Abstract
SRX246 is a potent, highly selective human vasopressin V1a antagonist that crosses the blood–brain barrier in rats. CNS penetration makes SRX246 an ideal candidate for potential radiolabeling and use in visualization and characterization of the role of the V1a receptor in multiple stress-related disorders. Before radiolabeling studies, cold reference analogs of SRX246 were prepared. This study describes the synthesis and in vitro screening for human V1a receptor binding and permeability of fluoro, iodo, and methyl reference compounds for SRX246 and the preparation of a tin precursor. For each compound, the potential utility of corresponding radiolabeled analogs for PET and SPECT imaging is discussed.
Keywords: Arginine vasopressin, Vasopressin V1a antagonist, Radiolabeling, PET, SPECT
1. Introduction
Arginine vasopressin (AVP) is a cyclic nonapeptide hormone that modulates a broad range of physiological and behavioral effects by binding to specific GPCRs in the central nervous system and certain peripheral tissues/sites.1,2 Three distinct AVP receptor subtypes have been identified—V1a, V1b, and V2. V1a is the predominant AVP receptor found in the brain. Especially high levels are found in the cerebral cortex, limbic system, hypothalamus, and brainstem.1 The V1b receptor is located in the pituitary gland and it is less widespread in the brain than the V1a receptor. The V2 receptor is found in kidneys, where it mediates the antidiuretic effects of vasopressin. It is not generally thought to be expressed in the nervous systems of adult animals and humans. These findings have led to considerable interest in V1a and V1b receptors as potential targets for CNS therapeutics. The effects of AVP and its mechanism of action have been extensively studied and discussed elsewhere.3–6
Selective V1a and V1b receptor antagonists that cross the blood–brain barrier have attracted considerable interest as potential therapeutics because the compounds represent a novel approach for the treatment of depression, anxiety, Post-Traumatic Stress Disorder (PTSD), and Intermittent Explosive Disorder.7–17
Our interest is in the V1a receptor, where there is strong evidence from preclinical animal models as well as observations in humans indicating that this subtype may well play a central role in these indications.1,3,4,6,18–32 We were the first group to report the discovery of potent, highly selective, CNS-penetrating human V1a (hV1a) receptor antagonists.18 The technology places us in a strong position to build upon our earlier discoveries to develop imaging agents for the hV1a system based in particular on SRX246, one of our clinical candidates.
Both PET and SPECT protocols have proved very useful as tools to investigate in vivo drug behavior including a recent example for the vasopressinergic system focusing on PET and the human V1b (hV1b) receptor.33,34 The current study presents our progress generating reference compounds and a versatile tin intermediate that would allow for the preparation of both PET and SPECT tracers for the hV1a receptor, together with preliminary work on 123I labeling.
2. Results and discussion
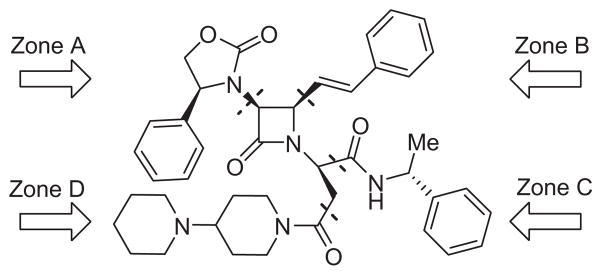
SRX246 is a mono beta-lactam derivative with an azetidone ring as a central pharmacophore core around which four distinct peripheral zones (Zone A, B, C and D) can be varied (Fig. 1). We intend to modify Zone B in SRX246 for radioisotope introduction (123I, 18F or 11C). This approach represents a less challenging synthetic pathway as compared to introducing the same tracers on the aryl group in either Zone A or C. We also know, based on previously described hV1a SARs,18 that Zone B is more tolerant of small structural modifications, especially at the meta-position of the phenyl ring.
Figure 1.
Key structural features of SRX246.
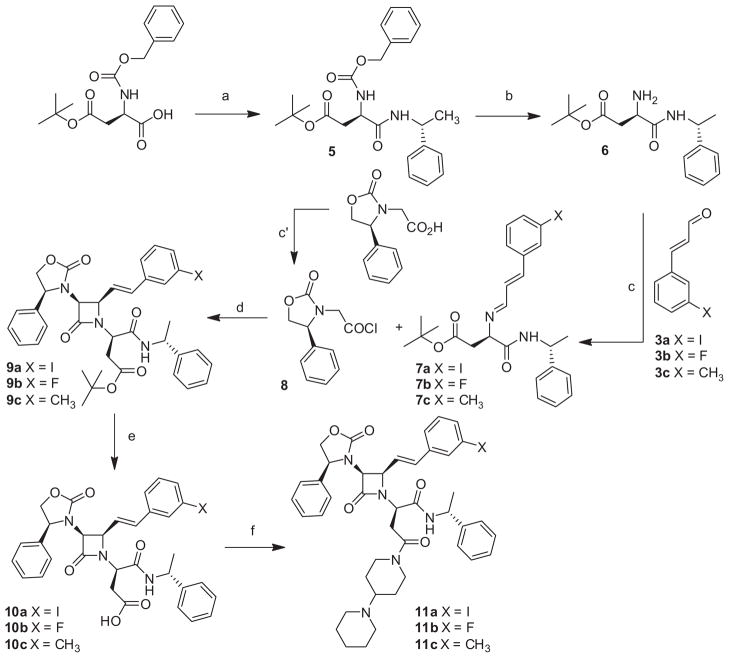
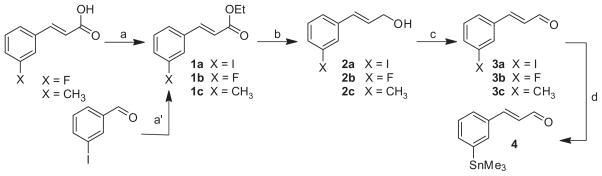
In practice, the synthetic route (Scheme 1) leading to compounds 11a, 11b and 11c was adapted from that previously published for the preparation of SRX246.18 The procedure is based on a chiral Staudinger 2+2 cyclo-addition reaction that allows the building of the azetidone ring. In this modified procedure, meta-substituted-trans-cinnamaldehydes were used instead of trans-cinnamaldehyde while the rest of the reaction sequence, reagents and conditions were kept the same. The three target compounds were obtained in consistent yield but required the preparation of the corresponding meta-substituted-trans-cinnamaldehydes. This was accomplished through a three step process (Scheme 2) from commercially available 3-fluoro or 3-methyl cinnamic acids.35 Because of the lack of a reliable commercial source for 3-iodocinnamic acid, 1a was obtained via a Horner–Wadsworth–Emmons reaction on the 3-iodobenzaldehyde instead.36 Reduction of the ethyl cinnamate esters to the alcohols 2a, 2b and 2c and further oxidation with pyridinium dichromate lead to the meta-substituted-trans-cinnamaldehyde 3a, 3b and 3c in high yields. This three step process led to the target halogenated or alkyl derivatives in much higher overall yields compared to published procedures,37 particularly for the iodo derivative. The absolute stereochemistry of the chiral methine carbons of the azetidone ring for compounds 11a, 11b and 11c was assigned based on previous findings and confirmed by the 1H and 13C NMR chemical shifts and coupling constants.18
Scheme 1.
Syntheses of fluoro, iodo and methyl analogs of SRX246. Reagents and conditions: (a) (R)-methylbenzylamine, HOBt, EDC·HCl, CH2Cl2, rt, 18 h; (b) H2, Pd/C 5%, MeOH, 18 h; (c) Na2SO4 anhydrous, CH2Cl2; (c′) oxalyl chloride, DMF cat., CH2Cl2; (d) NEt3, CH2Cl2, 0 °C to rt, 1 h; (e) HCO2H; (f) 4-(1-piperidinyl) piperidine, HOBt, EDC, HCl, CH2Cl2, rt, 18 h.
Scheme 2.
Preparation of meta-substituted-trans-cinnamaldehydes. Reagents and conditions: (a) EDC·HCl, DMAP, EtOH, CH2Cl2, rt, 18 h; (a′) triethyl phosphonoacetate, nBuLi in hexanes, THF, −78 °C, 10 min; 3-iodobenzaldehyde, THF, −78 °C, 40 min, to rt 45 min; (b) DIBAL, CH2Cl2, −78 °C, 2 h; 10% aqueous NaOH, −78 °C to rt, 15 min; (c) pyridinium dichromate, CH2Cl2; (d) hexamethylditin, Pd(PPh3)4, dioxane, 150 min.
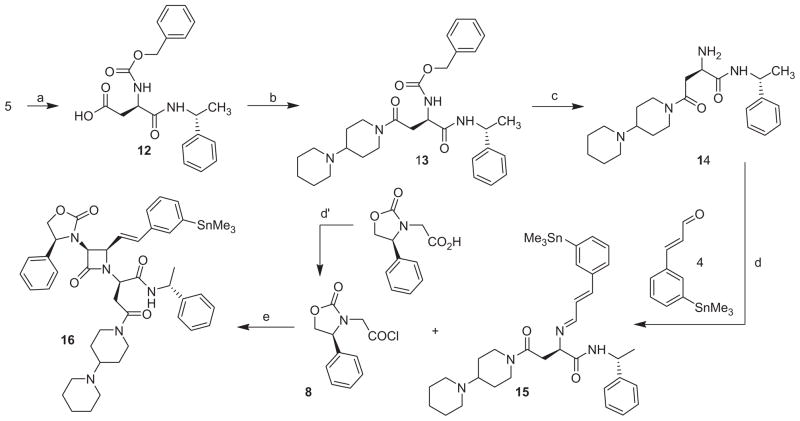
After an earlier attempt to directly convert 11a to 16, we decided to evaluate the utility of our general synthetic pathway for the preparation of such a tin compound. It became quickly apparent that the preparation of the tin analog (compound 16) represented a challenge because the conditions used for the synthesis of SRX246 were not directly transposable to the synthesis of the tin compound. In particular, we had concerns about the potential detrimental cleavage of the tin substituent in the presence of an acid such as the one used (formic acid) to cleave the t-Butyl ester distal carboxyl acid protecting group off the D-aspartic acid and after the cyclo-addition step. A distinct synthetic pathway was designed (Scheme 3) that would circumvent this potential problem. In this new approach, we decided to introduce the two amide functions prior to the cyclo-addition step, which allowed us to avoid putting our tin group in jeopardy because the use of formic acid was now warranted in an earlier step of the process. In summary, it allowed the conservation of the alkyl stannyl group through the multi-step synthesis and also resulted in the 2+2 cyclo-addition being the final step of the reaction sequence. To achieve this, we also had to develop a method to prepare the new meta-trimethyltin-trans-cinnamaldehyde (4) needed for this approach. It was synthesized by direct modification of 3-iodocinnamaldehyde (3a) via a Stille reaction with hexamethylditin in the presence of a catalytic amount of tetrakis(triphenylphosphine)palladium.38,39 The desired trimethylstannane aldehyde was obtained in high yield (80%) and was easily purified by silica gel filtration. Following the preparation of the aldehyde and implementing this strategy, we were indeed able to obtain the target compound 16 in satisfactory overall yield.
Scheme 3.
Synthesis of radiolabeling precursor 16. Reagents and conditions: (a) HCO2H; (b) 4-(1-piperidinyl) piperidine, HOBt, EDC·HCl, CH2Cl2, rt, 18 h; (c) H2, Pd/C 5%, MeOH, 18 h; (d) Na2SO4 anhydrous, CH2Cl2; (d′) oxalyl chloride, DMF cat., CH2Cl2; (e) NEt3, CH2Cl2, 0 °C to rt, 1 h.
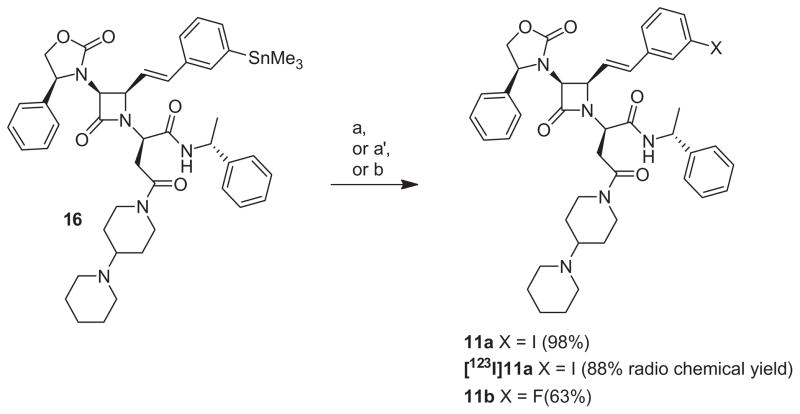
Conversion to the cold and hot reference compounds, 11a and [123I]11a, via demetallation reaction was probed to further validate the potential use of 16 for 123I radiolabeled ligands generation for SPECT imaging (Scheme 4). Preparation of 11a and [123I]11a from 16 was investigated following established methods.40 In the presence of chloramine T (CAT) as the oxidant and sodium iodide as the iodine source, 16 was successfully converted to the cold iodo compound 11a with a chemical yield superior to 98%. Radioiodination with [123I]INa was conducted to afford [123I]11a in 88% radiochemical yield. After RP-HPLC purification, the radiochemical purity of [123I]11a was determined at 94% with a specific activity of 20 mCi/nmol. The potential use of 16 for the generation of 18F and 11C radiolabeled ligands for PET imaging was also assayed in cold conditions only. Electrophilic fluorination of 16 with the well known fluorinated agent SelectFluor™, which has been highlighted recently as a promising fluorine source for 18F radiolabeling, afforded 11b with a chemical yield of 63% (Scheme 4).41 Alternative approaches leading to high specific activity radioligands will also be investigated in future studies.42–44 Conversion of 16 to 11c via a Stille coupling reaction used for 11C radiolabeling45 also showed promising results and confirmed the feasibility of 11C radiolabeling (data not presented).
Scheme 4.
Conversion of trimethyltin derivative 16 to 11a, [123I]11a and 11b. Reagents and conditions: (a) NaI, chloramine T, 1 M HCl, ethanol, rt, 5 min; (a′) [123I]NaI, chloramine T, 1 M HCl, ethanol, rt, 5 min; (b) Selectfluor™ (BF−4), AgOTf, acetone, rt, 30 min.
Having prepared compounds 11a, 11b and 11c the next step was to evaluate their biological activity in vitro against the human vasopressin V1a receptor and in the Parallel Artificial Membrane Permeability Assays for the Blood–Brain Barrier (PAMPA-BBB) assay. As expected, the modifications in the cinnamyl part (Zone B) of the clinical candidate SRX246 did not affect dramatically the biological activity for the hV1a receptor (SRX246’s hV1a Ki was reported previously at 0.3 nM).18 Compounds 11a, 11b and 11c displayed potent nanomolar activity and extremely high affinity in vitro for the hV1a receptor (Table 1). Compound 11b was the most active of the three potential radiolabeled ligands with a Ki of 0.61 nM. This is not surprising if one considers that fluorine is an excellent isostere for hydrogen,46 creating one of the closest possible structural analogs of SRX246. In a screening assay for binding to hV1b and hV2 receptors, all the tested compounds were found with Ki greater than 1000 nM demonstrating excellent selectivity for the V1a receptor over other vasopressin receptor subtypes (Table 1). The lipophilicity was mildly affected by the addition of a fluorine atom or a methyl group with cLogP increases of 0.16 and 0.49 units for 11b and 11c, respectively, compared to that of SRX246 (cLogP = 4.26, Spartan06). One can therefore reasonably predict that both compounds will exhibit bioavailability similar to SRX246, the first orally bioavailable, selective hV1a vasopressin antagonists with CNS penetration.47 A larger increase in lipophilicity is observed for 11a (1.4 units for cLogP) which is generally the case with the addition of an iodine atom.
Table 1.
Vasopressin receptor affinity and PAMPA-BBB results for potential SRX246 based PET and SPECT ligands
| Compound | hV1a Ki (nM) | hV1b Ki (nM) | hV2 Ki (nM) | cLogP | Pe (10−6 cm s−1) | Predicted CNS penetration |
|---|---|---|---|---|---|---|
| 11a | 1.1 | >1000 | >1000 | 5.62 | 2.2 | CNS± |
| 11b | 0.61 | 1154 | >10,000 | 4.42 | 5.3 | CNS+ |
| 11c | 0.63 | 1300 | >10,000 | 4.75 | 4.5 | CNS+ |
In vitro PAMPA-BBB were also carried out as a predictive tool for CNS penetration (Table 1). Both the fluorine and the methyl derivative 11b and 11c exhibited high permeability values (Pe >4.0 × 10−6 cm s−1), which strongly suggest high predicted passive Blood–Brain Barrier (BBB) permeation.48 Compound 11a exhibited a lower value (2.0 × 10−6 < Pe < 4.0 × 10−6 cm s−1), suggesting an uncertain predicted BBB permeation. However, BOLD (Blood-Oxygen- Level Dependence) imaging studies in rodents demonstrated that this particular compound has a CNS modulatory effect, demonstrating that it is capable of brain penetration.49
3. Conclusions
We have developed a reliable method to prepare a versatile precursor that should allow access to both PET (18F, 11C) and SPECT (123I) derivatives of SRX246, a highly selective and potent hV1a antagonist. Furthermore, we have synthesized the F, I, and Mebearing reference compounds and have shown that they exhibit strong hV1a receptor affinity, do not bind to V1b and V2 receptors, and have a high likelihood of brain penetration based on PAMPABBB results. These findings offer the potential to begin a broader investigation into the role of the human vasopressin V1a receptor in various CNS functions using relevant imaging tracers.
4. Experimental
4.1. Chemistry
4.1.1. (E)-Ethyl 3-(3-iodophenyl)acrylate (1a)
Triethyl phosphonoacetate (9.21 g, 41.12 mmol) in tetrahydrofuran (65 mL) was treated with 2.5 M n-butyl lithium in hexanes (12.82 mL, 32.07 mmol) at −78 °C. The resulting mixture was stirred for 10 min and added via a cannula to a pre-cooled solution at −78 °C of 3-iodobenzaldehyde (4.77 g, 20.56 mmol) in tetrahydrofuran (20 mL). The resulting mixture was stirred at −78 °C for 40 min and slowly warmed to ambient temperature for 45 min. The reaction was quenched with saturated aqueous NH4Cl (200 mL). The aqueous solution was extracted with diethyl ether. The resulting organic layer was dried over magnesium sulfate and evaporated. The residue was purified by silica gel chromatography (90:10 hexanes/ethyl acetate) to give 5.71 g (92%) of compound 1a as a yellow oil; 1H NMR (CDCl3) δ 1.32 (t, J = 7.1 Hz, 3H); 4.24 (q, J = 7.1 Hz, 2H); 6.39 (d, J = 16.0 Hz, 1H); 7.09 (dd, J = J′ = 7.8 Hz, 1H); 7.43–7.46 (m, 1H); 7.54 (d, J = 16.0 Hz, 1H); 7.64–7.69 (m, 1H); 7.83–7.86 (m, 1H).
4.1.2. General procedure for the formation of meta-substituted cinnamaldehyde (3 steps)
4.1.2.1. Step 1. General procedure for the formation of meta-substituted ethyl cinnamate ester
A solution of 1 equiv of meta-substituted cinnamic acid in dichloromethane (2 mL dichloromethane/mmol acid) was treated by sequential addition of 0.1 equiv of 4-dimethylaminopyridine, 1.05 equiv of 1-[3-(dimethylamino) propyl]-3-ethylcarbodiimide hydrochloride and 10 equiv of ethyl alcohol. The reaction was stirred at ambient temperature until all of the reactants were consumed as measured by thin layer chromatography (CH2Cl2 100%). When complete (approximately 18 h), the reaction mixture was concentrated under reduced pressure. Water was added to the residue and the aqueous layer was extracted with dichloromethane. The combined organic layers were washed successively with 1 N hydrochloric acid, saturated aqueous sodium bicarbonate, and saturated sodium chloride. The organic layer was dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was used directly for further reactions.
4.1.2.2. Step 2. General procedure for the reduction of meta-substituted ethyl cinnamate ester
A solution of 1 equiv of meta-substituted ethyl cinnamate ester in dichloromethane (2 mL dichloromethane/mmol ester) was treated slowly by the dropwise addition of 1 M diisobutylaluminium hydride (2.1 equiv) solution in dichloromethane at −78 °C. The resulting mixture was stirred 2 h at −78 °C and then quenched with 10% aqueous NaOH (25 mL/10 mmol ester). The resulting mixture was slowly warmup to ambient temperature and the layers separated. The aqueous layer was extracted with dichloromethane. The combined organic layers were washed successively with water, 1 N aqueous hydrochloric acid, and saturated aqueous sodium chloride. The organic layer was dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was used directly for further reactions or purified by chromatography from an appropriate solvent system before use.
4.1.2.3. Step 3. General procedure for the oxidation of meta-substituted allyl alcohol
A solution of 1 equiv of meta-substituted allyl alcohol in dichloromethane (2 mL dichloromethane/mmol alcohol) was treated with 1.5 equiv of pyridinium dichromate under inert atmosphere. The reaction was stirred at ambient temperature until all of the reactants were consumed as measured by thin layer chromatography (CH2Cl2 100%). The resulting mixture was diluted with diethyl ether/hexanes (50:50) and filtered through silica gel. The silica gel was rinsed with hexanes/ethyl acetate (60:40) and the combined filtrate evaporated. The residue obtained was dissolved in dichloromethane, washed with 0.1 N aqueous HCl, water and dried over magnesium sulfate. The residue was used directly for further reactions or purified by chromatography from an appropriate solvent system before use. The following compounds were prepared according to these procedures.
4.1.2.4. (E)-3-(3-Iodophenyl)prop-2-en-1-ol (2a)
Compound 1a (5.71 g, 18.90 mmol) was treated according to the General Procedure Step 2 to give 4.88 g (99%) of compound 2a as a yellow oil; 1H NMR (CDCl3) δ 1.55 (t, J = 5.9 Hz, 1H); 4.29–4.33 (m, 2H); 6.32 (dt, J = 15.8 Hz, J = 5.5 Hz, 1H); 6.49 (d, J = 15.9 Hz, 1H); 7.02 (dd, J = J′ = 7.8 Hz, 1H); 7.29–7.32 (m, 1H); 7.53–7.56 (m, 1H); 7.71–7.72 (m, 1H).
4.1.2.5. (E)-3-(3-Iodophenyl)acrylaldehyde (3a)
Compound 2a (4.88 g, 18.76 mmol) was treated according to the General Procedure Step 3 to give 4.26 g (88%) of compound 3a as a yellow solid. 1H NMR (CDCl3) δ 6.67 (dd, J = 16.0 Hz, J = 7.6 Hz, 1H); 7.15 (dd, J = J′ = 7.8 Hz, 1H); 7.35 (d, J = 16.0 Hz, 1H); 7.49–7.53 (m, 1H); 7.72–7.77 (m, 1H); 7.87–7.91 (m, 1H); 9.68 (d, J = 7.5 Hz, 1H). 13C NMR (CDCl3) δ 94.81, 127.44, 129.54, 130.69, 136.10, 137.22, 139.89, 150.59, 193.22.
4.1.2.6. (E)-Ethyl 3-(3-fluorophenyl)acrylate (1b)
3-Fluoro cinnamic acid (2 g, 12.04 mmol) was treated according to the General Procedure Step 1 to give 2.22 g (95%) of compound 1b as colorless oil. 1H NMR (CDCl3) δ 1.33 (t, J = 7.1 Hz, 3H); 4.27 (q, J = 7.1 Hz, 2H); 6.42 (d, J = 16.0 Hz, 1H); 7.07 (ddd, J = J′ = 8.2 Hz, J″ = 0.95 Hz, 1H); 7.14–7.24 (m, 1H); 7.28–7.31 (m, 1H); 7.32–7.38 (1H); 7.63 (d, J = 16.0 Hz, 1H).
4.1.2.7. (E)-Ethyl 3-(m-tolyl)acrylate (1c)
3-Methyl cinnamic acid (1.58 g, 9.77 mmol) was treated according to the General Procedure Step 1 to give 1.85 g (93%) of compound 1c as a white solid. 1H NMR (CDCl3) δ 1.32 (t, J = 7.1 Hz, 3H); 2.35 (s, 3H); 4.24 (q, J = 7.1 Hz, 2H); 6.40 (d, J = 16.0 Hz, 1H); 7.15–7.19 (m, 1H); 7.22–7.28 (m, 1H); 7.28–7.37 (2H); 7.66 (d, J = 16.0 Hz, 1H).
4.1.2.8. (E)-3-(3-Fluorophenyl)prop-2-en-1-ol (2b)
Compound 1b (2.2 g, 11.4 mmol) was treated according to the General Procedure Step 2 to give after flash column chromatography purification (hexanes/dichloromethane 50:50 to dichloromethane 100%) 1.4 g (82%) of compound 2b as colorless oil. 1H NMR (CDCl3) δ 1.48 (t, J = 5.9 Hz, 1H); 4.30–4.34 (m, 2H); 6.35 (dt, J = 15.8 Hz, J = 5.5 Hz, 1H); 6.57 (d, J = 15.9 Hz, 1H); 6.91 (ddd, J = J′ = 8.0 Hz, J′ = 2.2 Hz, 1H); 7.04–7.09 (m, 1H); 7.11–7.14 (m, 1H); 7.23–7.29 (m, 1H).
4.1.2.9. (E)-3-(m-Tolyl)prop-2-en-1-ol (2c)
Compound 1c (1.73 g, 9.09 mmol) was treated according to the General Procedure Step 2 to give 1.24 g (92%) of compound 2c as colorless oil. 1H NMR (CDCl3) δ 1.39 (t, J = 5.9 Hz, 1H); 2.33 (s, 3H); 4.28–4.32 (m, 2H); 6.34 (dt, J = 15.9 Hz, J = 5.7 Hz, 1H); 6.56 (d, J = 15.9 Hz, 1H); 7.03–7.08 (m, 1H); 7.15–7.25 (m, 3H).
4.1.2.10. (E)-3-(3-Fluorophenyl)acrylaldehyde (3b)
Compound 2b (1.4 g, 9.29 mmol) was treated according to the General Procedure Step 3 to give 1.3 g (96%) of compound 3b as a colorless oil. 1H NMR (CDCl3) δ 6.68 (dd, J = 16.0 Hz, J = 7.5 Hz, 1H); 7.12 (ddd, J = J′ = 8.2 Hz, J″ = 0.95 Hz, 1H); 7.22–7.26 (m, 1H); 7.31–7.34 (m, 1H); 7.37–7.42 (m, 1H); 7.42 (d, J = 16.0 Hz, 1H); 9.69 (d, J = 7.6 Hz, 1H). 13C NMR (CDCl3) δ 114.50 (d, 2JC-F = 22.6 Hz, 1C), 117.87 (d, 2JC-F = 21.3 Hz, 1C), 124.19 (d, 4JC-F = 2.5 Hz, 1C), 129.39, 130.48 (d, 3JC-F = 8.8 Hz, 1C), 150.79 (d, 4JC-F = 2.5 Hz, 1C), 162.78 (d, 1JC-F = 247.7 Hz, 1C), 193.13.
4.1.2.11. (E)-3-(m-Tolyl)acrylaldehyde (3c)
Compound 2c (1.24 g, 8.36 mmol) was treated according to the General Procedure Step 3 to give after flash column chromatography purification (hexanes 100% to hexanes/ethyl acetate 90:10) 0.68 g (55%) of compound 3c as a colorless oil. 1H NMR (CDCl3) δ 2.37 (s, 3H); 6.69 (dd, J = 15.9 Hz, J = 7.7 Hz, 1H); 7.22–7.40 (m, 3H); 7.43 (d, J = 16.0 Hz, 1H); 9.68 (d, J = 7.7 Hz, 1H). 13C NMR (CDCl3) δ 21.25, 125.69, 128.36, 128.93, 129.08, 132.10, 133.90, 138.78, 153.10, 193.81.
4.1.3. (E)-3-(3-(Trimethylstannyl)phenyl)acrylaldehyde (4)
Compound 3 (0.5 g, 1.93 mmol) dissolved in dioxane (20 mL) was treated with hexamethylditin (0.476 g, 1.45 mmol) and tetrakis(triphenylphosphine)palladium (0) (0.006 g, 0.0387 mmol). The reaction was refluxed for 150 min and shielded from light. The reaction mixture was then cooled down to ambient temperature and filtered through celite, the celite was washed with dichloromethane and the solvent concentrated. The resulting oil was filtered through silica gel (40 g) pre-equilibrated with a mixture of light ether/dichloromethane (90:10), rinsed with this mixture and dichloromethane and eluted with ethyl acetate/dichloromethane (30:70). The fractions collected were then evaporated to give 0.464 g (80%) of compound 4 as bright yellow oil. 1H NMR (CDCl3) δ 0.36 (s, with Sn satellites, d, J = 54.3 Hz, 9H); 6.73 (dd, J = 15.9 Hz, J = 7.7 Hz, 1H); 7.44–7.56 (m, 3H); 7.64 (br s, 1H); 9.69 (d, J = 7.7 Hz, 1H). 13C NMR (CDCl3) δ −9.54, 128.09, 128.48, 128.54, 133.41, 135.98, 138.78, 143.67, 153.24, 193.74.
4.1.4. General procedure for amide formation from a carboxylic acid
(R)-tert-Butyl 3-(((benzyloxy)carbonyl)amino)-4-oxo-4-(((R)-1-phenylethyl)amino)butanoate (5).18 A solution of 0.6g (1.75mmol) of N-benzyloxycarbonyl-D-aspartic acid β-t-butyl ester monohydrate in 10 mL dichloromethane was treated by sequential addition of 0.238mL (1.84 mmol) of (R)-α-methylbenzylamine, 0.237 g (1.84 mmol) of 1-hydroxy-7-benzotriazole, and 0.337 g (1.84 mmol) of 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride. After 18 h stirring at ambient temperature, the reaction mixture was washed sequentially with a saturated sodium bicarbonate solution and with saturated sodium chloride solution. The organic layer was evaporated to give 0.726 g (97%) of compound 5 as an off-white solid. 1H NMR (CDCl3) δ 1.38 (s, 9H); 1.43 (d, J = 6.9 Hz, 3H); 2.54 (dd, J = 17.2 Hz, J = 7.2 Hz, 1H); 2.87 (dd, J = 17.3 Hz, J = 4.0 Hz, 1H); 4.46–4.50 (m, 1H); 4.99–5.15 (m, 3H); 5.92–5.96 (m, 1H); 6.78–6.82 (m, 1H); 7.21–7.33 (m, 10 H).
4.1.5. General procedure for hydrolysis of a tert-butyl ester
A solution of tert-butyl ester derivative in formic acid, typically 1 g in 10 mL, is stirred at ambient temperature until no more ester is detected by thin layer chromatography (dichloromethane 95%/methanol 5%), a typical reaction time being around 3 h. The formic acid is then evaporated under reduced pressure to yield the corresponding carboxylic acid. The following compound was prepared according to this procedure.
4.1.5.1. (R)-3-(((Benzyloxy)carbonyl)amino)-4-oxo-4-(((R)-1-phenylethyl)amino)butanoic acid (12)
Compound 5 (0.726 g, 1.70 mmol) was hydrolyzed to give 0.614 g (97%) of compound 12 as a white solid; 1HNMR (MeOH-d4) δ 1.40 (br s, 3H); 2.56–2.81 (m, 2H); 5.0–5.15 (m, 3H); 7.11–7.41 (m, 10H).
4.1.6. Benzyl ((R)-4-([1,4′-bipiperidin]-1′-yl)-1,4-dioxo-1-(((R)-1-phenylethyl)amino)butan-2-yl)carbamate (13)
Compound 13 was prepared using the General procedure for amide formation from a carboxylic acid except that N-benzyloxycarbonyl-D-aspartic acid β-t-butyl ester was replaced with compound 12 (0.614 g, 1.65 mmol) and (R)-α-methylbenzylamine was replaced with 4-(1-piperidinyl)piperidine. Compound 13 was obtained as a pink solid (0.718 g, 83%). 1H NMR (CDCl3) δ 1.22–1.34 (m, 1H); 1.34–1.47 (m, 7H); 1.47–1.62 (m, 4H); 1.72–1.90 (m, 2H); 2.33–2.60 (m, 6H); 2.80–3.01 (m, 1H); 3.06–3.20 (m, 1H); 3.66–3.83 (m, 1H); 4.46–4.67 (m, 2H); 4.95–5.17 (m, 3H); 6.22–6.38 (m, 1H); 7.11–7.40 (m, 10H).
4.1.7. General procedure for hydrogenolysis of a benzyloxycarbonyl amine
4.1.7.1. (R)-4-([1,4′-Bipiperidin]-1′-yl)-2-amino-4-oxo-N-((R)-1-phenylethyl)butanamide (14)
A suspension of 0.718 g (1.37 mmol) of compound 13 and palladium (5 wt % on activated carbon, 0.35 g) in 20 mL methanol was held under an atmosphere of hydrogen for 18 h. The reaction was filtered to remove the palladium over carbon and the filtrate was evaporated to give 0.484 g (87%) of compound 14 as a colorless oil. 1H NMR (MeOH-d4) δ 1.23–1.39 (m, 1H); 1.39–1.52 (m, 7H); 1.52–1.76 (m, 4H); 1.80–1.92 (m, 2H); 2.42–2.61 (m, 7H); 2.61–2.69 (m, 1H); 2.69–2.80 (m, 1H); 2.87–3.03 (m, 1H); 2.64–3.74 (m, 1H); 3.88–4.00 (m, 1H); 4.49–4.59 (m, 1H); 4.92–5.10 (m, 1H); 7.16–7.36 (m, 5H). The following compound was obtained according to this procedure.
4.1.7.2. (R)-tert-Butyl 3-amino-4-oxo-4-(((R)-1-phenylethyl) amino)butanoate (6)
Compound 5 (0.618 g, 1.45 m mol) was hydrogenolized to give 0.407 g (96%) of 6 as an off-white solid; 1H NMR (CDCl3) δ 1.40 (s, 9H); 1.47 (d, J = 6.9 Hz, 3H); 1.98 (br s, 2H); 2.49 (dd, J = 7.9 Hz, J = 17.7 Hz, 1H); 2.83 (dd, J = 3.6 Hz, J = 16.7 Hz, 1H); 3.69 (br s, 1H); 4.99–5.10 (m, 1H); 7.19–7.33 (m, 5H); 7.65–7.68 (m, 1H).
4.1.8. General procedure for formation of a 2-azetidinone from an imine and an acetyl chloride
4.1.8.1. Step 1: General procedure for formation of an imine from an amino acid derivative
A solution of 1 equiv of an α-amino acid ester or amide in dichloromethane was treated sequentially with 1 equiv of an appropriate aldehyde, and a desiccating agent, such as magnesium sulfate or sodium sulfate, in the amount of about 14 equiv (2 g) of desiccating agent per gram of starting α-amino acid ester or amide. The reaction was stirred at ambient temperature until all of the reactants were consumed as measured by thin layer chromatography (CH2Cl2 95%/MeOH 5%). When complete, the reaction mixture was then filtered, the filter cake was washed with dichloromethane, and the filtrate concentrated under reduced pressure to provide the desired imine that was used directly in the subsequent step.
4.1.8.2. Step 2: General procedure for the [2+2] cyclo-addition of an imine and an acetyl chloride
A dichloromethane solution of the imine (10mL dichloromethane/1 g imine)was cooled to 0 °C. To this cooled solution was added 1.5 equiv triethylamine, followed by the dropwise addition of a dichloromethane solution of 1.1 equiv of an acetyl chloride 8 (10mL of dichloromethane/1 g of 8). The reaction mixture was allowed to warm to ambient temperature over 1 h and was then quenched by the addition of a saturated aqueous solution of ammonium chloride. The resulting mixture was partitioned between water and dichloromethane. The layers were separated and the organic layerwas washed successively with 1 N hydrochloric acid, saturated aqueous sodium bicarbonate, and saturated aqueous sodium chloride. The organic layer was dried over magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by chromatography (hexanes/ethyl acetate or dichloromethane/methanol) or by crystallization from dichloromethane/methanol. The following compounds were obtained according to this sequence of procedures.
4.1.8.3. (R)-4-([1,4′-Bipiperidin]-1′-yl)-4-oxo-2-((3S,4R)-2-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)-4-((E)-3-(trimethylstannyl) styryl)azetidin-1-yl)-N-((R)-1-phenylethyl)butanamide (16)
Imine 15 prepared from 0.445 g (1.15 mmol) of 14 and (E)-3-(3-(trimethylstannyl)phenyl)acrylaldehyde 4 was combined with 2-(4(S)-phenyloxazolidin-2-on-3-yl) acetyl chloride 8 to give 0.639 g (65%) of compound 16 as a yellow solid after flash column chromatography (CH2Cl2 99% to 90% gradient/MeOH 1% to 10% gradient, NH4OH <1%). 1H NMR (CDCl3) δ 0.29 (s, with Sn satellites, d, J = 54.2 Hz, 9H); 1.22–1.50 (m, 4H); 1.50–1.65 (m, 7H); 1.65–1.84 (m, 2H); 2.30–2.55 (m, 6H); 2.85–2.96 (m, 1H); 3.14–3.21 (m, 1H); 3.34–3.43 (m, 1H); 3.75–3.86 (m, 1H); 3.96–4.08 (m, 1H); 4.15 (t, J = 8.5 Hz); 4.18–4.26 (m, 1H); 4.41–4.55 (m, 1H); 4.61–4.67 (m, 1H); 4.67–4.77 (m, 2H); 5.0–5.13 (m, 1H); 6.33–6.41 (m, 1H); 6.76–6.84 (m, 1H); 7.15–7.49 (m, 15H); 8.21–8.29 (m, 1H). 13C NMR (CD3CN) δ −9.63, 25.38/25.42, 26.96/27.01, 28.32/28.40, 28.69/28.90, 34.13/34.19, 42.09/42.19, 45.61/45.47, 50.25/50.26, 50.78/50.83, 55.60, 55.80, 61.27, 63.06, 63.09/63.14, 63.88/63.92, 71.90/71.92, 123.43/123.52, 126.76, 127.04, 127.63, 128.31/128.36, 129.34, 130.09, 130.21, 135.76, 136.56/136.58, 136.96, 138.52/138.55, 138.97/139.04, 143.98, 145.75, 159.35/159.38, 164.62/164.62, 168.60/168.68, 169.15/169.21. HRMS (FAB) calcd for C45H58N5O5Sn 868.3464, found 868.3466 (M+H)+.
4.1.8.4. (R)-tert-Butyl 3-((2R,3S)-2-((E)-3-iodostyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-4-(((R)-1-phenylethyl)amino)butanoate (9a)
Imine 7a prepared from 0.815 g (2.78 mmol) of 6 and of 3a was combined with 2- (4(S)-phenyloxazolidin-2-on-3-yl) acetyl chloride 8 to give 1.462 g (71%) of compound 9a as a white solid after flash column chromatography (hexanes 95% to 25% gradient/ethyl acetate 5% to 75% gradient). 1HNMR(CDCl3) δ 1.35 (s, 9H); 1.58 (d, J = 7.1 Hz, 3H); 3.09 (dd, J = 3.6 Hz, J = 17.8 Hz, 1H); 3.17 (dd, J = 10.7 Hz, J = 17.8 Hz, 1H); 3.92 (dd, J = 3.5 Hz, J = 10.7 Hz, 1H); 4.16 (dd, J = 7.6 Hz, J = 8.5 Hz, 1H); 4.25 (d, J = 5.0 Hz, 1H); 4.58 (dd, J = 5.0 Hz, J = 9.3 Hz, 1H); 4.67 (t, J = 8.7 Hz, 1H); 4.73 (t, J = 8.2 Hz, 1H); 5.04–5.13 (m, 1H); 6.29 (dd, J = 9.3 Hz, J = 15.8 Hz, 1H); 6.62 (d, J = 15.8 Hz, 1H); 7.02 (t, J = 7.8 Hz, 1H); 7.11–7.28 (m, 4H); 7.34–7.48 (m, 7H); 7.58–7.65 (m, 2H); 8.05–8.11 (m, 1H).
4.1.8.5. (R)-tert-Butyl 3-((2R,3S)-2-((E)-3-fluorostyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-4-(((R)-1-phenylethyl)amino)butanoate (9b)
Imine 7b prepared from 0.330 g (1.13 mmol) of 6 and of 3b was combined with 2-(4(S)-phenyloxazolidin-2-on-3-yl) acetyl chloride 8 to give 0.539 g (76%) of compound 9b as a white solid after flash column chromatography (hexanes 100% to 70% gradient/ethyl acetate 0% to 30% gradient). 1H NMR (CDCl3) δ 1.35 (s, 9H); 1.59 (d, J = 7.0 Hz, 3H); 3.10 (dd, J = 3.5 Hz, J = 17.5 Hz, 1H); 3.19 (dd, J = 9.5 Hz, J = 18.0 Hz, 1H); 3.92 (dd, J = 3.2 Hz, J = 10.8 Hz, 1H); 4.15 (t, J = 8.2 Hz, 1H); 4.25 (d, J = 5.0 Hz, 1H); 4.58 (dd, J = 5.0 Hz, J = 9.5 Hz, 1H); 4.66 (t, J = 8.7 Hz, 1H); 4.73 (t, J = 8.2 Hz, 1H); 5.06–5.14 (m, 1H); 6.31 (dd, J = 9.2 Hz, J = 15.7 Hz, 1H); 6.68 (d, J = 16.0 Hz, 1H); 6.92–7.02 (m, 3H); 7.15–7.30 (m, 4H); 7.35–7.47 (m, 7H); 8.07–8.14 (m, 1H).
4.1.8.6. (R)-tert-Butyl 3-((2R,3S)-2-((E)-3-methylstyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-4-(((R)-1-phenylethyl)amino)butanoate (9c)
Imine 7c prepared from 0.40 g (1.37 mmol) of 6 and of 3c was combined with 2- (4(S)-phenyloxazolidin-2-on-3-yl) acetyl chloride 8 to give 0.469 g (55%) of compound 9c as a white solid after flash column chromatography (hexanes 95% to 25% gradient/ethyl acetate 5% to 75% gradient) 1H NMR (CDCl3) δ 1.35 (s, 9H); 1.59 (d, J = 7.1 Hz, 3H); 2.34 (s, 3H); 3.10 (dd, J = 3.5 Hz, J = 17.7 Hz, 1H); 3.19 (dd, J = 10.9 Hz, J = 17.8 Hz, 1H); 3.94 (dd, J = 3.5 Hz, J = 10.8 Hz, 1H); 4.14 (t, J = 8.5 Hz, 1H); 4.24 (d, J = 5.0 Hz, 1H); 4.58 (dd, J = 5.0 Hz, J = 9.4 Hz, 1H); 4.65 (t, J = 8.8 Hz, 1H); 4.73 (t, J = 8.4 Hz, 1H); 5.03–5.12 (m, 1H); 6.32 (dd, J = 9.5 Hz, J = 15.8 Hz, 1H); 6.72 (d, J = 15.8 Hz, 1H); 7.0–7.23 (m, 7H); 7.33–7.48 (m, 7H); 8.10–8.16 (m, 1H).
4.1.9. The following compounds were prepared according to the procedure ‘general procedure for hydrolysis of a tert-butyl ester’
4.1.9.1. (R)-3-((2R,3S)-2-((E)-3-Iodostyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-4-(((R)-1-phenyleth yl)amino)butanoic acid (10a)
Compound 9a (1.46 g, 1.98 mmol) was hydrolyzed to give 1.35 g (quantitative yield) of 10a as an off-white solid; 1H NMR (CDCl3) δ 1.57 (d, J = 7.1 Hz, 3H); 3.25 (d, J = 7.6 Hz, 2H); 3.92 (t, J = 7.1 Hz, 1H); 4.16 (dd, J = 8.4 Hz, J = 7.5 Hz, 1H); 4.27 (d, J = 5.0 Hz, 1H); 4.52 (dd, J = 9.2 Hz, J = 5.0 Hz, 1H); 4.66 (t, J = 8.7 Hz, 1H); 4.72 (t, J = 8.0 Hz, 1H); 5.03–5.11 (m, 1H); 6.25 (dd, J = 15.8 Hz, J = 9.3 Hz, 1H); 6.47 (d, J = 15.8 Hz, 1H); 7.02 (t, J = 7.8 Hz, 1H); 7.03–7.06 (m, 1H); 7.08–7.28 (m, 3H); 7.30–7.46 (m, 7H); 7.58–7.63 (m, 2H), 8.23–8.28 (m, 1H).
4.1.9.2. (R)-3-((2R,3S)-2-((E)-3-Fluorostyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-4-(((R)-1-phenylethyl) amino)butanoic acid (10b)
Compound 9b (0.535 g, 0.85 mmol) was hydrolyzed to give 0.487 g (quantitative yield) of compound 10b as an off-white solid; 1H NMR (CDCl3) δ 1.59 (d, J = 7.0 Hz, 3H); 3.25 (d, J = 7.0 Hz, 2H); 3.92 (t, J = 7.2 Hz, 1H); 4.16 (t, J = 8.0 Hz, 1H); 4.27 (d, J = 5.0 Hz, 1H); 4.52 (dd, J = 5.0 Hz, J = 9.5 Hz, 1H); 4.66 (t, J = 8.7 Hz, 1H); 4.72 (t, J = 8.0 Hz, 1H); 5.05–5.14 (m, 1H); 6.28 (dd, J = 15.5 Hz, J = 9.2 Hz, 1H); 6.66 (d, J = 16.0 Hz, 1H); 6.92–7.05 (m, 3H); 7.19–7.50 (m, 11H); 8.25–8.31 (m, 1H). 13C NMR (CDCl3) δ 21.66, 34.40, 49.83, 54.41, 61.02, 62.02, 63.44, 71.15, 113.29 (d, 2JC-F = 21.3 Hz, 1C), 115.76 (d, 2JCF = 21.3 Hz, 1C), 122.29, 122.83, 126.28, 127.19, 128.52, 129.76, 129.80, 130.33 (d, 3JC-F = 7.5 Hz, 1C), 135.82, 137.34 (d, 3JCF = 7.5 Hz, 1C), 138.10, 143.59, 158.04, 162.97 (d, 1JC-F = 246.5 Hz, 1C), 163.32, 167.28, 174.48.
4.1.9.3. (R)-3-((2R,3S)-2-((E)-3-Methylstyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-4-(((R)-1-phenyl ethyl)amino)butanoic acid (10c)
Compound 9c (0.126 g, 0.20 mmol) was hydrolyzed to give 0.116 g (quantitative yield) of compound 10c as an off-white solid; 1H NMR (CDCl3) δ 1.59 (d, J = 7.1 Hz, 3H); 2.34 (s, 3H); 3.22 (dd, J = 17.8 Hz, J = 8.1 Hz, 1H); 3.30 (dd, J = 17.8 Hz, J = 4.60 Hz, 1H); 3.92 (dd, J = 9.6 Hz, J = 4.6 Hz, 1H); 4.15 (t, J = 8.2 Hz, 1H); 4.26 (d, J = 5.0 Hz, 1H); 4.52 (dd, J = 9.4 Hz, J = 5.0 Hz, 1H); 4.66 (t, J = 8.7 Hz, 1H); 4.73 (t, J = 8.0 Hz, 1H); 5.04–5.14 (m, 1H); 6.29 (dd, J = 15.8 Hz, J = 9.6 Hz, 1H); 6.69 (d, J = 15.7 Hz, 1H); 7.01–7.47 (m, 14H); 8.29–8.34 (m, 1H). 13C NMR (CDCl3) δ 21.36, 21.98, 34.45, 49.86, 54.29, 60.99, 62.00, 63.68, 71.07, 120.37, 126.19, 126.91, 127.19, 127.78, 128.44, 128.96, 129.40, 129.71, 135.10, 135.82, 138.37, 139.60, 143.69, 158.03, 163.43, 167.39, 174.64.
4.1.10. Compounds 11a, 11b, and 11c were prepared according to the ‘general procedure for amide formation from a carboxyl ic acid’, except that N-benzyloxycarbonyl-D-aspartic acid α-t-bu tyl ester monohydrate was replaced with 10a, 10b and 10c and (R)-α-methylbenzylamine replaced by 4-(1-piperidinyl) piperid ine, all compounds exhibited an 1H and 13C NMR spectrum con sistent with the assigned structure
4.1.10.1. (R)-4-([1,4′-Bipiperidin]-1′-yl)-2-((2R,3S)-2-((E)-3-iodostyryl)- 4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1-yl)-4-oxo-N-((R)-1-phenylethyl)butanamide (11a)
Compound 11a was prepared using the ‘General procedure for amide formation from a carboxylic acid’, except that N-benzyloxycarbonyl- D-aspartic acid α-t-butyl ester monohydrate was replaced with 10a (0.60 g, 0.88 mmol) and 3-(trifluoromethyl)benzylamine was replaced with 4 (1-piperidinyl)piperidine. Compound 11a (0.72 g, 98%) was obtained as an off-white solid after flash silica gel column chromatography (CH2Cl2 99.5% to 88% gradient/MeOH 0.5% to 12% gradient, NH4OH <1%). 1H NMR (CDCl3) δ 1.24–1.49 (m, 4H); 1.52–1.65 (m, 7H); 1.74–1.85 (m, 2H); 2.30–2.60 (m, 6H); 2.84–2.96 (m, 1H); 3.12–3.21 (m, 1H); 3.31–3.40 (m, 1H); 3.75–3.83 (m, 1H); 3.98–4.04 (m, 1H); 4.16 (t, J = 7.8 Hz, 1H); 4.20–4.26 (m, 1H); 4.40–4.52 (m, 1H); 4.63–4.77 (m, 3H); 5.05–5.14 (m, 1H); 6.26–6.34 (m, 1H); 6.61–6.69 (m, 1H); 6.99–7.06 (m, 1H); 7.09–7.14 (m, 1H); 7.17–28 (m, 4H); 7.32–7.49 (m, 1H); 7.57–7.65 (m, 2H); 8.16–8.25 (m, 1H). 13C NMR (CD3CN) δ 22.88, 25.39/25.42, 26.95/27.00, 28.33/28.38, 28.69/28.89, 34.21/34.27, 42.11/42.19, 45.49/45.62, 50.10/50.11, 50.77/50.82, 55.52/55.71, 61.19, 63.05/63.08, 63.14/63.18, 63.62/63.64, 71.93/71.95, 95.23, 125.24/125.31, 126.85, 126.93, 127.82, 128.35/128.41, 129.46, 130.11, 130.22, 131.70, 136.47, 136.81/136.86, 138.20, 138.62/138.64, 139.46, 145.67, 159.27, 164.64/164.66, 168.59/168.66, 169.10/169.58. HRMS (FAB) calcd for C42H49IN5O5 830.2773, found 830.2782 (M+H)+.
4.1.10.2. (R)-4-([1,4′-Bipiperidin]-1′-yl)-2-((2R,3S)-2-((E)-3-fluorostyryl)- 4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin -1-yl)-4-oxo-N-((R)-1-phenylethyl)butanamide (11b)
Compound 11b was prepared using the ‘General procedure for amide formation from a carboxylic acid’, except that N-benzyloxycarbonyl-D-aspartic acid α-t-butyl ester monohydrate was replaced with 10b (0.030 g, 0.052mmol) and 3-(trifluoromethyl)benzylamine was replaced with 4 (1-piperidinyl)piperidine. Compound 11b (0.037 g, quantitative yield) was obtained as an off-white solid after flash silica gel column chromatography (CH2Cl2 99% to 90% gradient/MeOH 1% to 10% gradient, NH4OH<1%). 1HNMR(CDCl3) δ 1.26–1.47 (m, 4H); 1.49–1.65 (m, 7H); 1.70–1.82 (m, 2H); 2.33–2.55 (m, 6H); 2.83–2.95 (m, 1H); 3.11–3.22 (m, 1H); 3.33–3.42 (m, 1H); 3.74–3.82 (m, 1H); 3.95–4.02 (m, 1H); 4.15 (t, J = 8.1 Hz); 4.19–4.25 (m, 1H); 4.40–4.50 (m, 1H); 4.62–4.75 (m, 3H); 5.05–5.14 (m, 1H); 6.29–6.38 (m, 1H); 6.69–6.75 (m, 1H); 6.90–7.01 (m, 3H); 7.15–7.29 (m, 4H); 7.31–7.47 (m, 7H); 8.20–8.28 (m, 1H). 13C NMR (CD3CN) δ 22.79, 25.59, 27.21/27.18, 28.47/28.50, 28.81/29.03, 34.18/34.25, 42.20/42.28, 45.57/45.70, 50.09, 50.78/50.84, 55.64/55.84, 61.23, 62.96/62.98, 63.12/63.16, 63.63/63.67, 71.94/71.96, 114.00 (d, 2JC-F = 21.3 Hz, 1C), 115.99 (d, 2JC-F = 21.3 Hz, 1C), 123.72 (d, 4JC-F = 2.5 Hz, 1C), 125.19/125.27, 126.92, 127.83, 128.31/128.37, 129.38, 130.10, 130.22, 131.60 (d, 3JCF = 7.5 Hz, 1C), 137.21/137.25, 138.56/138.60, 139.53 (d, 3JCF = 6.3 Hz, 1C), 145.66, 159.30/159.33, 163.94 (1JC-F = 243.9Hz, 1C), 164.62/164.65, 168.59/168.66, 169.06/169.12. HRMS (FAB) calcd for C42H49FN5O5 722.3712, found 722.3703 (M+H)+.
4.1.10.3. (R)-4-([1,4′-Bipiperidin]-1′-yl)-2-((2R,3S)-2-((E)-3-meth ylstyryl)-4-oxo-3-((S)-2-oxo-4-phenyloxazolidin-3-yl)azetidin-1 -yl)-4-oxo-N-((R)-1-phenylethyl)butanamide (11c)
Compound 11c was prepared using the ‘General procedure for amide formation from a carboxylic acid’, except that N-benzyloxycarbonyl- D-aspartic acid α-t-butyl ester monohydrate was replaced with 10c (0.03 g, 0.053 mmol) and 3-(trifluoromethyl)benzylamine was replaced with 4 (1-piperidinyl)piperidine. Compound 11c (0.038 g, quantitative yield) was obtained as an off-white solid after flash silica gel column chromatography (CH2Cl2 99% to 90% gradient/MeOH 1% to 10% gradient, NH4OH <1%). 1H NMR (CDCl3) δ 1.29–1.53 (m, 4H); 1.56–1.76 (m, 7H); 1.83–1.94 (m, 2H); 2.33 (m, 3H); 2.41–2.69 (m, 6H); 2.85–2.98 (m, 1H); 3.11–3.22 (m, 1H); 3.32–3.42 (m, 1H); 3.78–3.89 (m, 1H); 3.97–4.06 (m, 1H); 4.13 (t, J = 8.3 Hz, 1H); 4.19–4.25 (m, 1H); 4.43–4.58 (m, 1H); 4.61–4.78 (m, 3H); 5.02–5.12 (m, 1H); 6.28–6.38 (m, 1H); 6.70– 6.79 (m, 1H); 6.99–7.23 (m, 1H); 7.29–7.47 (m, 7H); 8.21–8.30 (m, 1H). 13C NMR (CD3CN) δ 21.48, 22.92, 25.62, 27.23/27.25, 28.49/28.53, 28.83, 29.06, 34.18/34.25, 42.21/42.29, 45.59/45.29, 50.14, 50.80/50.86, 55.58/55.79, 61.25, 62.98/63.00, 63.09/63.14, 63.92/63.96, 71.91/71.94, 123.12/123.18, 124.67, 126.89, 127.75, 128.32/128.38, 128.49, 129.39, 129.71, 130.11, 130.19, 130.22, 137.04, 138.56/138.60, 138.82/138.87, 139.41, 145.70, 159.33, 164.65/164.68, 168.61/168.68, 169.12/169.18. HRMS (FAB) calcd for C43H52N5O5 717.3963, found 718.3954 (M+H)+.
4.2. Radiochemistry and conversions to cold analogs by destannylation
4.2.1. Cold conversion to 11a via iododestannylation
A solution of 16 (1.27 μmol) in ethanol (200 μL) was treated with 1 equiv NaI (1.27 μmol), 10 equiv of chloramine T trihydrate (12.7 μmol) and 100 μL aqueous HCl (1 M). The reaction was stirred for 5 min and quenched by the addition of sodium thiosulfate (100 μL) and sodium bicarbonate (200 μL). The mixture was loaded onto a conditioned Oasis HLB cartridge. The cartridge was washed three times with 250 μL water/methanol (95%/5%), eluted with acetonitrile (2 × 500 μL) and concentrated under reduced pressure. The mixture was analyzed by HPLC (method A) and compared to the previously prepared iodinated standard 11a. An estimated yield of 98% was determined by RP-HPLC. MS (ESI), calcd for C42H49IN5O5 830.27, found 830.29 (M+H)+. HPLC method A: instrument, Agilent 1120; mobile phase A, methanol 10%/water 90%/TFA 0.05%; mobile phase B, methanol 90%/water 10%/TFA 0.05%; linear gradient method, 20/80% A/B to 0/100% A/B in 3 min, hold at 100% B for 4 min; injection volume, 5 μL; flow rate: 1 ml/min; column, Eclipse XDB-C18 4.6 × 150 mm 5 μm; UV detection, 257 nm.
4.2.2. Cold conversion to 11b via electrophilic fluorodestannylation
Compound 16 (5 mg, 5.77 μmol) was treated with a solution of SelectFluor tetrafluoroborate and silver triflate in anhydrous acetone. The reaction was stirred 30 min at room temperature under inert atmosphere (argon). The resulting mixture was concentrated under reduced pressure and reconstituted in a mixture 1:1 methanol/water prior to loading onto a conditioned Oasis HLB cartridge. The cartridge was washed three times with 500 μL water/methanol (95%/5%), eluted with acetonitrile (2 × 500 μL) and concentrated under reduced pressure. The mixture was analyzed by HPLC (method A) and compared to the previously prepared fluorinated standard 11b. An estimated yield of 63% was determined by RP-HPLC. MS (ESI), calcd for C42H49FN5O5 722.36, found 722.38 (M+H) +.
4.2.3. Hot conversion to [123I]11a via iododestannylation
A solution of 16 (50 μg, 0.057 μmol) in ethanol (500 μL) was added to 500 μL [123I]NaI (11.0 mCi) in aqueous NaOH (0.01 N), 26 μg (0.11 μmol) chloramine T trihydrate in 25 μL H2O and 20 μL aqueous HCl (1 M). The reaction was stirred for 5 min, then 500 μL mobile phase A from HPLC was added and the resulting solution purified by HPLC (method B). [123I]11a was obtained with an estimated radiochemical yield of 88% and a specific activity of 9.7 mCi (94% radiochemical purity of 94%). HPLC method B: instrument, Agilent 1120; mobile phase A, 10 mM ammonium acetate in water; mobile phase B, 10 mM ammonium acetate in acetonitrile; gradient method, 100/0% A/B to 20/80% A/B in 30 min, hold for 10 min; injection volume, 10 μL; flow rate: 1 ml/min; column, Eclipse Plus C18 4.6 × 250 mm 5 μm; UV detection, 257 nm.
4.3. Biological evaluation
4.3.1. V1a receptor binding assay
The hV1a expressing cell line, cell culture conditions, and the cell-based receptor binding assay procedures were performed according to the methods described by Thibonnier et al.,50 with modifications as reported earlier.18
4.4. Permeability assay
4.4.1. General procedure for the PAMPA-BBB assay
Parallel Artificial Membrane Permeability Assays (PAMPA) to model the Blood–Brain Barrier (BBB) was run according to a published procedure.48 Stock solutions of test compounds and standards (5 mg/mL) were prepared in DMSO. Stock solution in DMSO (5 mg/mL) was diluted 200-fold (10 μL in 1.99 mL) in universal buffer pH 7.4.51 Three hundred microliters of secondary stock solution (25 μg/mL) were transferred to wells in donor plate. The donor plate used was a PTFE 96-well plate (catalog No. MSSACCEPT0R, Millipore). A PVDF filter membrane plate (catalog No. MAIPN4550, Millipore) was coated with 4 μL porcine brain lipid (Avanti Polar) in dodecane (20 mg/mL). A PVDF filter membrane plate served as the acceptor plate and was immediately filled with 300 μL universal buffer pH 7.4. The plate was left undisturbed at room temperature for 18 h. Verapamil and theophylline were used as standard for high and low permeability, respectively. Effective permeability (Pe) was calculated according to the equation reported by Faller.52
Acknowledgments
Supported in part by NIMH awards R44MH063663 and R44MH087001. The technical assistance of Carrie Garippa is recognized.
References and notes
- 1.Ring RH. Curr Pharm Des. 2005;11:205. doi: 10.2174/1381612053382241. [DOI] [PubMed] [Google Scholar]
- 2.Serradeil-Le Gal C, Wagnon J, Valette G, Garcia G, Pascal M, Maffrand JP, Le Fur G. Prog Brain Res. 2002;139:197. doi: 10.1016/s0079-6123(02)39017-4. [DOI] [PubMed] [Google Scholar]
- 3.Bunck M, Czibere L, Horvath C. PLoS One. 2009;4:e5129. doi: 10.1371/journal.pone.0005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goekoop JG, de Winter RP, de Rijk R, Zwinderman KH, Frankhuijzen-Sierevogel A, Wiegant VM. Psychiatry Res. 2006;141:201. doi: 10.1016/j.psychres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M. Neurosci Biobehav Rev. 2007;31:89. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Biol Psychiatry. 2006;60:892. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Plotsky PM, Owens MJ, Nemeroff CB. Psychiatr Clin North Am. 1998;21:293. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- 8.Tichomirowa MA, Keck ME, Schneider HJ, Paez-Pereda M, Renner U, Holsboer F, Stalla GK. J Endocrinol Invest. 2005;28:89. doi: 10.1007/BF03345535. [DOI] [PubMed] [Google Scholar]
- 9.Steckler T, Holsboer F, Reul JM. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:597. doi: 10.1053/beem.1999.0046. [DOI] [PubMed] [Google Scholar]
- 10.Dinan TG, Scott LV. J Anat. 2005;207:259. doi: 10.1111/j.1469-7580.2005.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott LV, Dinan TG. J Affect Disord. 2002;72:113. doi: 10.1016/s0165-0327(02)00026-5. [DOI] [PubMed] [Google Scholar]
- 12.Volpi S, Rabadan-Diehl C, Aguilera G. Stress. 2004;7:75. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- 13.Boudarene M, Legros JJ, Timsit-Berthier M. Encephale. 2002;28:139. [PubMed] [Google Scholar]
- 14.de Winter RF, van Hemert AM, DeRijk RH, Zwinderman KH, Frankhuijzen-Sierevogel A, Wiegant V, Goekoop JG. Neuropsychopharmacology. 2003;28:140. doi: 10.1038/sj.npp.1300002. [DOI] [PubMed] [Google Scholar]
- 15.Inder WJ, Prickett TC, Mulder RT, Donald RA, Joyce PR. Psychopharmacology (Berl) 2001;156:73. doi: 10.1007/s002130100737. [DOI] [PubMed] [Google Scholar]
- 16.van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel A, Wiegant VM, Van der velde EA, De Wied D. Neuropsychopharmacology. 1997;17:284. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- 17.van Londen L, Goekoop JG, Kerkhof GA, Zwinderman KH, Wiegant VM, De Wied D. Eur Neuropsychopharmacol. 2001;11:7. doi: 10.1016/s0924-977x(00)00124-3. [DOI] [PubMed] [Google Scholar]
- 18.Guillon C, Koppel G, Brownstein M, Chaney M, Ferris C, Lu SF, Fabio K, Miller M, Heindel N, Hunden D, Cooper R, Kaldor S, Dressman B, Clay M, Steinberg M, Bruns R, Simon N. Bioorg Med Chem. 2007;15:2054. doi: 10.1016/j.bmc.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holboer F, Engelmann M. J Neurosci. 1995;15:4250. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebsch G, Wotjak CT, Landgraf R, Engelmann M. Neurosci Lett. 1996;217:101. [PubMed] [Google Scholar]
- 21.Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. J Neurosci. 2001;21:7392. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber D, Veinante P, Stoop R. Science. 2005;308:245. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 23.Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Neuropsychopharmacology. 2004;29:483. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 24.Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O’Carroll AM, Young WS. Horm Behav. 2004;46:638. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Landgraf R, Wigger A. Stress. 2003:111. doi: 10.1080/1025389031000104193. [DOI] [PubMed] [Google Scholar]
- 26.Keck ME, Welt T, Müller MB, Uhr M, Ohl M, Ohl F, Wigger A, Toschi N, Holsboer F, Landgraf R. Neuropsychopharmacology. 2003;28:235. doi: 10.1038/sj.npp.1300040. [DOI] [PubMed] [Google Scholar]
- 27.Landgraf R. CNS Neurol Disord Drug Targets. 2006;5:167. doi: 10.2174/187152706776359664. [DOI] [PubMed] [Google Scholar]
- 28.Wigger A, Sánchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Neuropsychopharmacology. 2004;29:1. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- 29.Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Bédard T, Anisman H. Biol Psychiatry. 2006;59:594. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Arch Gen Psychiatry. 1996;53:137. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 31.Ferris CF, Lu S, Messenger T, Miller M, Koppel GA, Bruns FR, Simon NG. Pharmacol Biochem Behav. 2006;83:169. doi: 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JM, Kumar D, Mann JJ, Parsey RV. Curr Radiopharm. 2008;1:12. [Google Scholar]
- 34.Schönberger M, Leggett C, Kim SW, Hooker JM. Bioorg Med Chem Lett. 2010;20:3103. doi: 10.1016/j.bmcl.2010.03.108. [DOI] [PubMed] [Google Scholar]
- 35.Avery TD, Caiazza D, Culbert JA, Dennis K, Taylor DK, Edward RT, Tiekink ERT. J Org Chem. 2005;70:8344. doi: 10.1021/jo050806n. [DOI] [PubMed] [Google Scholar]
- 36.Vasudevan J, Yang R, Wang L, Liu X, Tsang KY, Li L, Takeuchi JA, Vu T, Beard RL, Bhat S, Chandraratna R. WO Patent 2005058301. 2005
- 37.Mahata PK, Barun O, Ila H, Junjappa H. Synlett. 2000;9:1345. [Google Scholar]
- 38.Harapanhalli RS, McLaughlin LW, Howell RW, Rao DV, Adelstein SJ, Kassis AI. J Med Chem. 1996;39:4804. doi: 10.1021/jm9602672. [DOI] [PubMed] [Google Scholar]
- 39.White JM, Lobachevsky PN, Karagiannis TC, Martin RF. WO Patent 2005082894. 2005
- 40.Pham TQ, Greguric I, Liu X, Berghofer P, Ballantyne P, Chapman J, Mattner F, Dikic B, Jackson T, Loc’h C, Katsifis A. J Med Chem. 2007;50:3561. doi: 10.1021/jm0701627. [DOI] [PubMed] [Google Scholar]
- 41.Teare H, Robins EG, Kirjavainen A, Forsback S, Sandford G, Solin O, Luthra SK, Gouverneur V. Angew Chem, Int Ed. 2010;49:6821. doi: 10.1002/anie.201002310. [DOI] [PubMed] [Google Scholar]
- 42.Kallmerten AE, Alexander A, Wager KM, Jones GB. Curr Radiopharm. 2011;14:343. doi: 10.2174/1874471011104040343. [DOI] [PubMed] [Google Scholar]
- 43.Ross T, Ermet J, Hocke C, Coenen HZ. J Am Chem Soc. 2007;129:8018. doi: 10.1021/ja066850h. [DOI] [PubMed] [Google Scholar]
- 44.Chun JH, Lu S, Lee YS, Pike V. J Org Chem. 2010;75:3332. doi: 10.1021/jo100361d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karimi F, Långström B. J Labelled Compd Radiopharm. 2002;45:423. [Google Scholar]
- 46.Meanwell NA. J Med Chem. 2011;54:2529. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- 47.Simon NG, Ferris CF, Lu S, et al. Poster. 51st Annual Meeting of the New Clinical Drug Evaluation Unit (NCDEU); Boca Raton, FL. 2011. [Google Scholar]
- 48.Di L, Kerns E, Fan K, McConnell O, Carter G. J Eur Med Chem. 2003;38:223. doi: 10.1016/s0223-5234(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 49.Ferris CF, Smerkers B, Simon N. Abstract of papers. 41st Annual Meeting, Society for Neuroscience; Washington, DC. 2011. [Google Scholar]
- 50.Thibonnier M, Auzan C, Madhun Z, Wilkins P, Berti-Mattera L, Clauser E. J Biol Chem. 1994;269:3304. [PubMed] [Google Scholar]
- 51.Ruell JA, Avdeef A. In: Optimization in Drug Discovery: In Vitro Methods. Yan Z, Caldwell GW, editors. Humana Press; 2004. pp. 37–63. [Google Scholar]
- 52.Wohnsland F, Faller B. J Med Chem. 2001;44:923. doi: 10.1021/jm001020e. [DOI] [PubMed] [Google Scholar]