Abstract
Hepatocellular carcinoma (HCC) is a global health burden with limited treatment options and poor prognosis. Silibinin, an antioxidant derived from the Milk Thistle plant (Silybum marianum), is reported to exert hepatoprotective and antitumorigenic effects in vitro and in vivo by suppressing oxidative stress and proliferation. Using a DEN-initiated mouse model of HCC, this study examined the effects of dietary silibinin supplementation alone, or in combination with chronic ethanol consumption on HCC progression. Our data demonstrate silibinin exerted marginal hepatoprotective effects in early stages of hepatocarcinogenesis but, when co-administered with ethanol, exacerbated the promotional effects of ethanol in HCC bearing mice, but only in males.
Keywords: Ethanol, Hepatocellular carcinoma, Diethylnitrosamine, Alcoholic liver disease, Sex, Silibinin
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer diagnosed and the third leading cause of cancer-related mortality in the world [1; 2]. The incidence of HCC is inextricably linked with exposure to known environmental risk factors of which hepatitis B and/or C virus (HBV/HCV) infection are the most common [1; 3]. However, chronic, heavy ethanol intake (daily ingestion of >40–60g ethanol) remains a primary risk factor in the United States and Europe [4; 5]. Following ingestion >80% of ethanol metabolism occurs in the liver via alcohol dehydrogenase (ADH), cytochrome P450 2E1 (CYP2E1), and catalase [5]. In the case of moderate or infrequent ethanol consumption ADH accounts for the majority of ethanol metabolism. However, in the setting of chronic, repeated ethanol consumption, CYP2E1 is induced and results in elevated acetaldehyde levels and oxidative damage which in turn can lead to the formation of nucleic acid, protein and/or lipid adducts, altered cell cycle progression, and pro-carcinogen activation [4; 5; 6]. Ethanol-induced DNA damage, coupled with net increases in reactive oxygen species (ROS) severely impairs parenchymal cell (hepatocyte) function and promotes cell transformation[5; 6].
Hepatocarcinogenesis is a multistage process involving initiation, promotion, and progression [3]. Hepatic oxidative stress influences all stages of HCC pathology including progression via clonal expansion and augmentation of tumor cell invasion and metastasis [6; 7]. In the healthy liver a balance between ROS generation and antioxidant defenses exists. In addition to increased ROS production, chronic, heavy ethanol consumption depletes endogenous antioxidant (glutathione; GSH) levels and further enhances potential for DNA damage and chromosomal aberrations [8; 9]. In addition, increased intrahepatic stress induces acute and chronic inflammation resulting in the activation of resident hepatic immune cells (Kupffer cells) and the recruitment of systemic immune cells [5; 10; 11]. Subsequently immune cells increase ROS-altering transcription factor response activity and generates aberrant pro-inflammatory cytokine and chemokine expression that acts to perpetuate epithelial and stromal cell damage [10]. In addition to the effects of ethanol during initiation of hepatocarcinogenesis, additional studies report toxic intermediates derived from ethanol metabolism promote progression via sustained stimulation of stress-activated cytokine cascades and continued depletion of cellular oxidative stress defenses [12].
Females are more susceptible to the deleterious hepatic effects of ethanol yet males more commonly develop cirrhosis [13] and progress to HCC. In fact, incidence of HCC in men is more than twice that of women. While men are more likely to consume ethanol than women, and consume larger amounts when drinking, other factors such as gender-dimorphic expression of enzymes, including CYP2E1, and antioxidants may also play a role in HCC incidence [14]. Similarly, ethanol-dependent changes in the hypothalamic pituitary-gonadal axis release of estrogens and androgens affects inflammation, oxidative stress, and ALD development [15], additional factors that may contribute to the dimorphism reported for HCC. In a recent study by our group we report similar sexual dimorphism exists in a mouse diethylnitrosamine (DEN)-initiated model of HCC, in which male mice are more susceptible to incidence of HCC and the effects of chronic ethanol consumption on rate of HCC progression [16].
The identification of ROS-oxidative stress as central mediators of the damaging effects of ethanol consumption in the liver has led to interest in antioxidants as hepatoprotectants. Considerable experimental evidence demonstrates that S-adenosyl methionine (SAMe), a precursor for GSH synthesis, is an effective hepatoprotectant against a wide range of hepatic insults, including ethanol [17]. However, clinical trials of SAMe have proved more controversial, leading to renewed interest in naturally occurring, plant derived antioxidants. Silymarin, a polyphenolic mixture of flavinoligands derived from the seeds of the Milk Thistle plant (Silybum marianum) has been described as an “hepatic elixir” for several millennia [18]. The reported protective effects silymarin exert on the liver have been attributed to suppression of ROS intermediates and lipid peroxidation, and the rebalancing of cellular REDOX status [18; 19]. Silibinin (a 1:1 mixture of silybin A and silybin B) is reported to inhibit pro-inflammatory signals, cellular proliferation and expression of survival proteins, suggesting additional benefits to protecting the liver against chemical insult [18; 19]. However, many experimental studies of Milk Thistle are designed such that animal models are treated with silibinin prior to hepatic insult. In contrast, the majority of individuals taking natural supplements do not do so until after being diagnosed with underlying liver disease. As a result, despite the historical and current use of Milk Thistle and encouraging experimental data, systematic reviews of randomized clinical trials are reluctant to draw conclusions as to the efficacy of silibinin on patient health [20]. Previous studies report the effects of sex and chronic ethanol feeding on HCC progression [16]. The aim of this study was to determine the effects of dietary silibinin on HCC progression in the absence or presence of chronic ethanol feeding in male and female mice.
2. MATERIALS AND METHODS
2.1 Animals
Male and female B6C3 mice (21–25 days old; Jackson Laboratories, Bar Harbor, ME) were used for these studies. All experiments were approved by the Institutional Animal Care and Use Committee and conformed to the NIH Guidelines for the Care and Use of Animals.
2.2 Materials
Diethylnitrosamine (DEN), p-nitrophenol, and ethanol (EtOH) were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against proliferating cell nuclear antigen (PCNA), ADH (alcohol dehydrogenase), aldehyde dehydrogenase (ALDH), glutathione S-transferase-placental isoform (GSTpi), and an avidin-biotin complex kit were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody against CYP2E1 was purchased from Millipore (Temecula, CA). For RNA extractions, RNeasy mini kits were purchased from Qiagen (Valencia, CA). RQ1 DNase and ImpromII RT-PCR based cDNA synthesis kits were purchased from Promega (Madison, WI). IQ SYBR green Supermix for Real Time PCR assays was purchased from BioRad (Hercules, CA). Serum alanine aminotransferase (ALT) levels were assessed using Infinity ALT (GTP) liquid stable reagent from ThermoScientific (Rockford, IL). Thiobarbituric acid reactive species (TBARS) and GSH assay kits were purchased from Cayman Chemical (Ann Arbor, MI).
2.3 In vivo model of hepatocarcinogenesis
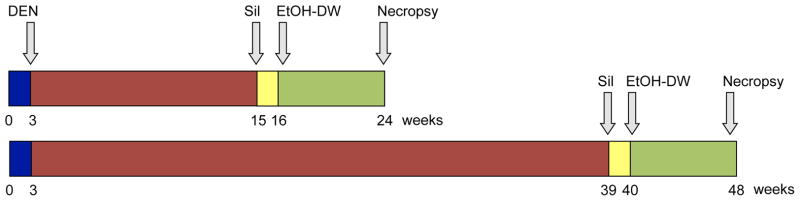
B6C3 mice (21–25 days old) were weighed and randomized to DEN and non-DEN-initiated groups. Animals were injected with a single dose of DEN (1 mg/kg body weight dissolved in sterile olive oil) or vehicle (olive oil) at 3-wks old. Mice were next randomized to receive control diet (AIN93-M) or diet supplemented with silibinin (AIN93-M + 0.5% (w/w) silibinin) for a period of 9 weeks beginning at 15 or 39 wks old (Fig. 1).
Fig. 1. Experimental animal model.
Juvenile mice (21–25 days old) were given a single (i.p.) injection of vehicle (olive oil) or DEN (1 mg/kg). Silibinin diet was introduced at 15 or 39 weeks, continuing for 9 weeks with or without ethanol drinking-water initiation at 16 or 40 weeks. Animals were sacrificed at 24 or 48 weeks.
Ethanol feeding
Mice were randomized to receive control (drinking water) or chronic ethanol feeding (10/20% (v/v) ethanol in drinking water (EtOH-DW), alternate days) regimes as previously reported [16]. Mice were initiated onto EtOH-DW 1 wk after receiving control or silibinin diets (16- or 40-wks) and maintained in this manner for a further 8-wks (Fig. 1). Animals were weighed twice weekly and data recorded.
2.4 Necropsy
At 24- or 48-wks mice were weighed, anesthetized and sacrificed by exsanguination. Livers were harvested, weighed and examined for gross pathology. Liver sections were snap frozen in liquid nitrogen or fixed in neutral buffered formalin prior to transfer to 70% EtOH for histological analysis.
2.5 Histology and immunohistochemistry
Sections (4–6μm) were stained with Mayer’s hematoxylin and eosin (H and E) or Picrosirius red and analyzed as previously reported [16]. Independent injury scores in the categories of steatosis, necrosis, inflammation, and fibrosis were utilized to generate a total liver injury score (TLIS) [16]. Tissues were examined for multiplicity and area of altered hepatic foci (AHF) using GSTpi immunohistochemistry.
2.6 Quantitative Real Time PCR and immunoblot analysis
Total RNA was extracted from liver tissue and cDNA synthesized [16]. Real Time PCR was performed using 50 ng of cDNA template and SYBR Green Supermix. Mouse specific primers for T-bet, GATA3, and SMAD3 were designed and validated prior to use in an iCycler IQ detection system. Data were analyzed according to the comparative Ct method using b-2 microglobulin (b2M) as an invariant control and normalized to pair-matched silibinin controls. Ct values were converted into relative quantities of mRNA expression (Q) using the delta-Ct (ΔCt) method by the formula Q=2Δ−Ct. For protein detection standard immunoblotting techniques were performed using lysates prepared from ≈25 mg of tissue in radioimmunoprecipitation assay (RIPA) buffer [16]. Signal intensity was quantitated using ImageJ software.
2.7 Liver function and oxidative stress status
Serum ALT levels and hepatic lipid peroxidation (evaluated by TBARS assay and expressed as malondialdehyde [MDA]) and GSH were determined as previously reported [16]. Oxidation rate of p-nitrophenol to 4-nitrocatechol in the presence of NADPH and O2 was used as an indicator of CYP2E1 activity.
2.8 Serum silibinin levels
Determination of silibinin levels in serum and tissue were conducted at the David H. Murdock Research Institute (DHMRI) by mass spectrometry, UPLC-tandem MS system (Acquity UPLC-Quattro XE MS, Waters Corp., Milford, MA). Serum was pooled from each treatment group and liver tissue prepared and homogenized in KCl (0.15 mol/L) or Tris-HCl (pH 7.4) buffers. Homogenates were centrifuged and supernatants used for analysis. Silibinin from conjugated and un-conjugated fractions in serum and liver were digested with β-glucuronidase and sulphatase at 37°C for 1.5 hrs. After digestion, 3 volumes of internal standard were added and then mixtures centrifuged. A UPLC-tandem MS system was used for quantification following chromatographic separation on an analytical column.
2.9 Statistical analysis
Data were analyzed using Student’s t-test, one-way analysis of variance (ANOVA), or two-way ANOVA and Tukey’s post-hoc test where appropriate. A p-value <0.05 was considered significant. Quantitative data are presented as mean ± SEM. All experiments were performed a minimum of three times with n≥5 animals per group per experiment.
3. RESULTS
3.1 Effect of silibinin on gross pathology and hepatic injury
At necropsy, animal weights were recorded, livers resected, photographed and weighed to evaluate liver (L): body weight (BW) ratio. Both male and female mice tolerated silibinin and ethanol diets without significant differences in dietary consumption among groups. While males maintained a higher body weight compared to females, no marked differences in L:BW ratio were noted among experimental groups at 24 wks (Suppl. Table 1). Upon gross examination, no visible lesions were observed at 24 wks (data not shown); however, in latter stages of hepatocarcinogenesis (48 wks), both sexes presented with varying degrees of visible tumor burden (Fig. 2a). Similarly, histological analysis showed widespread signs of hepatic injury in DEN-initiated animals predominantly at 48 compared to 24 wks (Suppl. Fig. 1). Assessment of tumor-burden demonstrated moderate, though significant, protective effects of silibinin in tumor bearing female mice, this effect being limited to differences in mean foci area. (Suppl. Fig. 1a–e) In the absence of visible lesions, coupled with unremarkable histology during early stages of carcinogenesis (24 wks); only those animals progressing to advanced HCC (48 wk time points) will be discussed in detail.
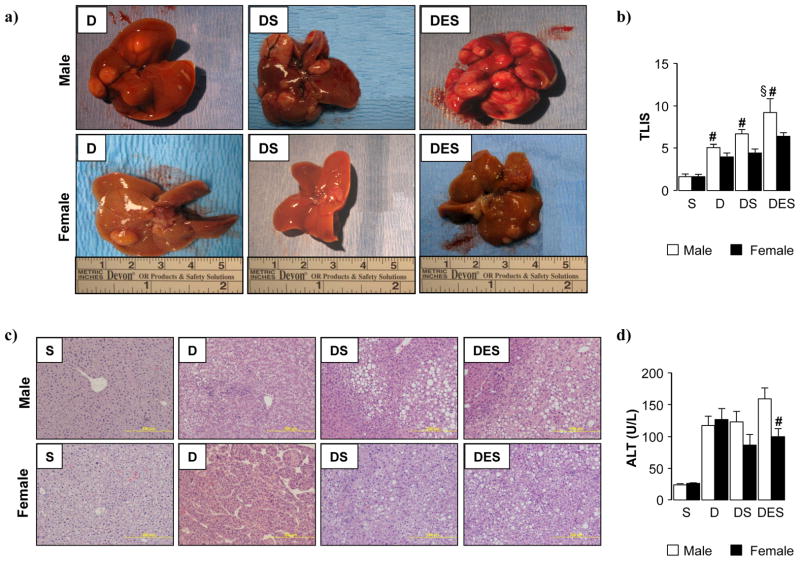
Fig. 2. Silibinin feeding provided marginal hepatoprotective effects in male and female DEN-initiated mice, and exacerbated carcinogenesis when combined with ethanol.
(a) Representative images of livers resected from male and female DEN-initiated mice (48 weeks) maintained on dietary silibinin alone or in combination with ethanol feeding. (b) Liver injury was blind-scored from representative H and E (c) and Picosirius red sections (Suppl. Fig. 2) (2 lobes/mouse, 5 fields/lobe) from each experimental group to generate a total liver injury score (TLIS) (d) Serum alanine aminotransferase (ALT) activity was measured. (S; dietary silibinin, D; DEN-initiated, DS; DEN-initiated/dietary silibinin, DES; DEN-initiated/dietary silibinin/EtOH). n≥5 animals/group, p<.05 S vs D, DS and DES in male and female groups, # p<.05 male vs female, § p<.05 DES vs D and DS.
Tumors were detected in all DEN-initiated animals (D). When examining tumor-bearing females, neither dietary silibinin (DS) or silibinin combined with ethanol (DES) affected tumor burden in females. However, DEN-initiated males fed silibinin diet (DS) displayed an increase in both tumor size and number, an effect further amplified with chronic ethanol consumption (DES) (Fig. 2). L:BW ratios mirrored visual assessment of tumor burden (Suppl. Table 1). Compared to silibinin control group (S), L:BW ratios increased by ≈50% in both male D and DS groups, and doubled in male DES animals (Sup. Table 1). In female experimental groups, only DEN-initiated females (D) displayed a minor increase in L:BW ratio compared to silibinin (S) (Suppl. Table 1).
Blind scoring of H and E and Picrosirius red stained slides (Fig. 2c, Suppl. Fig. 2) for total fat, necrosis, inflammatory cell infiltration and collagen deposition was used to generate a total liver injury score (TLIS) (Fig. 2b). Increased hepatic injury was observed as a result of DEN-initiation/HCC development (D, DS, DES) compared to silibinin-fed controls (S). However, dietary silibinin did not abrogate the damage associated with HCC progression (DS vs. D) in either sex (Fig. 2b). Female mice, irrespective of treatment group, displayed significantly less hepatic damage compared to males. While the potentiating effects of ethanol were relatively minor in females, male DES mice exhibited a significant increase in hepatic damage compared to other tumor-bearing groups (D and DS) (Fig. 2b); supporting gross pathology observations (Fig. 2a). When dissecting individual components of hepatic injury, increased micro- and macrovesicular steatosis were identified in DS and DES male mice, corresponding to increased foamy degeneration, necrosis, and increased inflammatory cell infiltrate (Fig. 2c). While silibinin feeding was associated with lipid accumulation and inflammation in females (DS), measures of necrosis were decreased compared to tumor-bearing mice not given silibinin (D). Additionally, pattern of tumor development was divergent, with a microtrabecular arrangement identified in males as opposed to the macrotrabecular pattern in females (D) (Fig. 2c). However, tumors in silibinin-fed females (DS and DES) were histologically similar to that of males not receiving dietary silibinin (D).
As a consequence of tumor development/progression serum alanine aminotransferase (ALT) levels were markedly increased in tumor-bearing males and females, and were not significantly altered by the presence of dietary silibinin (DS vs D) (Fig. 2d). Corroborating TLIS data, the enhancing effects of ethanol were associated with increased ALT levels in male DES mice compared to DEN-initiated males with or without silibinin (D and DS) and initiated males fed silibinin (DS) (Fig. 2d). Additionally, silibinin feeding (DES group) does not resolve hepatic damage we previously reported in DEN-initiated male mice receiving ethanol alone (DE; ALT measured 140.0±18.2; males, 107.0±13.9; females [16]). However the current study showed ALT levels were dampened in females receiving silibinin (DS vs. D) (Fig. 2d).
3.2 Effect of silibinin on tumor burden and proliferation
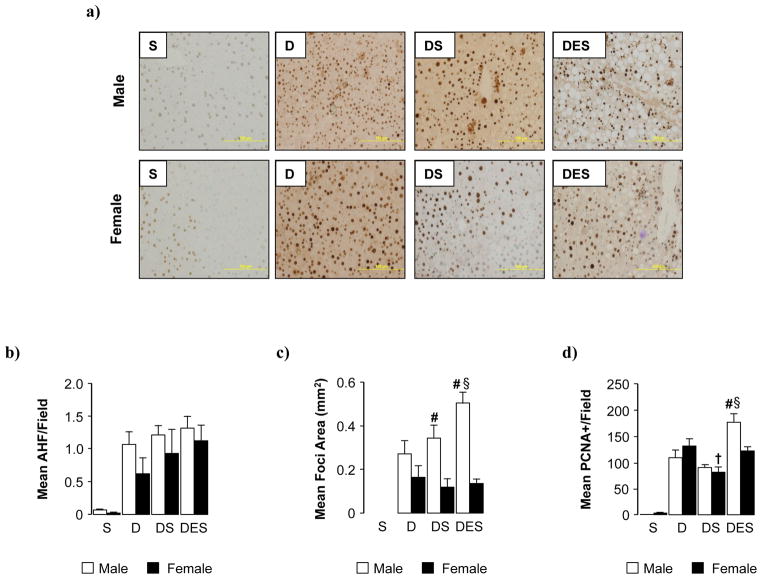
Multiplicity and area of altered hepatic foci (AHF) were assessed by GSTpi immunohistochemical staining (Fig. 3a,b,c). No statistically significant differences were observed in multiplicity of AHF across all DEN-initiated groups (D, DS, DES) (Fig. 3b). Compared to pair-matched females, area of AHF was significantly increased in tumor-bearing males fed silibinin (DS) (Fig. 3c). However, irrespective of sex, area of AHF was not significantly reduced by dietary silibinin (DS). In addition to presenting with larger foci, promotional effects of ethanol were only observed in male DES mice compared to females. The addition of dietary silibinin was unable to suppress previously reported effects of ethanol alone in DEN-initiated animals (DE; area AHF measured 0.48±0.03 in males and 0.13±.03 in females [16]). Examination of PCNA staining (Fig. 3d) indicated proliferation associated with tumor incidence was decreased in silibinin-fed animals (DS). However, this effect was abrogated with concomitant ethanol consumption (DES) with a more pronounced effect observed in male DES mice compared to females.
Fig. 3. Dietary silibinin did not reduce tumor burden in male or female mice.
(a) Representative glutathione S-transferase-placental isoform (GSTpi) immunohistochemical staining used to calculate tumor multiplicity (b) and mean area (mm2) (c) of altered hepatic foci/field (AHF) (d) Number of PCNA-positive cells per microscopic field measured in representative sections (2 lobes/mouse, 5 fields/lobe) at 48 weeks post-DEN and mean values calculated. Data are presented as mean ± SEM. (S; dietary silibinin, D; DEN-initiated, DS; DEN-initiated/dietary silibinin, DES; DEN-initiated/dietary silibinin/EtOH). n≥5 animals/group. p<.05 S vs D, DS and DES in male and female groups, # male vs female, § DES vs D and DS, † DS vs D.
3.3 Effect of dietary silibinin on ethanol metabolism and hepatic REDOX status
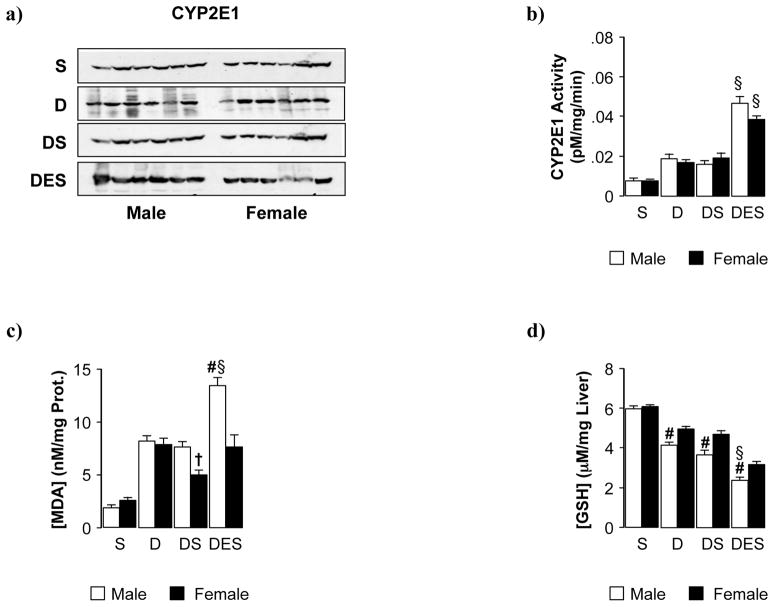
We previously reported silibinin provided protective effects via suppression of proliferation and expression/activity of CYP2E1 in vitro [21]. However, in this in vivo model CYP2E1 protein expression, in both male and female tumor-bearing mice, was not significantly reduced by dietary silibinin (DS vs D) (Fig. 4a). Hydroxylation of p-nitrophenol was measured as an indicator of CYP2E1 biochemical activity. Similar to protein results, dietary silibinin did not reduce activity of CYP2E1 induced by DEN-initiation/tumor progression (DS vs. D) (Fig. 4b). In contrast to previous data, sex bias was not observed in the aggravating effects of ethanol on CYP2E1 activity (DES). Both male and female DES animals displayed similar levels of end product p-nitrocatechol (Fig. 4b) and were not statistically different from DEN-initiated animals that received ethanol alone (DE; CYP2E1 activity measured 0.051 ± 0.004 in males and 0.037 ± 0.003 in females [16]). Expression of ethanol metabolizing factors ADH/ALDH were assessed by immunoblot and no significant effects of silibinin or sex were measured (data not shown).
Fig. 4. Silibinin feeding did not significantly inhibit cytochrome P4502E1 or alter hepatic oxidative stress status in DEN-initiated male and female mice.
(a) Representative immunoblots for detection of CYP2E1 protein in control and DEN-initiated animals fed silibinin alone or in combination with ethanol. Equal loading was verified by b-actin expression (data not shown). (b) Quantitative analysis of the effect of silibinin on CYP2E1 activity. (c) malondialdehyde (MDA) and (d) glutathione (GSH) levels were measured 48 weeks post-DEN initiation in male and female mice fed dietary silibinin with or without ethanol in the drinking water. Data are presented as mean ± SEM. (S; dietary silibinin, D; DEN-initiated, DS; DEN-initiated/dietary silibinin, DES; DEN-initiated/dietary silibinin/EtOH). n≥5 animals/group, p<.05 S vs D, DS and DES in male and female groups, # male vs female, § DES vs D and DS, † DS vs D.
Malondialdehyde (MDA) and glutathione (GSH) were measured to indicate lipid peroxidation levels/intrahepatic REDOX status (Fig. 4c,d). While lipid peroxidation was increased in male and female mice initiated with DEN (D), a significant decrease in MDA was present in tumor-bearing females fed dietary silibinin (DS). Chronic ethanol consumption significantly promoted oxidative stress, predominantly in male mice, and abrogated the protective effect of silibinin in females (DES) (Fig. 4c). Hepatic GSH levels were significantly depleted in tumor-bearing animals (D vs S), with dietary silibinin providing no protective effects (DS) (Fig. 4d). Concomitant ethanol consumption aggravated this effect in both male and female mice (DES vs D and DS). Additionally, tumor-bearing males had significantly less GSH compared to pair-matched females in all treatment groups.
3.4. Immunological characterization of silibinin-fed male and female mice during late hepatocarcinogenesis
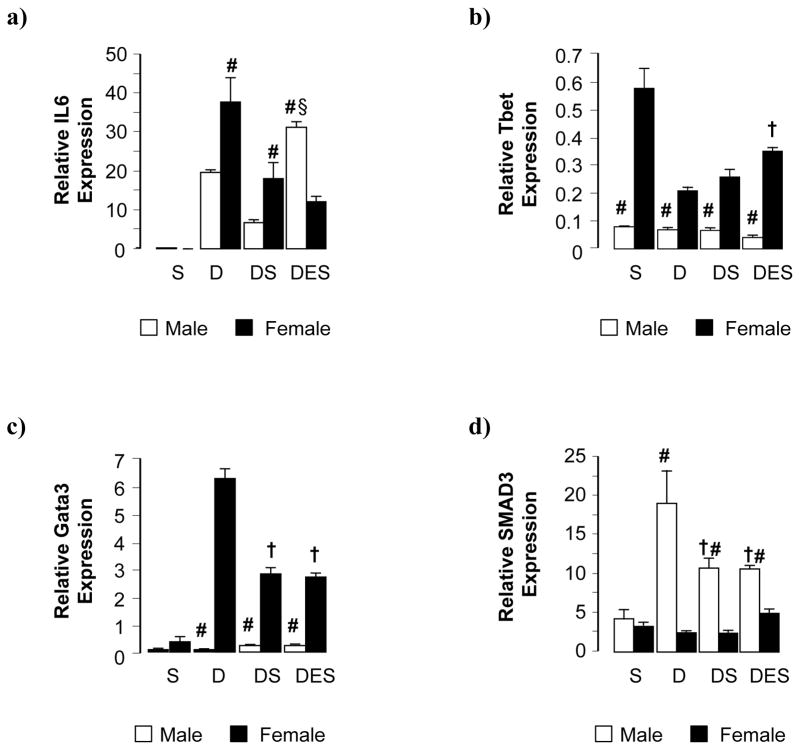
To assess hepatic inflammatory state in experimental groups, interleukin-6 (IL-6) expression was measured by quantitative RT-PCR (Fig. 5a). DEN-initiated animals (D) showed a dramatic increase in hepatic IL-6 expression compared to silibinin controls (S), this increase being approximately double in females compared to male counterparts (Fig 5a). While still elevated above control, silibinin diet diminished hepatic IL-6 expression in tumor-bearing animals (DS) while maintaining sex differences (Fig 5a). Chronic ethanol consumption only affected IL-6 expression in male DES mice where the protective effect of silibinin diet on hepatic inflammation was abrogated (DES vs D and DS) (Fig. 5a).
Fig. 5. Silibinin feeding evoked differential immunological effects in male and female mice in the setting of HCC alone and concomitant with ethanol feeding.
(a,b,c,d) Relative expression of IL6, Tbet, Gata3 and SMAD3 mRNA in total liver as assessed by qRT-PCR. Data are presented as mean ± SEM. (S; dietary silibinin, D; DEN-initiated, DS; DEN-initiated/dietary silibinin, DES; DEN-initiated/dietary silibinin/EtOH). n≥5 animals/group, p<.05 S vs D, DS and DES in male and female groups (a, c and d; b – female groups only), # male vs female, § DES vs D and DS, † DS vs D.
To characterize the phenotype of inflammatory processes associated with HCC development-progression in DEN-initiated animals, hepatic T-bet (TH1 “cellular” inflammation) and Gata3 (TH2 “humoral” inflammation) mRNAs were quantified (Fig. 5b,c). Female mice (DEN groups) expressed higher levels of both T-bet and Gata3 transcription factors across experimental groups, compared to male animals, although expression levels were low (Fig. 5b, c). T-bet expression decreased in DEN-induced female mice (D and DS). A weak pro-TH1 effect was observed as a result of concomitant ethanol consumption (DES); however, expression levels remained lower than that of control (S) (Fig. 5b). Female mice were also characterized by increased Gata3 expression, a pro-TH2 effect, which was partly inhibited by dietary silibinin (DS and DES) (Fig. 5c).
Finally, hepatic Smad3 expression was used as an indicator of TGF-β signaling, an anti-inflammatory pathway (Fig. 5d). Irrespective of experimental group, female animals showed lower hepatic Smad3 mRNA compared to males (Fig. 5d). Conversely, DEN-initiation alone led to a dramatic increase in hepatic Smad3 expression in male mice (D). Silibinin diet alone, or in combination with chronic ethanol intake, was associated with decreased hepatic Smad3 expression in both male DS and DES groups (Fig 5d).
3.5 Hepatic tissue and serum levels of silibinin
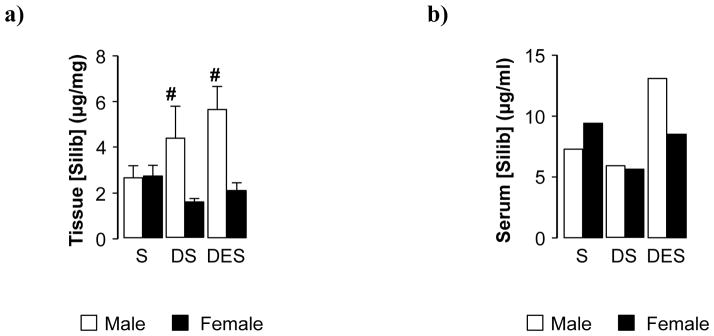
In late stage hepatocarcinogenesis DEN-initiated females (DS) maintained lower hepatic levels of silibinin compared to controls (S) (Fig. 6a). Conversely, tissue sequestered silibinin was markedly increased in DEN-initiated male mice (DS vs S), an effect further enhanced by ethanol (DES). When examining circulating levels of the flavinoligand in pooled serum samples, silibinin concentrations were markedly reduced in tumor-bearing females (DS) compared to silibinin controls (S), an effect abolished by ethanol (DES) (Fig. 6b). Corresponding to tissue data, elevated serum silibinin was observed in male DES mice compared to tumor-bearing males fed silibinin (DS).
Fig. 6. Tissue and serum silibinin levels were preferentially increased in later stages of hepatocarcinogenesis in male mice.
(a) Silibinin levels in liver tissue following 9 weeks of dietary consumption in male and female mice. (b) Silibinin levels in pooled serum samples collected from male and female mice with or without DEN-initiated hepatocarcinogenesis. Data are presented as mean ± SEM. (S; dietary silibinin, D; DEN-initiated, DS; DEN-initiated/dietary silibinin, DES; DEN-initiated/dietary silibinin/EtOH). n≥5 animals/group. p<.05 S vs DS and DES in male and female groups, # male vs female.
4. DISCUSSION
This study investigated the effects of dietary silibinin on HCC progression in mice in the absence or presence of chronic ethanol consumption. Using a well-characterized model of chemically-induced (DEN) carcinogenesis [22], male mice developed HCC at a rate of 2:1 compared to female mice, a rate comparable with that of humans [1]. Previous studies by our group and others report chronic alcohol feeding in this DEN model of HCC leads to increased tumor progression in males, exacerbates oxidative stress, and accelerates hepatic damage [16; 23; 24]. In contrast to previous animal studies, where Milk Thistle derivatives were administered prior to hepatic insult [25], in our model dietary silibinin was introduced after foci formation. This approach more closely mimics typical silibinin consumption in patients. For example, a survey of liver disease clinics in the US reported ≈40% of patients used a form of complementary and alternative medicine (CAM) following initial diagnosis, with Milk Thistle (silibinin) the most popular herbal supplement taken [26; 27]. Herein we report, silibinin feeding preferentially dampened tumor growth during early hepatocarcinogenesis (Suppl. Fig. 1), and to a lesser extent at 48 wks, in DEN-initiated females. These data suggest that in this mouse model of HCC progression timing of silibinin consumption, relative to foci formation and tumor development, may be central in mediating potential health benefits associated with silibinin. Of note, the moderate beneficial effects of silibinin in this model were not evidenced in pair-matched male mice. Additionally, concomitant ethanol feeding abrogated the antioxidant and anti-tumorigenic effects in male mice, indicating potential drug-drug hepatotoxic interactions. Previous studies do not report significant side effects or adverse health effects associated with flavinoligands derived from Milk Thistle. Interestingly, the potentially hepatoprotective effects of silibinin in early, foci development stages were no longer evidenced at later HCC progression stages, either in the presence or absence of concomitant chronic ethanol feeding. These data further support the suggestion that timing of silibinin feeding may be the critical factor in determining silibinin efficacy in slowing HCC tumor progression.
Oxidative stress is a central component of progressive liver injury and hepatocarcinogenesis [23]. We originally hypothesized that antioxidant properties ascribed to silibinin could protect against hepatic injury and HCC progression. However, when compared to DEN-initiated groups, silibinin failed to increase intrahepatic GSH levels, corroborating reports that silibinin does not reverse GSH depletion, in contrast to other antioxidants such as S-adenosyl-L-methionine [28]. Among factors known to induce hepatic oxidative stress, chronic ethanol consumption, and CYP2E1-dependent metabolism, increases ROS production [23]. We have previously reported silibinin suppresses ethanol-induced proliferation of HCC cells in vitro via inhibition of CYP2E1 mediated-oxidative stress [29]. Conversely, in the current study, dietary silibinin did not alter expression or activity of CYP2E1 in vivo. One possible explanation of this finding may be higher “basal” expression of CYP2E1 in H4IIE cells compared to hepatocytes induced with ethanol [29].
The liver is a dimorphic organ responsive to both estrogens and androgens with distinct sex differences associated with gene expression, mitochondrial function, membrane lipid composition, and immune response [30; 31]. In the setting of HCC, DEN initiates a greater increase in serum IL-6 in male compared to female mice [32; 33]. In these studies, sex differences were attributed to estrogen-mediated inhibition of IL-6 production. In contrast, a retrospective clinical study by Nakagawa et al. reported a weak correlation between serum IL-6 and estradiol levels in chronic HCV cases [34]. In this study, hepatic IL-6 mRNA was increased in DEN-initiated female mice, an effect abrogated by silibinin feeding. Chronic ethanol consumption evokes hepatic inflammation through increased gastrointestinal permeability and subsequent translocation of bacterial endotoxin (lipopolysaccharide; LPS) to the liver. Increased hepatic LPS levels stimulate pro-inflammatory signaling pathways, including Toll-like receptor 4 (TLR4), inducing activation of Kupffer cells and thus IL-6 production [30]. In DEN models of HCC, IL-6 production by Kupffer cells requires Toll-like receptor adaptor protein MyD88 [32]. In our model a silibinin diet, concomitant with chronic ethanol consumption, led to a dramatic increase in hepatic inflammation (histological assessment) in DEN-initiated male mice with concurrent IL-6 production. Taken together those findings suggest hepatic IL-6 expression may be predominantly regulated by tissue damage associated oxidative stress, as opposed to sex hormones.
Plant-derived antioxidants have been proposed as a means of ameliorating increased oxidative stress and inflammation (ethanol-derived) associated with fibrosis/cirrhosis, potentially preventing progression to HCC [35]. Silibinin is the most biologically active component contained in the silymarin mixture (Milk Thistle) [36; 37]. Following absorption in the gut, intrahepatic silibinin components are rapidly conjugated to sulfate and glucoronic acid, excreted into bile and hydrolyzed by intestinal flora to undergo re-uptake in the intestine [38; 39]. Increased serum and tissue silibinin levels were measured in DEN-initiated animals chronically consuming ethanol, suggesting a possible impairment of hepatic silibinin processing that may account for to the observed lack in silibinin-mediated protection against HCC progression in this model. One possible mechanism of decreased silibinin efficacy in this model may relate the ability of ethanol to suppress glucoronic acid/β-glucoronidase [40], potentially interfering with biliary excretion of silibinin. In addition to altered hepatic silibinin metabolism, resulting from diminished functional mass in tumor-bearing animals, the altered metabolic capacity of transformed hepatocytes (HCC) may also directly contribute to decreased silibinin clearance in an established model of HCC.
While silibinin is not currently reported to induce deleterious effects, extensive drug-drug interaction/toxicity studies are lacking, and its therapeutic efficacy against a range of hepatic disorders, including cirrhosis and acute/chronic hepatitis, remains to be fully elucidated [19; 41; 42]. In addition, the lack of manufacturing regulatiuon and variations in chemical composition of Milk Thistle products, arising from factors such as geographical source and processing procedures [43; 44], can also influence composition and potency of the flavinoligand components consumed. Of note, a recent study analyzing quality and purity of US Milk Thistle reported aflatoxin, a known risk factor for HCC, contamination in 19% of samples [45], raising an additional potential risk associated with consumption.
In conclusion, in our model of chemically induced HCC, the effects of dietary silibinin, consumed after administration of a known hepatocarcinogen (DEN), did not effectively inhibit hepatic tumor progression. Furthermore, chronic alcohol feeding in this model further exacerbated the rate of tumor progression, an effect limited to male mice. Many individuals diagnosed with disease utilize CAM regimes for health benefits, in addition to prescription drug-based care. The current perception of many of these plant-derived CAMs is that, at worst, these drugs will “do no harm”, even if they do not provide any health benefit. In light of reports suggesting that ≈27% of patients do not disclose CAM use to physicians [42], the data we present herein suggest further research is required to determine the efficacy of silibinin in treating pre-cancerous (e.g. NASH and NAFLD) and cancerous states.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was funded in part by a Grant from the National Institutes of Health (IHM; Grant# AA016858).
The authors thank the David H. Murdock Research Institute for their mass spectrometry services.
Footnotes
CONFLICT OF INTEREST
The authors declare they have no financial and/or personal relationships with other people or organization that could inappropriately influence (bias) their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKillop IH, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006;136:125–135. doi: 10.1016/j.jss.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Cornella H, Alsinet C, Villanueva A. Molecular pathogenesis of hepatocellular carcinoma. Alcoholism, clinical and experimental research. 2011;35:821–825. doi: 10.1111/j.1530-0277.2010.01406.x. [DOI] [PubMed] [Google Scholar]
- 5.McKillop IH, Schrum LW. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29:222–232. doi: 10.1055/s-0029-1214377. [DOI] [PubMed] [Google Scholar]
- 6.Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis. 2010;28:802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–1444. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 8.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 10.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Molec Asp of Med. 2008;29:9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Müller C. Liver, Alcohol and Gender. WMW Wiener Medizinische Wochenschrift. 2006;156:523–526. doi: 10.1007/s10354-006-0348-8. [DOI] [PubMed] [Google Scholar]
- 14.Harada S, Tachiyashiki K, Imaizumi K. Effect of sex hormones on rat liver cytosolic alcohol dehydrogenase activity. J Nutr Sci Vitaminol (Tokyo) 1998;44:625–639. doi: 10.3177/jnsv.44.625. [DOI] [PubMed] [Google Scholar]
- 15.Ellefson WM, Lakner AM, Hamilton A, McKillop IH, Bonkovsky HL, Steuerwald NM, Huet YM, Schrum LW. Neonatal and rogenization exacerbates alcohol-induced liver injury in adult rats, an effect abrogated by estrogen. PLoS One. 2011;6:e29463. doi: 10.1371/journal.pone.0029463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandon-Warner E, Walling TL, Schrum LW, McKillop IH. Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcoholism, clinical and experimental research. 2012;36:641–653. doi: 10.1111/j.1530-0277.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cederbaum AI. Hepatoprotective effects of S-adenosyl-L-methionine against alcohol- and cytochrome P450 2E1-induced liver injury. World journal of gastroenterology: WJG. 2010;16:1366–1376. doi: 10.3748/wjg.v16.i11.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytotherapy research: PTR. 2010;24:1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs B, Dennehy C, Ramirez G, Sapp J, Lawrence V. Milk thistle for the treatment of liver disease: A systematic review and meta-analysis. The American journal of medicine. 2002;113:506–515. doi: 10.1016/s0002-9343(02)01244-5. [DOI] [PubMed] [Google Scholar]
- 20.Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15:9–20. doi: 10.1159/000113648. [DOI] [PubMed] [Google Scholar]
- 21.Brandon-Warner E, Sugg JA, Schrum LW, McKillop IH. Silibinin inhibits ethanol metabolism and ethanol-dependent cell proliferation in an in vitro model of hepatocellular carcinoma. Cancer Lett. 2010;291:120–129. doi: 10.1016/j.canlet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fausto N, Campbell JS. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30:87–98. doi: 10.1055/s-0030-1247135. [DOI] [PubMed] [Google Scholar]
- 23.McKillop I, Shrum LW. Alcohol and liver cancer. Alcohol. 2005;35:195–203. doi: 10.1016/j.alcohol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010;31:1409–1420. doi: 10.1038/aps.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayaraj R, Deb U, Bhaskar AS, Prasad GB, Rao PV. Hepatoprotective efficacy of certain flavonoids against microcystin induced toxicity in mice. Environmental toxicology. 2007;22:472–479. doi: 10.1002/tox.20283. [DOI] [PubMed] [Google Scholar]
- 26.Seeff LB, Lindsay KL, Bacon BR, Kresina TF, Hoofnagle JH. Complementary and alternative medicine in chronic liver disease. Hepatology. 2001;34:595–603. doi: 10.1053/jhep.2001.27445. [DOI] [PubMed] [Google Scholar]
- 27.Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, Wright EC, Allen J, Khokar MF, Hoofnagle JH, Seeff LB. Use of complementary and alternative medicine in patients with liver disease. The American journal of gastroenterology. 2002;97:2391–2397. doi: 10.1111/j.1572-0241.2002.05993.x. [DOI] [PubMed] [Google Scholar]
- 28.Thompson KJ, Lakner AM, Cross BW, Tsukada S, Rippe RA, McKillop IH, Schrum LW. S-adenosyl-L-methionine inhibits collagen secretion in hepatic stellate cells via increased ubiquitination. Liver international: official journal of the International Association for the Study of the Liver. 2011;31:891–901. doi: 10.1111/j.1478-3231.2011.02512.x. [DOI] [PubMed] [Google Scholar]
- 29.Brandon-Warner E, Sugg JA, Schrum LW, McKillop IH. Silibinin inhibits ethanol metabolism and ethanol-dependent cell proliferation in an in vitro model of hepatocellular carcinoma. Cancer Lett. 2010;291:120–129. doi: 10.1016/j.canlet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kono H, Wheeler M, Rusyn I, Lin M, Seabra V, Rivera C, Bradford B, Forman D, Thurman R. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kB, and TNFa. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2000;278:G652–G661. doi: 10.1152/ajpgi.2000.278.4.G652. [DOI] [PubMed] [Google Scholar]
- 31.Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60:790–797. doi: 10.1002/iub.124. [DOI] [PubMed] [Google Scholar]
- 32.Naugler W, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 33.Prieto J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J Hepatol. 2008;48:380–381. doi: 10.1016/j.jhep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa H, Maeda S, Yoshida H, Tateishi R, Masuzaki R, Ohki T, Hayakawa Y, Kinoshita H, Yamakado M, Kato N, Shiina S, Omata M. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. International journal of cancer. Journal International du Cancer. 2009;125:2264–2269. doi: 10.1002/ijc.24720. [DOI] [PubMed] [Google Scholar]
- 35.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- 36.Gazak R, Walterova D, Kren V. Silybin and silymarin--new and emerging applications in medicine. Curr Med Chem. 2007;14:315–338. doi: 10.2174/092986707779941159. [DOI] [PubMed] [Google Scholar]
- 37.Saller R, Melzer J, Reichling J, Brignoli R, Meier R. An updated systematic review of the pharmacology of silymarin. Forsch Komplementmed. 2007;14:70–80. doi: 10.1159/000100581. [DOI] [PubMed] [Google Scholar]
- 38.Wu JW, Lin LC, Hung SC, Lin CH, Chi CW, Tsai TH. Hepatobiliary excretion of silibinin in normal and liver cirrhotic rats. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:589–596. doi: 10.1124/dmd.107.017004. [DOI] [PubMed] [Google Scholar]
- 39.Zhao J, Agarwal R. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention. Carcinogenesis. 1999;20:2101–2108. doi: 10.1093/carcin/20.11.2101. [DOI] [PubMed] [Google Scholar]
- 40.Jovic A, Howell B, Cote M, Wade SM, Mehta K, Miyawaki A, Neubig RR, Linderman JJ, Takayama S. Phase-locked signals elucidate circuit architecture of an oscillatory pathway. PLoS computational biology. 2010;6:e1001040. doi: 10.1371/journal.pcbi.1001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 42.Wabuyele MB, Yan F, Vo-Dinh T. Plasmonics nanoprobes: detection of single-nucleotide polymorphisms in the breast cancer BRCA1 gene. Analytical and bioanalytical chemistry. 2010;398:729–736. doi: 10.1007/s00216-010-3992-1. [DOI] [PubMed] [Google Scholar]
- 43.Martin DRLRJ, Smith WA, Jensen DJ, Deo B, Douglas JA. Factors influencing silymarin content and composition in variegated thistle (Silybum marianum) New Zealand Journal of Crop and Horticulture Science. 2006;34:239–245. [Google Scholar]
- 44.Lee J, Hsu BH, Wu D, Barrett JS. Separation and characterization of silybin, isosilybin, silydianin and silychristin in milk thistle extract by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2006;26:57–68. doi: 10.1016/j.chroma.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 45.Tournas VH, Sapp C, Trucksess MW. Occurrence of aflatoxins in milk thistle herbal supplements. Food additives & contaminants Part A, Chemistry, analysis, control, exposure & risk assessment. 2012;29:994–999. doi: 10.1080/19440049.2012.664788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.