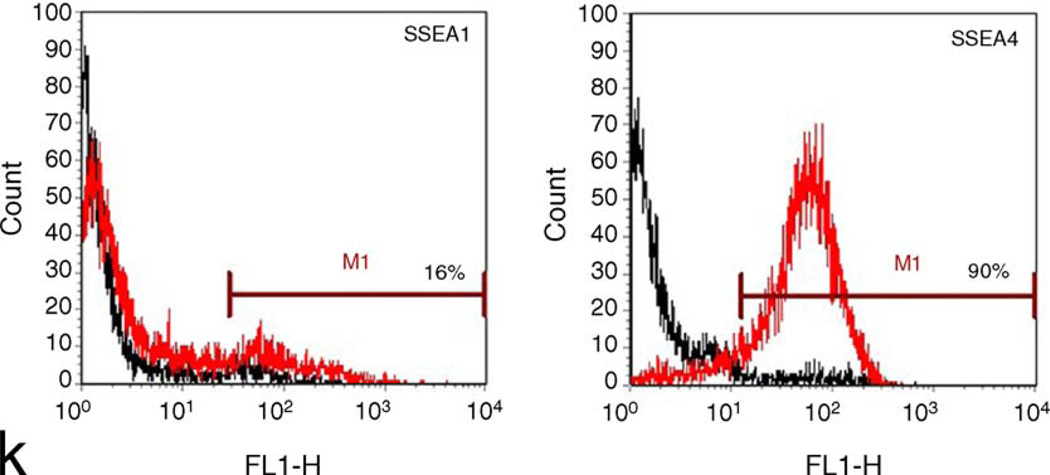
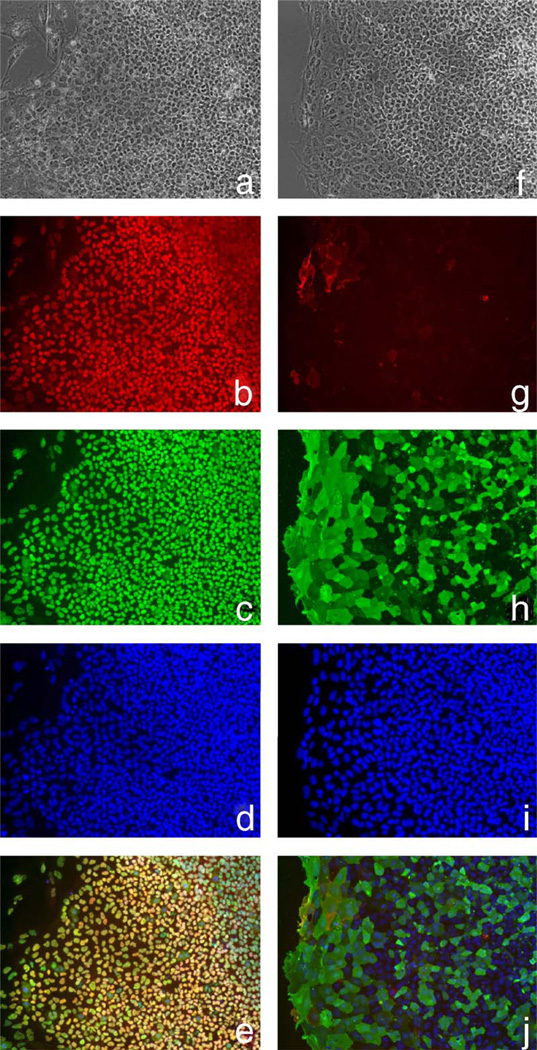
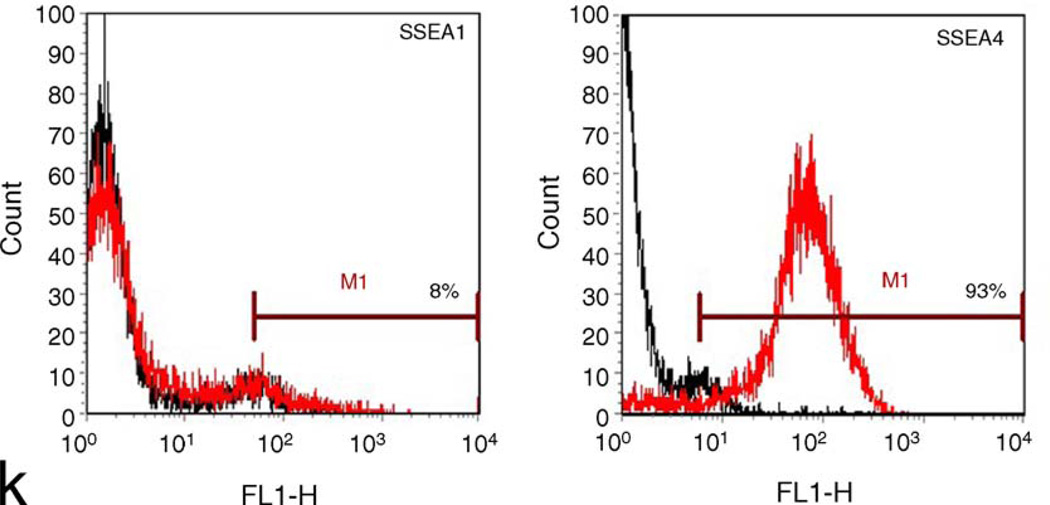
In the NIH Stem Cell Unit, we have tested the 40 ng/ml bFGF/Matrigel combination with two cell lines different to those used in the above studies—I3 and HSF-6 (TE03 and UC06). Despite initial promising findings, these cells also showed extensive differentiation after about three and seven passages, respectively. Current studies with 100 ng/ml bFGF on Matrigel show undifferentiated morphology and marker expression up to 11 and 15 passages using I3 and HSF-6 cells (TE03 and UC06), respectively, or up to 6 passages with 40 ng/ml bFGF plus 100 ng/ml Noggin using I3 cells (TE03). Both cell lines have also shown promising growth on mouse laminin (Roche) with 100 ng/ml bFGF up to three passages. Figs. 1 and 2 show phase images, immunostaining and FACS analysis of TE03 cells cultured on Matrigel for nine passages or on laminin for three passages, respectively. Under both conditions the cells express high levels of Oct-4, Nanog and SSEA4 with low expression of SSEA1. These data demonstrate that I3 cells (TE03), under these conditions, remain undifferentiated.
Fig. 1.
I3 cells (TE03) cultured on Matrigel for nine passages in 100 ng/ml bFGF. (a and f) Phase images of immunostained colonies shown in (b–e) and (g–j), respectively (10×); (b) Nanog (ReproCell Inc); (c) Oct-4 (Santa Cruz Biotechnology Inc.); (d) Hoescht stain (Sigma), (e) Nanog/Oct-4 merged image; (g) SSEA1 (gift from Peter Andrews, Sheffield University); (h) SSEA4 (Santa Cruz Biotechnology Inc.); (i) Hoescht stain (Sigma); (j) SSEA1/SSEA4 merged image; (k) FACS analysis with SSEA1 (left) or SSEA4 (right). The marker line was drawn to include approximately 5% of the negative control. Data were collected with the BD FACSCalibur system and analyzed using FCS Express software.
Fig. 2.
I3 cells (TE03) cultured on laminin for three passages in 100 ng/ml bFGF. (a and f) Phase images of immunostained colonies shown in (b–e) and (g–j), respectively (10×); (b) Nanog (ReproCell Inc); (c) Oct-4 (Santa Cruz Biotechnology Inc.); (d) Hoescht stain (Sigma); (e) Nanog/Oct-4 merged image; (g) SSEA1 (gift from Peter Andrews, Sheffield University); (h) SSEA4 (Santa Cruz Biotechnology Inc.); (i) Hoescht stain (Sigma); (j) SSEA1/SSEA4 merged image; (k) FACS analysis with SSEA1 (left) or SSEA4 (right). The marker line was drawn to include approximately 5% of the negative control. Data were collected with the BD FACSCalibur system and analyzed using FCS Express software.
Matrigel has been extremely useful in establishing feeder-free cultures but, since it may contain unknown factors and is of mouse origin, alternative substrates of human origin are being investigated in the push toward a totally xeno-free culture system. These efforts are summarized in Table 1. Amit, Shariki, Margulets, and Itskovitz-Eldor (2004) have reported a method that utilizes human fibronectin-coated plates and medium supplemented with 15% serum replacement in the presence of TGFβ1, LIF and bFGF to successfully support the growth of I3, I6 and H9 cells (TE03, TE06 and WA09). They found that the best conditions were a combination of TGFβ1 and bFGF with or without LIF. Although they discovered some karyotypic abnormalities at later passages, the authors stated that these were no more common than have been discussed elsewhere under standard culture conditions. Moreover, although the parameters tested were inferior to culture on MEFs, the difference was found to be marginal. This was manifested as a lower growth rate and cloning efficiency and a higher rate of spontaneous differentiation. Cells were maintained for over 50 passages.
Beattie et al. (2005) have shown that hESCs grown on laminin in the presence of activin A, nicotinamide (NIC) and keratinocyte growth factor (KGF) remain undifferentiated during continuous growth over 20 passages. Activin A is thought to block differentiation as removal of activin A from the growth medium resulted in a rapid change in cell morphology to a differentiated phenotype within a week with loss of Nanog expression. In contrast, when either KGF or NIC was removed from the medium, the cells maintained their undifferentiated phenotype. However, there was a significant difference in cell proliferation when cells were cultured with activin, KGF or NIC alone compared with a combination of the three factors. The authors noticed a gradual change of the hESC appearance from the usual tight colony formation to an irregular monolayer of uniformly shaped cells. The cells appeared larger than those observed in the original colonies. With continuous growth, they eventually formed a continuous monolayer and mounded up in the dish. However, these changes were reversible; when cells were placed back on feeder layers, they gradually resumed colony formation similar to that previously observed on feeder layers.
The role of activin has also been investigated by James, Levine, Besser, and Hemmati-Brivanlou (2005). They found that, in the undifferentiated state maintained by growth in MEF-CM, SMAD2/3 was phosphorylated and localized to the nucleus of hESC, indicating activation of the TGFβ/activin/Nodal pathways. Furthermore, phosphorylation and nuclear localization were reduced in cells allowed to differentiate by growth in non-conditioned medium (nCM). The authors also found that, in the undifferentiated state, SMAD1/5 phosphorylation was barely evident but upon differentiation, phosphorylation was globally increased and localized to the nucleus. Activation of SMAD2/3 by the addition of activin A to nCM not only increased phosphorylation of SMAD2/3 but also reduced phosphorylation of SMAD1/5 to levels comparable with those of hESC grown in MEF-CM, demonstrating an input of SMAD2/3-mediated signaling on the suppression of SMAD1/5 activation. Activin A in nCM also restored high level expression of both Oct-4 and Nanog. A morphological change of the hESC through extended culture was noted.
Most recently, Vallier, Alexander, and Pederson (2005) have demonstrated the role of the activin/Nodal/TGFβ pathway in the maintenance of pluripotency of H9 cells (WA09) cultured on FBS-coated plates in a chemically defined medium (CDM). It was found that inhibition of activin/Nodal signaling, but not inhibition of Nodal alone, resulted in increased differentiation of the cells. Addition of activin, but not TGFβ1, in these feeder-free conditions was sufficient to maintain cells in their undifferentiated state in the short term (three passages). However, for longer term growth, it was found that bFGF in combination with activin or Nodal was best and a role for FGF as a competence factor was proposed. These data are in concert with those ofAmit et al. (2004) who found that a combination of TGFβ and bFGF could maintain the undifferentiated status of hESCs cultured on human fibronectin. In the NIH Stem Cell Unit, we have also tested the use of activin A in feeder-free culture. HSF-6 cells (UC06) have been grown for seven passages in nCM containing 10 ng/ml bFGF and 25 ng/ml activin A and remain morphologically undifferentiated.
A purely defined medium would be of great advantage with respect to enhancing reproducibility and standardization of a culture protocol. However, most of the described culture conditions still use KSR which contains bovine serum albumin and other animal-derived products. Li, Powell, Brunette, Lebkowski, and Mandalam (2005) have investigated the use of a medium previously used for hematopoietic stem cell culture which is composed solely of human derived and recombinant proteins (X-VIVO 10). Cultures of H1 cells (WA01) were originally adapted to this culture medium on Matrigel but were subsequently adapted to growth on human laminin. Cells were cultured for over 20 passages in these conditions without detriment to the expression of undifferentiated markers, karyotype and pluripotency as determined by teratoma and EB formation. These culture conditions have been tested in the NIHSCU using I3 cells (TE03) with little initial success. However, the advantage of a medium or a supplement which is completely human or recombinant sourced is clear and should be investigated further.
These reports demonstrate that undifferentiated culture of hESCs in a more defined medium is certainly possible and gives some insight into the pathways important for self-renewal and pluripotency. However, the cost of the described reagents is currently quite prohibitive for large-scale culture in the doses described. This could be addressed in the future by more effective manipulation of the pathways involved.
3.3. Other substrates
Apart from standard extracellular matrix molecules, some groups have tested alternative substrate sources which are listed in Table 1.Klimanskaya et al. (2005) have developed a serum- and feeder-free culture system based on tissue culture plates coated with extracellular matrix (ECM) derived from MEFs. This substrate was tested with several established lines (WA01, WA07 and WA09) among others and was found to support the undifferentiated growth of all. In addition, a novel cell line was established on this matrix and was supported for more than 30 passages at time of going to press. Once prepared, the ECM may be stored at 4 °C or even sterilized by prolonged heat exposure. The advantages of this include some labor-saving in that feeder layers may be prepared ahead of time and stored but also in that sterilization will eliminate potential pathogenic contaminants. This may also prove to be a more cost-effective alternative to other expensive matrices. In the NIH Stem Cell Unit, we have derived a clonal line from I3 cells (TE03) on a similarly prepared ECM. This line was expanded in MEF-CM for two passages before switching to standard culture medium containing 20% KSR and 4 ng/ml bFGF. Cells grew as discrete colonies as described by Klimanskaya and stained with appropriate hESC markers after four passages (data not shown).
Stojkovic et al. (2005b) have described a novel method of hESC culture using a combination of human serum (HS) matrix and conditioned medium from hESC-derived fibroblasts (see Stojkovic et al., 2005a for derivation). The HS matrix contains ECM components, such as hyaluronic acid, fibronectin and vitronectin, as well as other unknown human factors and supports the attachment and growth of undifferentiated cells. This substrate was capable of supporting the undifferentiated growth of H1 cells (WA01) for 27 passages. Further defining the human factors could lead to discovery of key regulatory molecules that control the differentiated or undifferentiated status of hESCs.
There have also been some recent advances in the use of synthetic matrices for the culture of stem cells. A synthetic three-dimensional (3D) matrix, composed of cellulose acetate, has been used in the study of human embryonic germ cell derivatives which share some properties with hESCs (Yim & Leong, 2005). When immobilized with growth factors or cell-adhesion molecules such 3D scaffolds can mimic the characteristics of a natural extracellular matrix. The authors found that the 3D culture not only enhanced cell culture, but lengthened the proliferation period. However, the cells used in this study were germ cell derivatives and it is unclear if hESCs would be able to maintain an undifferentiated state on this matrix. Indeed, most studies with synthetic substrates and stem cells are largely concerned with their differentiation (reviewed in Lutolf & Hubbell, 2005). The first report of such a scaffold being used for undifferentiated culture of ESCs comes from Nur-E-Kamal, Ahmed, Kamal, Schindler, and Meiners (2005) who have recently used a commercially available synthetic polyamide matrix (UltraWeb) for the growth of mouse ESCs. The authors showed that proliferation and self-renewal, as well as Nanog expression, were enhanced relative to 2D surfaces and suggested that dimensionality may play a role in ‘stemness’ in mESCs. It will be very interesting to see if these observations apply to hESCs. One caveat to the use of these scaffolds for routine culture is that the authors do not describe further passaging of the cells after initial attachment and growth. It could be that recovery is not simple or may require trypsin which is not preferred in hESC culture.
Although the MEF-ECM and HS matrices may be useful in addressing some xenogeneic contamination issues, they are still subject to variability. Concerns regarding variability and sources of these and the other substrates described may be overcome by using some form of synthetic matrix and we anticipate that this field will see significant advances shortly.
4. Analyzing the MEF contribution
It is clear that MEFs provide critical materials both in terms of substrate as well as soluble molecules, such as growth factors, which maintain the hESCs in their undifferentiated, pluripotent state. However, our understanding of the precise role of MEFs in this maintenance is somewhat limited. Some efforts have been made to dissect the potential players in this role, particularly with respect to the components of MEF-CM (Lim & Bodnar, 2002), which have been shown to help cultures retain their self-renewal properties on Matrigel and other substrates. This proteomics-driven investigation, although preliminary, revealed the complexity of proteins found in the CM that included intracellular proteins as well as extracellular matrix proteins and those associated with cell adhesion and growth. However, MEF-CM alone is not sufficient to maintain hESCs in the undifferentiated state—there is also a requirement for a substrate such as laminin, fibronectin or Matrigel. Xie, Lin, Luo, Luo, and Lu (2004) have compared the differences in total proteins expressed in MEFs, not just those in the CM, before and after irradiation. These authors found proteins that were thought to be involved in ECM formation, cell differentiation and apoptosis among others. A fuller understanding of the role that MEFs play in the maintenance of hESCs will allow more precise manipulation of these cells in a feeder-free environment.
Other studies have taken a more fundamental approach to understanding the signaling pathways involved in hESC self-renewal. It is well known that mouse ES cells can remain undifferentiated without feeder layers when the medium is supplemented with LIF. This, however, is not the case with hESCs (Thomson et al., 1998). It is thought that activation of STAT3 via the LIF receptor is sufficient to maintain mouse ES cells in the undifferentiated state but, despite the demonstration of an active LIF/STAT3 pathway in hESC, it has been shown that this is not sufficient in these cells (Daheron et al., 2004). In addition, MEF-CM did not induce Y705 phosphorylation of STAT3, suggesting that CM triggers other pathways to self-renewal in these cells. One pathway implicated in hESC pluripotency is the canonical Wnt signaling pathway. Using a GSK-3-specific inhibitor, BIO, Sato, Meier, Skalsounis, Greengard, and Brivanlou (2004) showed that activation of this pathway was able to maintain pluripotency in both mouse and human ES cells. TGFβ has also been demonstrated to help maintain the pluripotent state of hESCs, a role which is enhanced by the use of bFGF (Amit et al., 2004). It has recently been proposed that FGF acts as a competence factor for activin/Nodal/TGFβ in the maintenance of pluripotency/self-renewal and that the Wnt signaling cascade feeds into this pathway (Vallier et al., 2005). This role for FGF may involve activation of the PI-3-kinase/Akt pathway (Kim et al., 2005) although direct evidence for this has to be established. Finally, inhibition of the BMP signaling pathway, which is suggested to drive differentiation of hESCs, may also be important (Xu, Peck, et al., 2005). Investigation and manipulation of these pathways will be useful in the drive to feeder-free culture in defined culture environments.
5. Contaminant sources
One of the most critical reasons for pursuing the undifferentiated culture of hESCs, without mouse feeders or conditioned medium, lies in preventing contamination of the culture by non-human pathogens which would rule out transplantation studies in the future. A recent study showed that there was no infection of hESCs by murine leukemia viruses (MuLV) derived from mouse feeders (Amit et al., 2005) despite evidence of humantropic MuLV in the feeder systems and expression of receptors for several MuLVs in hESCs. This may be a cause of some optimism.
Feeder cells, however, may not be the only route by which foreign material is introduced to the culture. This was demonstrated by another report which showed that hESCs carried a sialic acid (Neu5Gc) on their surface that was not human. This molecule was shown to originate, not from the MEFs as may have been expected, but rather from the commercial serum replacement in the medium (Martin et al., 2005). As most healthy people have circulating antibodies to this sugar molecule, the possibility exists that it could elicit an immune response if cells cultured in this medium were transplanted to humans.
Other possible non-human components of the commonly used KSR include transferrin and Albumax—a lipid-rich derivative of bovine serum albumin. This may raise some concern over the standard hESC culture medium and may support a move to human-derived components such as human serum. Unfortunately, some of the problems in substituting human serum in the existing culture protocols involve a concern over its inherent variability and the potential for transmission of more serious pathogens than those carried by mouse feeders or bovine serum. Any overlooked or undetected pathogen in human serum does not need to cross a species barrier to cause untold harm to the transplant recipient and could indeed be more likely to cause an immune response.
This may underscore the need to address the issue of recombinant components such as those described byLi et al. (2005). The proprietary ‘X-VIVO 10’ medium used in this study may also have other benefits as the authors describe proliferation being increased under those culture conditions. However, only total cell counts at inoculation and harvest are monitored and the authors do not address the questions of cell death and survival.
6. Summary
This review has summarized the move from standard culture on MEFs, through immortalized mouse fibroblast cell lines and human cell lines to the use of conditioned medium in feeder-free cultures and ultimately a totally defined culture system. Each approach has its own unique advantages and disadvantages which we summarize in Table 2.
Table 2.
Advantages and disadvantages of different approaches to xeno-free culture of hESCs
| Alternative method | Advantages | Disadvantages |
|---|---|---|
| Human feeders | Eliminates xenogeneic feeder component | No reduction in work load |
| Immortalized cells as feeders | Reduces work load slightly | Xenogeneic feeder component |
| Reduces feeder lot variability | ||
| ECM + MEF-CM | Reduces work load moderately | Xenogeneic feeder component |
| Less variable substrate | Still need to grow fibroblasts—possible variability in CM | |
| ECM + HEF-CM | Reduces work load moderately | Still need to grow fibroblasts—possible variability in CM |
| Less variable substrate | ||
| ECM + high GFs or signaling molecules | Reduces work load significantly | High cost |
| Less variable substrate | ||
| Can eliminate xenogeneic feeder component |
In general, the culture systems described have successfully supported the attachment and growth of undifferentiated hESCs. The hESCs grown under feeder-free conditions retained expression of specific cell-surface and intracellular hESC markers such as SSEA3, SSEA4, Tra-1–60, Tra-1–81, Oct-4 and Nanog. Pluripotency was most commonly evaluated by the formation of embryoid bodies (EBs) in vitro or of teratomas in SCID mice upon transplantation (see Table 1). Although it has been found that prolonged feeder-free culture can lead to karyotypic abnormalities (Draper et al., 2004b), most of the currently reviewed studies describe no aneuploidy in the hESCs, where tested. Still, this must remain a concern and should be routinely monitored for any future transplantation studies.
Of the feeder-free methods tested in the NIH Stem Cell Unit, we find the most reproducibly successful has been the use of 100 ng/ml bFGF on Matrigel. However, apart from the murine origin of the substrate, this culture system is very costly and efforts are underway to understand the mechanisms involved in maintaining the undifferentiated state with a view to manipulating it directly in a more cost-effective manner. Ultimately, a medium containing human-derived or recombinant components on a synthetic or standardized human-derived substrate is the goal.
This is a very exciting and dynamic time in the human ES cell field with great strides being made in our understanding of the basis for self-renewal, pluripotency and directed differentiation of hESCs. It is important, therefore, to reinvent culture conditions to keep abreast of current and future requirements. The potential impact on regenerative medicine and drug discovery in the coming years may be unrivaled and is certainly worthy of the current efforts.
Note added in proof
The Thomson group has most recently published research showing that cell lines may be derived and propagated in a culture system that includes protein components solely derived from recombinant sources or purified from human material. The authors have published the complete formulation of their novel medium which does not include the commonly used KSR (Ludwig et al., Nat. Biotechnol. 2006 (online), doi:10.1038/nbt1177).
Acknowledgements
We would like to thank Dr. Jeanette Beers for help with the flow cytometry. The NIH Stem Cell Unit is supported by NIH funds.
Contributor Information
Barbara S. Mallon, Email: mallonb@mail.nih.gov.
Kye-Yoon Park, Email: parkky@mail.nih.gov.
Kevin G. Chen, Email: cheng@mail.nih.gov.
Rebecca S. Hamilton, Email: rebeccahamilton@mail.nih.gov.
Ronald D.G. McKay, Email: ronmckay@mail.nih.gov.
References
- Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- Amit M, Winkler ME, Menke S, Bruning E, Buscher K, Denner J, et al. Noevidence for infection of human embryonic stem cells by feeder cell-derived murine leukemia viruses. Stem Cells. 2005;23:761–771. doi: 10.1634/stemcells.2004-0046. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG, et al. Karyotypic stability, genotyping, differentiation, feeder-free maintenance and gene expression sampling in three human embryonic stem cell lines derived prior to August 9. Stem Cells Dev. 2001;13:585–597. doi: 10.1089/scd.2004.13.585. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79–88. doi: 10.1089/153623003321512193. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- Daheron L, Opitz SL, Zaehres H, Lensch WM, Andrews PW, Itskovitz-Eldor J, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- Draper JS, Moorer HD, Ruban LN, Gokhale PJ, Andrews PW. Culture and characterization of human embryonic stem cells. Stem Cells Dev. 2004;13:325–326. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat. Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- Heng BC, Liu H, Cao T. Feeder cell density—A key parameter in human embryonic stem cell culture. In Vitro Cell. Dev. Biol. Anim. 2004;40:255–257. doi: 10.1290/0407052.1. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Mikkola M, Gertow K, Stromberg A, Inzunza J, Hreinsson J, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum. Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Cheon SH, Yoo SJ, Kwon J, Park JH, Kim CG, et al. Contribution of the PI3K/Akt/PKB signal pathway to maintenance of self-renewal in human embryonic stem cells. FEBS Lett. 2005;579:534–540. doi: 10.1016/j.febslet.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR, et al. Isolation and characterization of Type IV procollagen, laminin and heparin sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R, et al. Human embryonic stem cells derived without feeder cells. Lancet. 2005;365:1636–1641. doi: 10.1016/S0140-6736(05)66473-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol. Bioeng. 2005;91:688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- Lim JWE, Bodnar A. Proteome analysis of conditioned medium from mouse embryonic fibroblast feeder layers which support the growth of human embryonic stem cells. Proteomics. 2002;2:1187–1203. doi: 10.1002/1615-9861(200209)2:9<1187::AID-PROT1187>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Nur-E-Kamal A, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells Express. 2005;24:426–433. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- Park SP, Young JL, Lee KS, Shin HA, Cho HY, Chung KS, et al. Establishment of human embryonic stem cell lines from frozen-thawed blastocysts using STO feeder layers. Hum. Reprod. 2004;19:676–684. doi: 10.1093/humrep/deh102. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A, et al. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- Rosler ES, Fisk GJ, Ares X, Irving J, Miura T, Rao MS, et al. Long-term culture of human embryonic stem cells in feeder-free conditions. Dev. Dyn. 2004;229:259–274. doi: 10.1002/dvdy.10430. [DOI] [PubMed] [Google Scholar]
- Sato N, Meier L, Skalsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Przyborski S, Stewart R, Armstrong L, Evans J, et al. Human-serum matrix supports undifferentiated growth of human embryonic stem cells. Stem Cells. 2005;23:895–902. doi: 10.1634/stemcells.2004-0326. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Stewart R, Pryzborski S, Armstrong L, Evans J, et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro S, Waknitz MA, Sweirgiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pederson RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell. Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhang H, Zhao Y, Li J, Cai J, Wang P, et al. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem. Biophys. Res. Commun. 2005;330:934–942. doi: 10.1016/j.bbrc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- Xie CQ, Lin G, Luo KL, Luo SW, Lu GX. Newly expressed proteins of mouse embryonic fibroblasts irradiated to be inactive. Biochem. Biophys. Res. Commun. 2004;315:581–588. doi: 10.1016/j.bbrc.2004.01.089. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK, et al. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972–980. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, O’Sullivan C, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- Yim EK, Leong KW. Proliferation and differentiation of human embryonic germ cell derivatives in bioactive polymeric fibrous scaffold. J. Biomater. Sci. Polym. Ed. 2005;16:1193–1217. doi: 10.1163/156856205774269485. [DOI] [PubMed] [Google Scholar]