Abstract
Vascular endothelial growth factor (VEGF) has been implicated in breast tumor angiogenesis. And tumor necrosis factor-α (TNF-α) is a positive regulator of VEGF. This study was aimed to identify the signalling pathway of TNF-α in VEGF expression regulation in breast cancer cell line MCF7. Using luciferase reporter assays, we demonstrated that TNF-α significantly increased activator protein-1 (AP-1) transcriptional activity in the MCF7 cells. The expression of the AP-1 family members c-Jun, c-Fos and JunB and phosphorylation levels of c-Jun were upregulated by TNF-α, whereas other AP-1 family members Fra-1, Fra-2, and JunD were unaffected. The activation of AP-1 was associated with the formation of p-c-Jun-c-Jun and p-c-Jun-JunB homodimers. Furthermore, the phosphorylation levels of c-Jun N-terminal kinase (JNK) but not P38 and ERK were elevated by TNF-α in MCF7 cells. TNF-α potently upregulated the mRNA and protein levels of VEGF, which were significantly reversed by JNK inhibitor SP600125. Finally using chromatin immunoprecipitation (CHIP) assays, we found that p-c-Jun bound to the VEGF promoter and regulated VEGF transcription directly. These data suggest that the pro-inflammatory cytokine TNF-α is a critical regulator of VEGF expression in breast cancer cells, at least partially via a JNK and AP-1 dependent pathway.
Keywords: AP-1, JNK, MCF7, VEGF
1. Introduction
Vascular endothelial growth factor (VEGF) is a potent endothelial mitogen that is upregulated in a number of tumor types, including breast cancer [1,2]. Because it can stimulate macrophage migration [3], VEGF appears to be important for breast cancer growth and neovascularization. The VEGF promoter region includes several potential binding sites for the transcription factors SP-1, AP-1 and AP-2 [4]. Hypoxia up-regulates VEGF via the binding of hypoxia inducible factor-1 (HIF-1) to a hypoxia response element in the VEGF promoter [5]. VEGF gene expression is also enhanced by cytokines such as TNF-α, EGF, PDGF and TGF-β [6].
The cytokine TNF-α appears to have different roles depending upon whether it is studied during breast cancer pathogenesis or when used as a therapeutic agent. High-dose local administration of TNF-α selectively destroys tumor blood vessels, and has powerful anti-cancer actions [7]. However, when TNF-α is chronically elevated endogenously during breast cancer development, it appears to act as a tumor enhancer, contributing to the tissue remodeling and stromal development necessary for tumor growth and spread [8]. The effects of TNF-α are mediated by several signal transduction pathways, including the NF-κB pathway and mitogen-activated protein kinase (MAPK) pathways. TNF-α has also been shown to acts as positive regulator of VEGF in human glioma cells [9], although the details of their relationship in breast cancer pathogenesis are not yet clear.
AP-1 is known to be involved in the TNF-α receptor signalling pathway, enabling TNF-α to influence the expression of many genes [10], some of which may be important in tumor invasion [8]. The AP-1 complex is composed of heterodimers of members of the Jun (c-Jun, JunB and JunD) and Fos (c-Fos, FosB, Fra-1 and Fra-2) families, or Jun-Jun homodimers [11–13]. In particular, c-Jun has been implicated in events leading to tumor development [14], whereas c-Fos appears to be involved in the malignant conversion of benign papillomas [15].
The activation of AP-1 is mediated by at least two regulatory events [16,17]. First, some AP-1 proteins (e.g. c-Jun) are encoded by immediate early genes that are transcriptionally induced following treatment. Second, AP-1 activity can also be regulated by post-translational modification, including phosphorylation by MAPKs. The MAPK family comprises the extracellular signal regulated kinase (ERK), p38 MAPK and c-Jun NH2-terminal kinase (JNK). However, the exact mechanism of specific condition or treatment on AP-1 activation and the relative role of different MAPKs in these processes are diverse. TNF-α regulates AP-1 activity, in part, by increasing the expression of members of the Jun and Fos families. An important role for the JNK signalling pathway in AP-1 activation by TNF-α has also been proposed [18]. Although some links have been made between TNF-α and the activation of JNK and AP-1, it remains unclear whether these events are linked to TNF-α-mediated VEGF expression in breast cancer.
This study was designed to identify the signalling pathway of VEGF expression regulation in the MCF7 breast cancer cell line. Our results demonstrate, for the first time, that the inflammatory cytokine TNF-α is a critical regulator of VEGF expression in breast cancer cells through the JNK/AP-1 dependent pathway.
2. Materials and methods
2.1. Reagents
TNF-α was from R&D Laboratories, Inc. (Minneapolis, MN, USA). DMSO was from Pierce (Rockford, IL, USA). Antibodies against c-Jun (H-79), JunD (329), JunB (N-17), c-Fos (H-125), Fra-1 (N-17), Fra-2 (L-15) and FosB (102) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Ser63-phosphorylated-c-Jun and Ser73-phosphorylated-c-Jun antibodies were from Cell Signalling Technology (New England Biolabs, Beverly, MA, USA) and Upstate Biotechnology (Lake Placid, NY, USA), respectively. The Dual-Luciferase reporter assay system was purchased from Promega (Madison, WI, USA). The Plasmid extraction and DNA Gel extraction kits were purchased from Qiagen (Hilden, Germany). Fugene 6 was purchased from Roche (Indianapolis, IN, USA).
2.2. Cell culture
MCF7 cells were purchased from American Type Culture Collection (Manassas, VA, USA) and were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS, Hyclone), 2 mM l-glutamine, 50 units/ml penicillin and 50 µg/ml streptomycin. The cells were incubated at 37 °C in a humidified atmosphere containing 95% air and 5% CO2.
2.3. MTT assay
Cytotoxicity and cell survival was determined by MTT assay. Briefly, about 15,000 cells per well were plated on 96-well plates. The next day, cells were treated with TNF-α (20 ng/ml) and incubated at 37 °C for 24–48 h. 10 µl MTT was then added to each well and the plates were incubated at 37 °C for an additional 3 h. At the end of the incubation, 0.1 ml DMSO was added to each well to dissolve the formazan crystals and the absorbance was read at 570 nm in a microplate reader. All assays were done in triplicate and were repeated three times.
2.4. Transient transfection and luciferase reporter assay
The AP-1 luciferase reporter construct (AP-1-LUC) driven by four copies of the AP-1 binding sites and the VEGF luciferase reporter construct (VEGF-LUC) driven by VEGF promoter were used to assessed the effects of TNF-α on AP-1 transactivation and VEGF transcriptional activities, respectively. Plasmid containing the β-galactosidase gene driven by the cytomegalovirus promoter (Clontech Laboratories, Palo Alto, CA, USA) was used as an internal control. Using the Fugene 6 reagent, MCF7 cells were transiently cotransfected with AP-1-LUC or VEGF-LUC and β-galactosidase. To determine the role of c-Jun in TNF-α induced AP-1 activation, the AP-1-LUC and β-galactosidase were cotransfected with pCMV-TAM-67 or its control vector pCMV-neo into MCF7 cells. Eight hours after tranfection, cells were serum starved for 24 h, and then treated with TNF-α or vehicle (0.1% DMSO) for varying time intervals. Cells were lysed and luciferase activity was measured using the Dual-Luciferase Assay Kit (Promega). β-Galactosidase activity was detected to normalise any variations in the transfection efficiency. Each experiment was performed in triplicate and repeated at least three times.
2.5. Nuclear protein extraction
MCF7 cells were grown in 15 cm diameter dishes. Cells were treated with vehicle (0.1% DMSO) or TNF-α (20 ng/ml) for different periods of time. After washing with ice-cold PBS, cells were harvested with a scraper and centrifuged. Nuclear protein was extracted using the Nuclear Protein Extraction Kit (Pierce Chemical Co., IL, USA) according the manufacturer’s instructions. The protein concentration of each sample was determined using a DC Protein Assay kit (Bio-Rad).
2.6. Western blot
Protein samples were boiled and denatured in sample buffer containing SDS and DTT, then separated by gel electrophoresis using NuPage 10% Bis-Tris prepacked gels in MOPS buffer. An equal amount of protein was loaded in each lane. The gel proteins were transferred to a nitrocellulose membrane using a semi-dry transfer blotting system. The membrane was then blotted with the indicated antibodies and visualized using ECL reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
2.7. Immunoprecipitation (IP) and immunoblotting (IB)
Cells were lysed in IP lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40 and 10% glycerol. Protease inhibitor cocktail tablets (one tablet per 10 ml; Roche, USA) were added to the IP lysis buffer immediately before use. Antibodies were added to the lysis buffer, and the precipitated proteins were analyzed by Western blot as described above.
2.8. RNA isolation and RT-PCR
Total RNA was isolated using The RNeasy Mini Kit from Qiagen (Hilden, Germany). Triplicate RNA samples were independently prepared for each treatment group. MCF7 cells were cultured in a 6-well plate until they reached 85–90% confluence. The cell culture medium was replaced with DMEM containing 0.1% FBS and cultured for 24 h, then cells were exposed to TNF-α for different periods of time. Cells were extracted for whole RNA with Trizol reagent according to the manufacturer’s instructions. cDNA was synthesized from 1 µg of RNA using the First-Strand Synthesis System (AMV, Promega) for RT-PCR. PCR was performed using 2 µl of synthesized cDNA and primers specific to VEGF (forward: 5′-GCGGGCTGCCTCGCAGTC-3′; reverse: 5′TCACCGCCTTGGCTTGTCAC-3′) or GAPDH (internal control; forward: 5′-GGCTCTCCAGAACATCATCCCTGC-3′; reverse: 5′-GGGTGTCGCTGTTGAAGTCAGAGG-3′). PCR products were separated on 1.8% agarose gels. RT-PCR was conducted within the linear ranges of PCR cycles and RNA input.
2.9. Chromatin immunoprecipitation assay
Chromatin Immunoprecipitation (CHIP) assay was done using a CHIP assay kit (Upstate Biotechnology), according to the supplied protocol. Briefly, cells (1 × 107)were exposed to TNF-α for either 3 h or 24 h. Chromatin was cross-linked by adding formaldehyde (1%) to the culture medium for 10 min at 37 °C. A fraction of the soluble chromatin (1%) was saved for measurement of total chromatin input. Precleared chromatin was incubated with Ser73-phosphorylated-c-Jun antibody or nonimmune IgG overnight at 4°C. DNA recovered from the immunoprecipitated product was used as a template for PCR with VEGF-promoter-specific primers: forward: 5′-GAGACGAAACCCCCATTTCT-3′, reverse: 5′-AGATGTTGCCAGGGAACTGA-3′. PCR mixtures were denatured for 1 cycle at 94 °C for 2 min, followed by 35 cycles (94 °C for 30 s, annealing at 55 °C for 30 s, elongation in was incubated with p-c-j IB at 68 °C for 30 s), and then subjected to a final elongation at 68 °C for 5 min.
2.10. Statistical analysis
Results are expressed as mean ± SEM. Data were analyzed using one-way analysis of variance between groups with least significant difference. Statistical analysis was performed with statistical analysis software SPSS 10.0. P < 0.05 was considered to be significant.
3. Results
3.1. TNF-α increases AP-1 transactivation activity
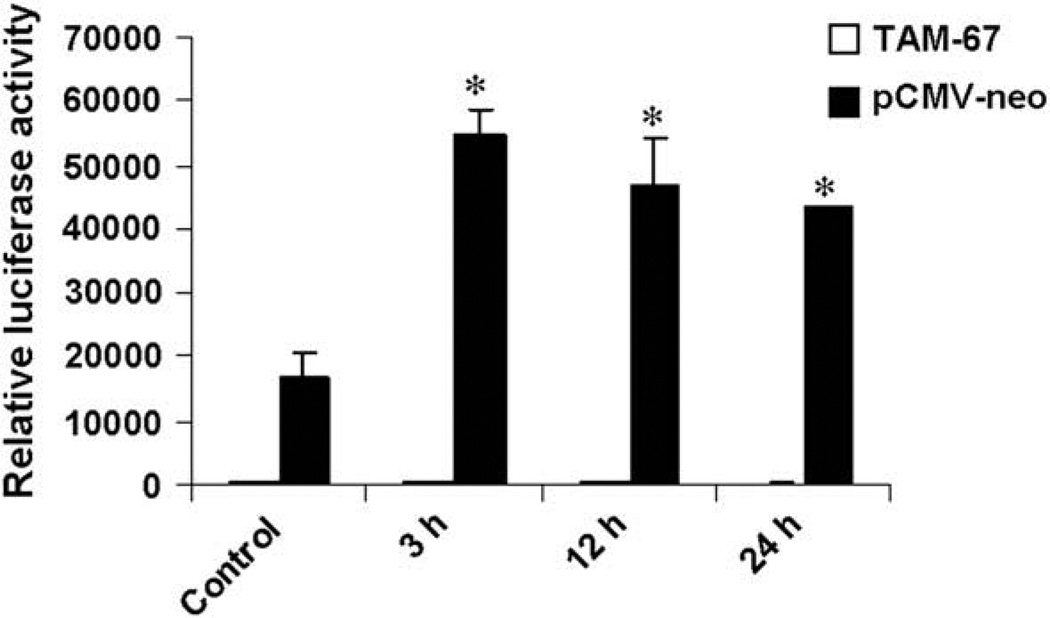
Previous studies have shown AP-1 is involved in TNF-α receptor signalling pathway in some types of cells [10]. To determine whether AP-1 transcription factor complexes are also activated by TNF-α in breast cancer cell MCF7 cells, transient transfection and luciferase reporter assays were used. As shown in Fig. 1, the AP-1 transactivation activity was significantly increased by TNF-α (20 ng/ml), peaked at 3 h but remained elevated 24 h after treatment.
Fig. 1.
TNF-α elevates AP-1 luciferase reporter activity. MCF7 cells were transiently cotransfected with the firefly luciferase reporter construct AP-1-LUC and β-galactosidase plasmid (as an internal control) plus pCMV-TAM-67 or pCMV-neo vector. Eight hours after transfection, cells were serum starved for 24 h, and then treated with TNF-α (20 ng/ml) or vehicle (Control) for the indicated periods of time. Then cells were harvested for luciferase reporter assays. The relative values of AP-1 luciferase activity to β-galactosidase are shown as means ± SEM of three independent experiments. *P < 0.05 vs. levels in cells cotransfected with pCMV-neo vector and treated with vehicle.
To assess the role of c-Jun in TNF-α induced AP-1 activation, a dominant-negative c-Jun mutant pCMV-TAM-67 or its control vector pCMV-neo was used. TAM-67 is a mutant form of c-Jun in which the transactivation domain has been deleted, leaving the DNA binding and the leucine zipper domains intact. The pCMV-TAM-67 or pCMV-neo vector was cotranfected with AP-1-LUC and β-galactosidase into MCF7 cells. As shown in Fig. 1, basal AP-1 luciferase activity was potently inhibited by TAM-67 overexpression. Moreover, the upregulation effects of TNF-α on AP-1 transactivation activity were reversed by TAM-67. These results suggest the involvement of c-Jun in TNF-α induced AP-1 activation.
3.2. TNF-α induces c-Jun, c-Fos and JunB expression but not other Jun or Fos proteins
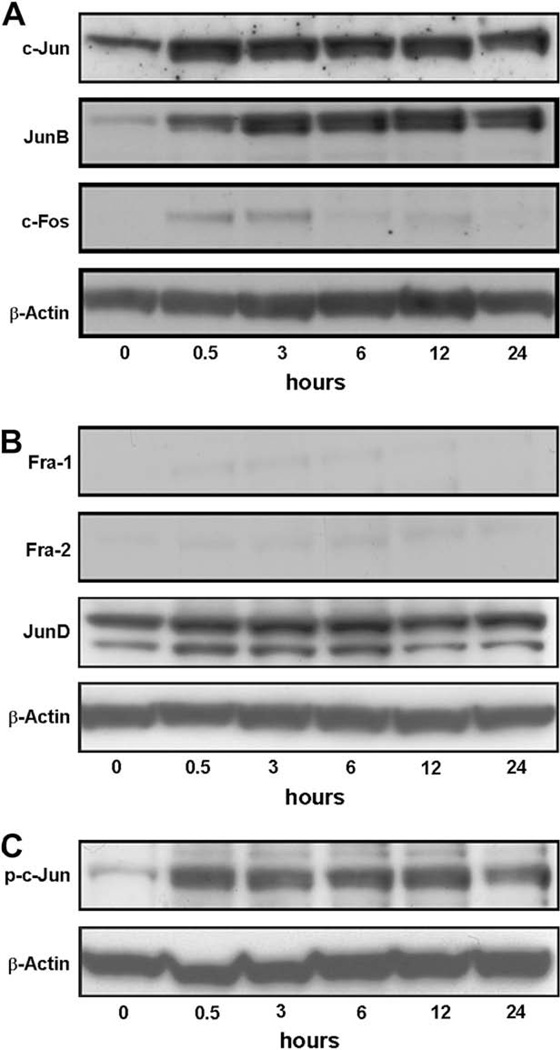
To investigate whether TNF-α alter the protein levels of AP-1 family members in MCF7 cells, nuclear lysates from cells treated with TNF-α (20 ng/ml) for various time intervals were subjected to Western blot analysis. Low levels of c-Jun, c-Fos and JunB proteins were detected in MCF7 cells before treatment. TNF-α treatment induced a substantial increase in the nuclear protein levels of c-Jun, c-Fos and JunB (Fig. 2A). However, protein levels of other AP-1 family members were not affected (Fig. 2B). Specifically, Fra-1 and Fra-2 proteins were expressed at very low levels in MCF7 cells both before and after TNF-α treatment (Fig. 2B).
Fig. 2.
TNF-α induces the expression of c-Jun, JunB and c-Fos, but not other Jun or Fos proteins. MCF7 cells were serum starved for 24 h and treated with TNF-α (20 ng/ml) for indicated periods of time. Nuclear protein samples were harvested for Western blot analyses to determine the protein levels of c-Jun, JunB, c-Fos (A), Fra-1, Fra-2, JunD (B) and Ser73-phosphorylated-c-Jun (p-c-Jun; C). The protein levels of β-actin were assessed simultaneously in each group as a loading control. Immunoblots are representative of three separate experiments.
c-Jun transcriptional activation, which is necessary for tumor development, is regulated by a variety of post-translational modifications, the most important of which is thought to be the phosphorylation of Ser63 and Ser73 within the N-terminus of c-Jun [19,20]. We therefore determined the phosphorylation status of c-Jun protein in MCF7 cells after TNF-α treatment by probing nuclear extracts with antibodies against Ser63-phosphorylated-c-Jun and Ser73-phosphorylatedc-Jun. Although Ser63-phosphorylated-c-Jun was not detectable in these extracts (data not shown), measurable levels of Ser73-phosphorylated-c-Jun were observed and were further increased by TNF-α treatment in MCF7 cells (Fig. 2C).
3.3. AP-1 is composed of p-c-Jun-c-Jun and p-c-Jun-JunB homodimers after TNF-α treatment
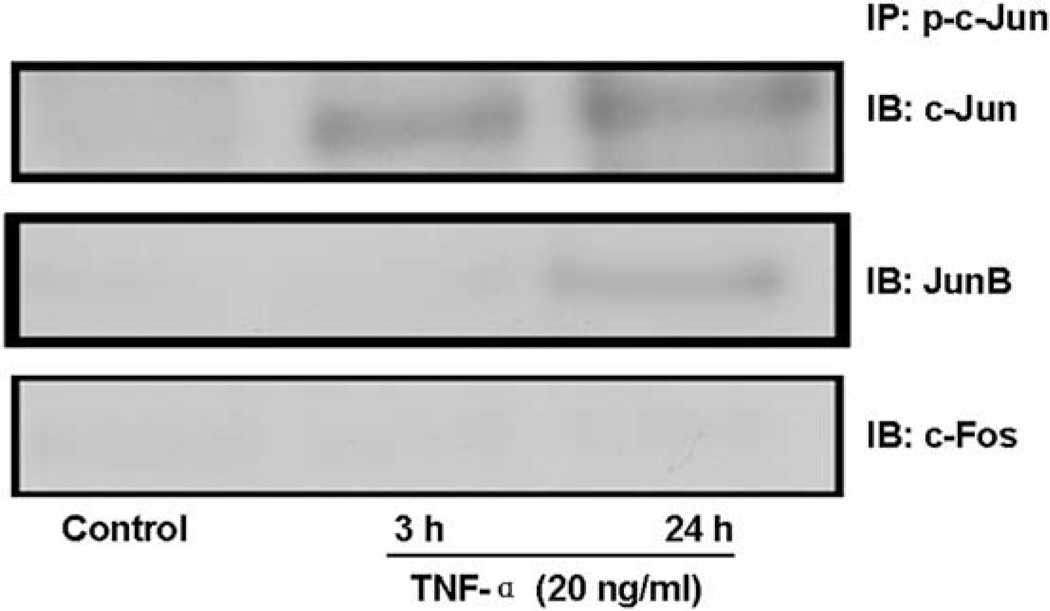
AP-1 proteins are composed principally of homodimers of Jun family members (c-Jun, JunB, JunD) or heterodimers of the Jun family members with the Fos family members (c-Fos, FosB, Fra-1, and Fra-2) [11–13]. To determine the pattern of AP-1 complex in TNF-α-treated MCF7 cells, we therefore carried out immunoprecipitation experiments in these cells targeting toward activated AP-1 complex family members. We found that activated AP-1 complex is composed of p-c-Jun-c-Jun and p-c-Jun-JunB homodimers after TNF-α treatment (Fig. 3). However, we were unable to detect any p-c-Jun-c-Fos heterodimer.
Fig. 3.
AP-1 complex is composed of p-c-Jun-c-Jun and p-c-Jun-JunB homodimers after TNF-α treatment. MCF7 cells were serum starved for 24 h and treated without (Control) or with TNF-α (20 ng/ml) for 3 h or 24 h. Cell nuclear lysates were subjected to immunoprecipitation (IP) with an antibody against p-c-Jun followed by immunoblotting (IB) with antibodies against c-Jun, c-Fos and JunB. Representative Immunoblots of three separate experiments are shown.
3.4. Effects of TNF-α on the activities of ERK, JNK and p38 MAPK pathways
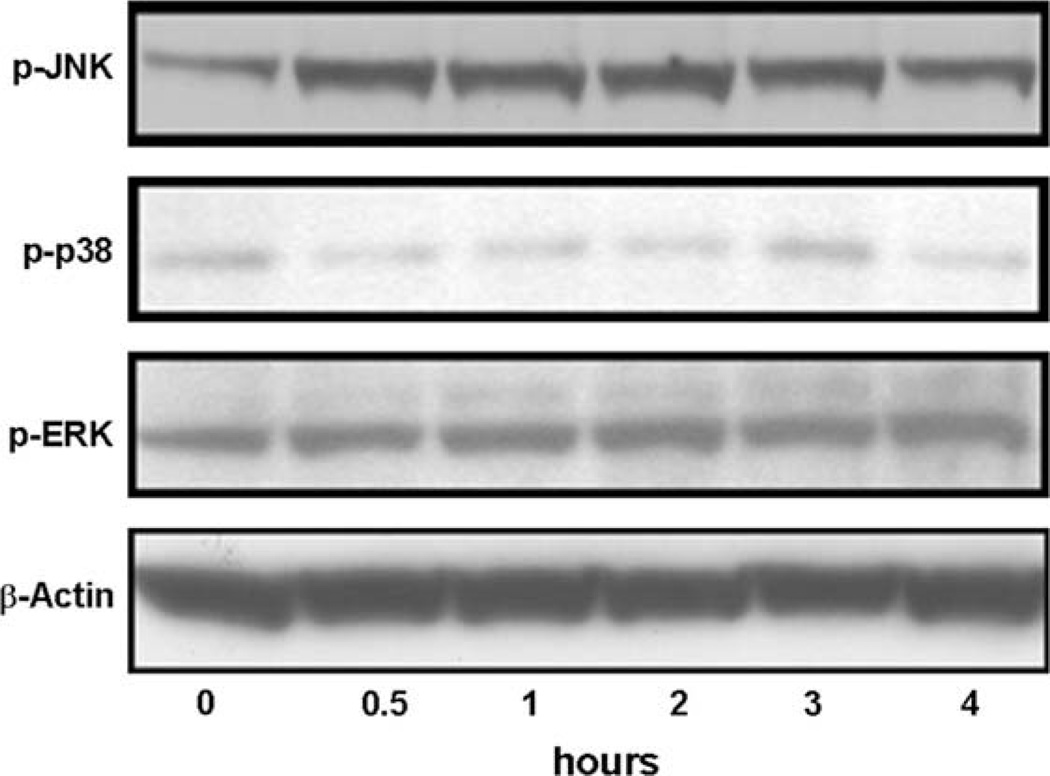
TNF-α stimulates the activation of the MAPK pathways, and AP-1 acts as a major downstream effector of MAPKs in other cell types. Three related MAPK cascades have been described: the ERK, JNK and p38 MAPK pathways [21]. We therefore explored which MAPK pathway was essential for TNF-α induced AP-1 activation in MCF7 cells. Cells were serum starved for 24 h and treated with TNF-α for the indicated periods of time, and JNK, P38 and ERK activation was assessed using phosphorylation specific antibodies (Fig. 4). TNF-α strongly stimulated the phosphorylation of JNK, but had no effect on the phosphorylation of P38 and ERK. These data indicate that JNK but not P38 and ERK pathways might be involved in TNF-α induced AP-1 activation.
Fig. 4.
Effects of TNF-α on the activities of MAPK pathways. MCF7 cells were serum starved for 24 h and treated with TNF-α (20 ng/ml) for the indicated periods of time, and JNK (p-JNK), P38 (p-p38) and ERK (p-ERK) activation was assessed using phosphorylation specific antibodies. The levels of β-actin protein in each group were used as the references. Representative immunoblots of three separate experiments are shown.
3.5. TNF-α stimulates VEGF gene transcription in MCF7 cells
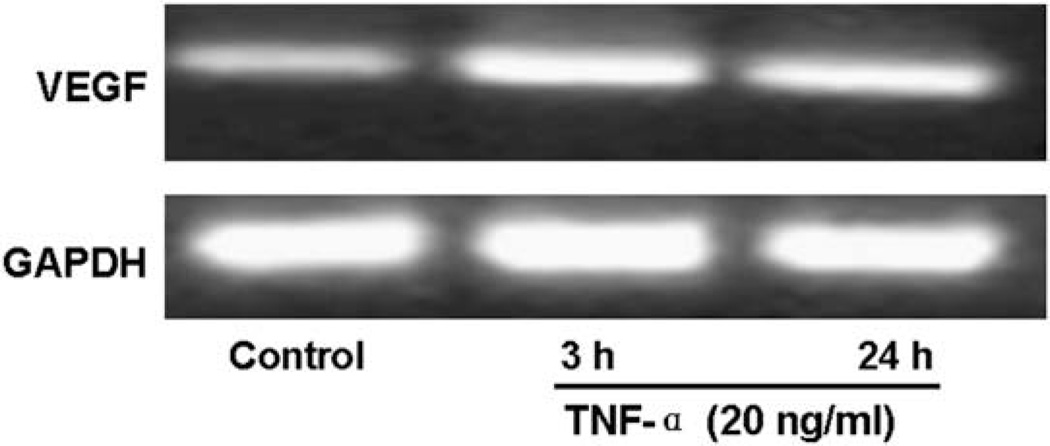
VEGF has been reported to be important for breast cancer growth and neovascularization. And TNF-α has also been shown to acts as positive regulator of VEGF [9]. We then investigated the effect of TNF-α (20 ng/ml) on VEGF gene expression in MCF7 cells. Firstly, MCF7 cells were cotranfected with the VEGF luciferase reporter construct (VEGF-LUC) driven by VEGF promoter and β-galactosidase plasmid, and treated with TNF-α (20 ng/ml) for varying time periods, followed by luciferase activity determination. As shown in Fig. 5, TNF-α significantly increased the VEGF promoter transcriptional activity in MCF7 cells. Additionally, using RT-PCR, we further examined the mRNA levels of VEGF in TNF-α treated MCF7 cells. After treating with TNF-α (20 ng/ml) for 3 h or 24 h, MCF7 cells were collected for RNA extraction and RT-PCR to determine the mRNA levels of VEGF and GAPDH (loading control). Consistent with the luciferase reporter activity assay, TNF-α obviously increased the mRNA levels of VEGF in MCF7 cells (Fig. 6), indicating that TNF-α could increased VEGF mRNA levels through transcriptional mechanism.
Fig. 5.
TNF-α induces VEGF luciferase reporter activity. MCF7 cells were transiently transfected with VEGF-LUC construct and β-galactosidase plasmid (as an internal control). Eight hours after transfection, cells were serum starved for 24 h, and then treated with TNF-α (20 ng/ml) or vehicle (Control) for the indicated periods of time. Then cells were collected for luciferase reporter assays. The relative values of LXRE luciferase activity to β-galactosidase are shown as means ± SEM of three independent experiments. *P < 0.05 vs. control.
Fig. 6.
TNF-α stimulates VEGF mRNA transcription in MCF7 cells. MCF7 cells were treated with 20 ng/ml of TNF-α for the indicated time periods after 24 h serum starvation. Total RNA was isolated and 1 µg RNA was used for RT-PCR analyses of VEGF and GAPDH (as a reference) mRNA levels. PCR products were revealed by ethidium bromide staining under UV after agarose gel electrophoresis. Representative graphs of three separate experiments are shown.
3.6. TNF-α induces VEGF protein production in MCF7 cells, which is reversed by JNK inhibitor
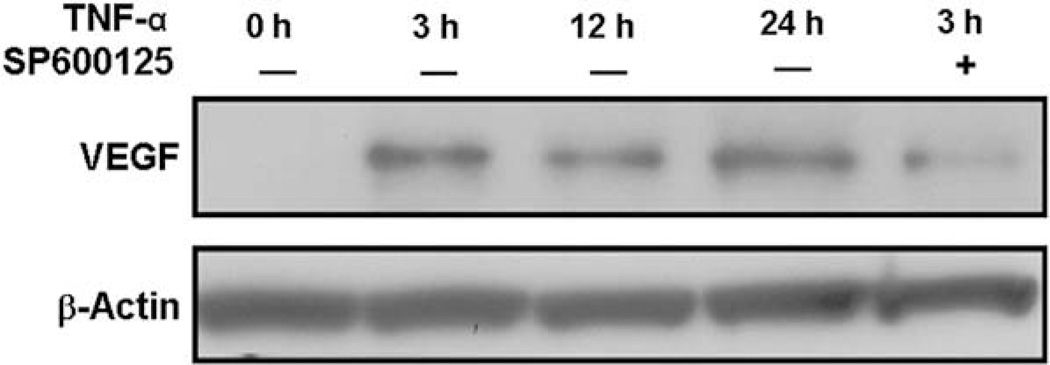
We next investigated whether TNF-α upregulated VEGF protein levels. Serum starved MCF7 cells were treated with 20 ng/ml of TNF-α for the indicated periods of time and collected for total protein extraction and Western blot analyses. As shown in Fig. 7, VEGF protein level was very low before treatment, but was markedly upregulated after 3 h of TNF-α treatment. To determine the role of JNK signalling pathway in TNF-α-stimulated VEGF expression, MCF7 cells were pretreated with the JNK inhibitor SP600125 (5 µM) for 0.5 h, followed by TNF-α treatment for another 3 h. Pretreatment of cells with SP600125 markedly suppressed TNF-α stimulated VEGF protein production (Fig. 7).
Fig. 7.
TNF-α increases VEGF protein levels in MCF7 cells. After serum starvation for 24 h, MCF7 cells were pretreated without or with SP600125 for 30 min, and treated with TNF-α (20 ng/ml) for the indicated time periods. Cell lysates were collected for Western blot analyses with antibodies against VEGF or β-actin (as a loading control). Representative Immunoblots of three separate experiments are shown.
3.7. p-c-Jun is recruited to the VEGF promoter following TNF-α treatment and regulates VEGF mRNA transcription directly
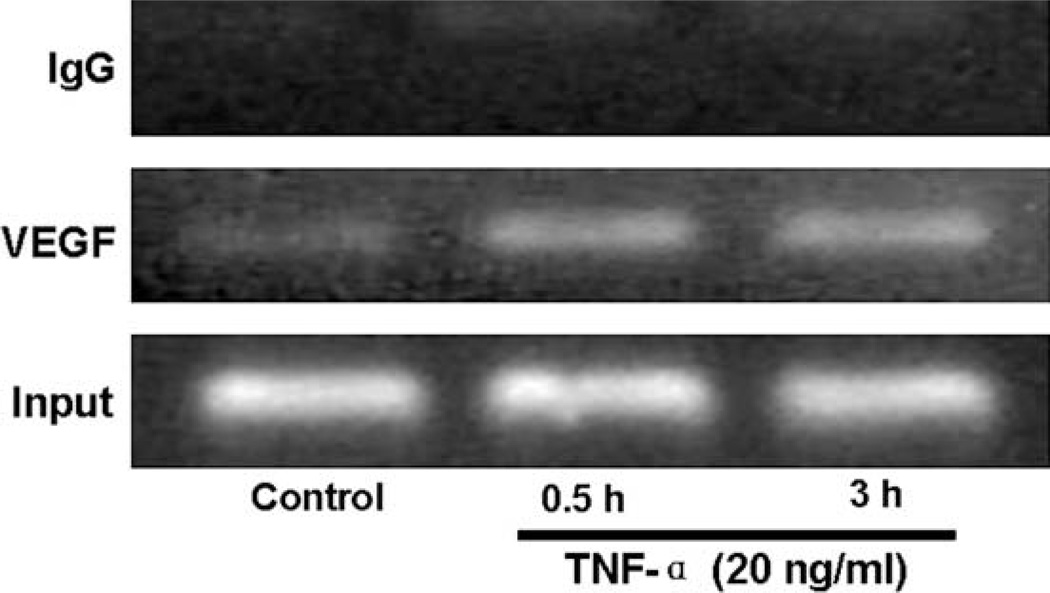
To further investigate the mechanism TNF-α induced VEGF expression and determine whether p-c-Jun directly bind to the endogenous VEGF gene promoter following TNF-α treatment, ChIP assays were performed. As depicted in Fig. 8, TNF-α treatment promoted p-c-Jun binding to VEGF promoter as early as 0.5 h (Fig. 8, lane 2), and it remained high until 3 h of treatment (Fig. 8, lane 3). We chose these time points because AP-1 activity was maximal within 3 h after TNF-α stimulation (Fig. 1). In contrast, ChIP assays of nonimmune IgG showed no amplification of the VEGF promoter. Collectively, these results indicate a critical role of p-c-Jun in the regulation of TNF-α induced VEGF transcription.
Fig. 8.
p-c-Jun is recruited to the VEGF promoter following TNF-α treatment. After serum starvation for 24 h, MCF7 cells were treated without (Control) or with TNF-α (20 ng/ml) for the indicated time periods, and chromatin protein-DNA complexes were cross-linked using formaldehyde. The purified nucleoprotein complexes were immunoprecipitated with Ser73-phosphorylated-c-Jun antibody (p-c-Jun) or nonimmune IgG and DNA samples recovered were amplified by PCR as detailed in Materials and Methods. Representative graphs of three separate experiments are shown.
4. Discussion
TNF-α has been proposed as a potent angiogenesis-promotingfactor in two in vivo angiogenesis models using the cornea and chorioallantoic membrane [22], and in an in vitro system with human microvascular endothelial cells [23]. In these models, antibodies against VEGF were able to block TNF-α-dependent angiogenesis. However, the molecular mechanisms by which TNF-α induces VEGF are not yet understood. In this study, treatment of MCF7 cells with TNF-α resulted in a significant activation of AP-1. Overexpression of TAM-67 blocked AP-1 induction by TNF-α, suggesting that c-Jun is involved in TNF-α signalling pathway in MCF7 cells. Results from Western blot assays further demonstrated that, as transcription factors, the AP-1 family members c-Jun, c-Fos, JunB and Ser73-phosphorylated-c-Jun were increased in MCF7 cells treated with TNF-α, but Fra-1, Fra-2 and JunD expression did not change significantly following treatment. We also found that activated AP-1 is composed of p-c-Jun-c-Jun and p-c-Jun-JunB homodimers after TNF-α treatment. Treatment of MCF7 cells with TNF-α resulted in markedly induction of VEGF expression. Inhibition of JNK activation by JNK specific inhibitor SP600125 resulted in impairment of VEGF induction, suggesting that JNK is responsible for VEGF induction by TNF-α. Further studies using ChIP assays demonstrated that, as a transcription factor, p-c-Jun recognized and bound to the cis-elements of the VEGF promoter and, in turn, activated VEGF transcription. These results suggest that VEGF induction by TNF-α is through JNK/AP-1-dependent pathways in breast cancer MCF7 cells.
Substantial evidence implicates the AP-1 group of transcription factors in many of the non apoptotic cellular responses to TNF-α [24]. AP-1 activation involves increased expression [16] and phosphorylation [25], but how TNF-α regulates these functions is unclear. However, TNF-α mediated JNK-dependent regulation of AP-1 activation has been proposed [18]. One mechanism by which chronic inflammation predisposes to cancer might be that the proinflammatory cytokine TNF-α is a critical mediator of tumor promotion, acting via a PKC-α and AP-1-dependent pathway [24]. Ventura demonstrated that JNK is critical for TNF-α regulation of the AP-1 group of transcription factors [26]. Both the AP-1 proteins and MAPKs have been implicated as important mediators in immunity and inflammation [10]. In the present study, we found that TNF-α markedly increased the phosphorylation levels of JNK but not ERK (p-ERK) or p38 (p-p38) in MCF7 cells. In agreement with our results, when MCF7 cells were cocultured with microphages, the invasive capacity of the MCF7 cell was significantly increased. c-Jun, c-Fos and JunB activities significantly increased and were inhibited by the neutralizing TNF-α antibody. However, p38 and ERK pathways have either no or a limited role in this system [27].
VEGF expression was controlled by several transcriptional factors, such as AP-1, AP-2 and SP-1 [4]. The enhancement of VEGF expression by TNF-α stimulation appeared to be mediated through the transcription factor SP-1 in glioma cells [9]. The VEGF gene promoter region contains AP-1 binding sites, which can be recognized by activated AP-1 transcription factor, so it is proposed that AP-1 may regulate VEGF expression [28,29]. It has been reported that activation of the MEK/ERK/AP-1 pathway is involved in VEGF expression induced by EGF in human head and neck squamous cell carcinoma lines [30]. In addition, Shih et al. [31] showed that TGF-β potently induces VEGF expression in human HT-1080 fibrosarcomas primarily through both AP-l and HIF-1 dependent mechanisms. Using a dominant negative mutant of c-Jun (pCMV-TAM-67), we found here overexpression of TAM-67 blocked AP-1 activation induced by TNF-α. And results from ChIP assays showed that TNF-α induced activated c-Jun directly bound to the AP-1 binding site of the VEGF promoter region, which is critical for VEGF induction by TNF-α. These data suggest that c-Jun is a critical regulator in TNF-α induced VEGF upregulation. Furthermore, we demonstrated that JNK is essential for TNF-α-induced VEGF expression by using JNK inhibitor SP600125. In addition, we found that TNF-α treatment did not affect p38 and ERK activation. Therefore, our studies strongly demonstrate that JNK/AP-1 dependent and p38 and ERK independent pathways specifically mediate VEGF induction by TNF-α in breast cancer cells.
In summary, we have found that TNF-α exposure leads to VEGF over-expression in MCF7 cells, and that this induction is primarily mediated through JNK/AP-1 dependent pathways. Although the detailed molecular mechanisms of the signalling pathways initiated by TNF-α are still under investigation, identification of these important mediators in this signalling pathway will not only deepen our understanding of tumor-promoting effects of inflammatory cytokine, but may also provide valuable information for the prevention and therapy of cancers caused by TNF-α.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30772474 and 30771041), the Special Funds for Major State Basic Research Program of China (973 Program, 2006CB503908) to X. Han and Jiangsu Provincial Natural Science Foundation (NO.BK2008477).
Abbreviations
- AP-1
activator protein-1
- ChIP
chromatin immunoprecipitation
- ERK
extracellular signal regulated kinase
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-κB
- TNF-α
tumor necrosis factor-α
- VEGF
Vascular endothelial growth factor
Footnotes
Duality of interest
The authors declare that they have no duality of interest
References
- 1.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 2.Leek RD, Harris AL, Lewis CE. Cytokine networks in solid human tumors: regulation of angiogenesis. J Leukoc Biol. 1994;56(4):423–435. doi: 10.1002/jlb.56.4.423. [DOI] [PubMed] [Google Scholar]
- 3.Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172(6):1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266(18):11947–11954. [PubMed] [Google Scholar]
- 5.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, et al. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269(9):6271–6274. [PubMed] [Google Scholar]
- 7.Lejeune FJ, Ruegg C, Lienard D. Clinical applications of TNF-alpha in cancer. Curr Opin Immunol. 1998;10(5):573–580. doi: 10.1016/s0952-7915(98)80226-4. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 9.Ryuto M, Ono M, Izumi H, Yoshida S, Weich HA, Kohno K, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271(45):28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 10.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11(9):372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 11.Hartl M, Vogt PK. Oncogenic transformation by Jun: role of transactivation and homodimerization. Cell Growth Differ. 1992;3(12):899–908. [PubMed] [Google Scholar]
- 12.Abate C, Curran T. Encounters with Fos and Jun on the road to AP-1. Semin Cancer Biol. 1990;1(1):19–26. [PubMed] [Google Scholar]
- 13.Curran T, Franza BR., Jr Fos and Jun: the AP-1 connection. Cell. 1988;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 14.Li JJ, Cao Y, Young MR, Colburn NH. Induced expression of dominant-negative c-jun downregulates NFkappaB and AP-1 target genes and suppresses tumor phenotype in human keratinocytes. Mol Carcinog. 2000;29(3):159–169. doi: 10.1002/1098-2744(200011)29:3<159::aid-mc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Saez E, Rutberg SE, Mueller E, Oppenheim H, Smoluk J, Yuspa SH, et al. c-fos is required for malignant progression of skin tumors. Cell. 1995;82(5):721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- 16.Brenner DA, O’Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- 17.Westwick JK, Weitzel C, Minden A, Karin M, Brenner DA. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994;269(42):26396–26401. [PubMed] [Google Scholar]
- 18.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 19.Vogt PK. Jun, the oncoprotein. Oncogene. 2001;20(19):2365–2377. doi: 10.1038/sj.onc.1204443. [DOI] [PubMed] [Google Scholar]
- 20.Behrens A, Sabapathy K, Graef I, Cleary M, Crabtree GR, Wagner EF. Jun N-terminal kinase 2 modulates thymocyte apoptosis and T cell activation through c-Jun and nuclear factor of activated T cell (NF-AT) Proc Natl Acad Sci U S A. 2001;98(4):1769–1774. doi: 10.1073/pnas.98.4.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- 22.Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Bohlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shono T, Ono M, Izumi H, Jimi SI, Matsushima K, Okamoto T, et al. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol Cell Biol. 1996;16(8):4231–4239. doi: 10.1128/mcb.16.8.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnott CH, Scott KA, Moore RJ, Hewer A, Phillips DH, Parker P, et al. Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway. Oncogene. 2002;21(31):4728–4738. doi: 10.1038/sj.onc.1205588. [DOI] [PubMed] [Google Scholar]
- 25.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 26.Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003;23(8):2871–2882. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175(2):1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 28.Enholm B, Paavonen K, Ristimäki A, Kumar V, Gunji Y, Klefstrom J, et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene. 1997;14(20):2475–2483. doi: 10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- 29.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270(21):12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 30.Bancroft CC, Chen Z, Yeh J, Sunwoo JB, Yeh NT, Jackson S, et al. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappaB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer. 2002;99(4):538–548. doi: 10.1002/ijc.10398. [DOI] [PubMed] [Google Scholar]
- 31.Shih SC, Claffey KP. Role of AP-1 and HIF-1 transcription factors in TGF-beta activation of VEGF expression. Growth Factors. 2001;19(1):19–34. doi: 10.3109/08977190109001073. [DOI] [PubMed] [Google Scholar]