Abstract
Myosuppressin peptides dramatically diminish contractions of the gut and heart. Thus, delineating mechanisms involved in myosuppressin signaling may provide insight into peptidergic control of muscle contractility. Drosophila myosuppressin (DMS, TDVDHVFLRFamide) structure-activity relationship (SAR) was investigated to identify an antagonist and explore signaling. Alanyl-substituted, N-terminal truncated, and modified amino acid analogs identified residues and peptide length required for activity. Immunochemistry independently provided insight into myosuppressin mechanisms. DMS decreased gut motility and cardiac contractility dose dependently; the different effective concentrations at half maximal-response were indicative of tissue-specific mechanisms. Replacement of aspartic acid 2 (D2) generated an analog with different developmental- and tissue-specific effects; [A2] DMS mimicked DMS in adult gut (100% inhibition), yet decreased larval gut contractions by only 32% with increased potency in pupal heart (126% inhibition). The DMS active core differed across development and in tissues; adult (DHVFLRFamide) and larval gut (TDVDHVFLRFamide), and adult (VFLRFamide) and pupal heart (VFLRFamide). Substitution of D2 and D4 with a modified amino acid, p-benzoyl-phenylalanine, produced developmental-and tissue-specific antagonists. In the presence of protease inhibitors, DMS and VFLRFamide were more effective in adult gut, but lower or unchanged in pupal heart compared to peptide or analog alone, respectively. DMS-specific antisera stained neurons that innervated the gut or heart. This study describes novel antagonists and data to identify developmental- and tissue-specific mechanisms underlying the pleotropic effects of myosuppressin in muscle physiology.
1. Introduction
Muscle contractions are important for survival; failure to properly regulate contractility may result in a serious disease or death. Peptides regulate muscle contractions, yet much remains to be discovered regarding the mechanisms underlying peptidergic regulation of contractility. Establishing structure-activity relationship (SAR) is a powerful approach to design molecular tools to delineate mechanisms and design antagonists to a myoactive peptide which serves as a high-affinity ligand. Antagonism of peptidergic ligands which interact with G protein-coupled receptors (GPCRs) can provide a promising avenue to identify progenitors and develop strategies to address muscle contractility-related disorders.
Myosuppressins are invertebrate, myoactive peptides [5, 7, 8, 10, 18]. The consensus structure representing insect myosuppressins is X1DVX2HX3FLRFamide, where X1 = pE, P, T, A; X2 = D, G, V; X3 = V, S. Myosuppressins are members of a peptide family which contain an identical RFamide C terminus. The first RFamide peptide identified was the cardioactive tetrapeptide, FMRFamide [15]. The FMRFamide-related peptide (FaRP) family is subdivided based on XRFamide, where X defines the subgroup. Myosuppressins contain an LRFamide.
The first myosuppressin identified, leucomyosuppressin (LMS), was isolated based on its ability to decrease the frequency and amplitude of gut contractions [5]. The structurally-similar Drosophila melanogaster myosuppressin (dromyosuppressin, DMS, TDVDHVFLRFamide) was identified as a naturally-occurring peptide [10]. Following the discovery of LMS as a brain peptide which decreased gut motility, myosuppressins were found to regulate cardiovascular function [17]. DMS binds to two expressed GPCR proteins, DMS receptor-1 (DMSR-1) and -2 (DMSR-2) [4].
Numerous vertebrate FaRPs are known. The first vertebrate FaRP identified was an LRFamide-containing peptide, chicken brain LPLRFamide [2]. Human RFamide-related peptide-1 (hRFRP-1; MPHSFANLPLRFamide), also an LRFamide, dramatically decreases mammalian cardiac performance [13]. The cross-species conservation of structure and bioactivity provides the opportunity to utilize an interdisciplinary approach to delineate peptidergic regulation of muscle contractility. However, no published report describes a detailed SAR, functional antagonist, and developmental- and/or tissue-specific analysis of an LRFamide. Thus, DMS SAR was established in D. melanogaster adult and larval gut, and in adult, pupal, and larval heart. Additionally, analogs containing a modified amino acid were designed to identify antagonists. Finally, DMS-specific antisera were used to immunochemically map peptide expression.
This study tested the hypothesis that residues throughout the peptide may be involved in signaling. This prediction was based on the myosuppressin consensus structure. Furthermore, it was hypothesized that mechanisms underlying the influence of myosuppressin may be developmentally and/or tissue-specifically regulated. Additionally, it was hypothesized that unique brain cells innervated either the gut or heart to regulate peptidergic function. These predictions were based on the needs of an animal to respond to variant physiological and environmental cues and requirements for nutrient absorption and circulation across development and in tissues. Myosuppressin SAR, active cores, antagonists, and antisera provide powerful information and molecular tools to dissect mechanisms and establish function. Knowledge of mechanisms underlying the influence of myosuppressin aids in identifying molecular targets to develop strategies to regulate muscle contractility.
2. Materials and methods
2.1. Flies
D. melanogaster Oregon R strain flies were maintained on cornmeal molasses media at 24°C under a 12 hour light/dark cycle. Animals selected for analysis were wandering 3rd instar larvae for gut and heart bioassays, and white prepupae for heart bioassays. In addition, 4-7 day and newly eclosed to 2 hour adults were chosen for gut or heart bioassays, respectively. The effects of DMS, analogs, and physiological saline, the carrier, tested without peptide or analog as a control, were measured on both females and males; no sex-specific response was observed.
2.2. Chemicals
Alanyl-substituted, truncated, p-benzoyl-phenylalanine (Bpa)-containing, and free acid DMS analogs were synthesized using a standard Fmoc protocol and purified by reversed phase HPLC. Structures were confirmed by amino acid analysis and mass spectrometry. The nomenclature for the alanyl-substituted and N-terminal truncated analogs is found in Tables 1-5. Peptides were diluted in series with physiological saline to obtain the working solutions. The cocktail of water-soluble protease inhibitors had broad specificity for serine, cysteine, and metalloproteases (Sigma-Aldrich Co.; SKU P2714).
Table 1.
The effects of alanine-substituted analogs on adult and larval gut are reported as mean values calculated as percent inhibition relative to the effect of DMS. The symbol † indicates p ≤0.00001.
| % Inhibition (gut) | ||||||
|---|---|---|---|---|---|---|
| Adult | p value | Larval | p value | |||
| DMS |
|
TDVDHVFLRFamide | 100 | 100 | ||
| [A1]DMS |
|
ADVDHVFLRFamide | 100 | 1 | 95 | 0.6 |
| [A2]DMS |
|
TAVDHVFLRFamide | 100 | 1 | 32 | 0.002 |
| [A3]DMS |
|
TDADHVFLRFamide | 100 | 1 | 26 | 0.00008 |
| [A4]DMS |
|
TDVAHVFLRFamide | 91 | 1 | 10 | 0.00002 |
| [A5]DMS |
|
TDVDAVFLRFamide | 78 | 0.01 | 14 | † |
| [A6]DMS |
|
TDVDHAFLRFamide | 100 | 1 | 91 | 0.5 |
| [A7]DMS |
|
TDVDHVALRFamide | 70 | 0.008 | 10 | † |
| [A8]DMS |
|
TDVDHVFARFamide | 14 | † | 42 | 0.0005 |
| [A9]DMS |
|
TDVDHVFLAFamide | 100 | 1 | 74 | 0.1 |
| [A10]DMS |
|
TDVDHVFLRAamide | 90 | 0.08 | 26 | 0.0002 |
| DMS-OH |
|
TDVDHVFLRF-OH | 71 | 0.002 | 19 | † |
| Saline |
|
23 | † | 8 | † | |
Table 5.
The effects of DMS and an analog in the presence of protease inhibitor (PI) on adult gut (0.1 nM) and pupal heart (1 uM) are reported as mean values calculated as percent inhibition relative to the effect of DMS. The p values compare DMS, [6-10] DMS, or saline with versus without PI. The symbol † indicates p ≤ 0.00001.
| % Inhibition | ||||
|---|---|---|---|---|
| Adult gut | p value | Pupal heart | p value | |
| DMS | 100 | 100 | ||
| DMS + PI | 146 | 0.003 | 86 | 0.4 |
| [6-10]DMS | 78 | 103 | ||
| [6-10]DMS + PI | 124 | 0.004 | 100 | 0.8 |
| Saline | 20 | 0 | ||
| Saline + PI | 39 | 0.8 | 22 | 0.05 |
2.3 Bioassays
An adult gut assay was performed according to a previously reported protocol [3]. An in vivo larval gut assay was established. To measure the effect of substances on gut contractions wandering 3rd instar larvae were removed from media, washed, dried, and placed on double-sided sticky tape attached to a glass microscope slide. A micromanipulator was used to deliver 40 nl of experimental solution or saline, during and after which contractions were continuously recorded for a 10-minute time period. A minimum of 7 animals were analyzed for each developmental stage and each injectant. Gut contractions of each animal were recorded prior to application of the peptide, analog, or saline to yield basal gut motility.
Heart beat was observed in each developmental stage using a previously described microscope-based assay [12]. The rate of heart contractions for each animal was recorded prior to application of the peptide, analog, or saline to yield basal heart rate. Animals (n ≥ 7) were injected with 40 nl of solution using drawn out glass micropipettes; each animal was used only once. Injections were made anterior of the CNS and dorsal vessel to promote diffusion of injectant in the hemolymph and avoid damage to neural and cardiac tissues. During and after delivery of the injectant, contractions were continuously recorded for a 10-minute time period.
To test whether an inactive analog acted as an antagonist it was applied and the 30-second and 1-minute data points were recorded, at which time DMS was applied and the response was recorded for 10 minutes. Controls included saline applied in place of the inactive analog or DMS or both.
A cocktail of protease enzyme inhibitors was included at a final concentration of 1% to investigate whether its presence led to a difference in the activity of DMS or VFLRFamide on adult gut and pupal heart. Saline in the presence of 1% inhibitor cocktail was a control. The application and recording were done as described above for DMS, analog, or saline alone.
2.4 Data analysis
Data analyzed were the maximum responses of peptide, analogs, or saline within the 10-minute recording period reported as mean values ± S.E.M. Data in the dose-response curves and graphs are reported as % basal gut motility or heart rate where the frequency of contractions before application of peptide, analog, or saline is considered a measure of the baseline or basal value.Data in the tables are reported as % inhibition relative to the effect of DMS.
Dose-response curves were generated and effective concentration at half-maximal response (EC50) values were calculated using a non-linear regression (GraphPad Prism version 3.0). Activities of analogs and saline were compared to DMS using Single Factor ANOVA (Microsoft Excel) with significance at p value ≤ 0.01. In the presence of protease inhibitors, DMS, analogs, and saline were compared to injectant alone to calculate a p value.
2.5 Immunochemical localization
Indirect immunochemical localization was performed according to a protocol previously described [8, 11, 14]. Second antibodies included cyanine 3-labeled goat anti-rabbit antibody (Jackson ImmunoResearch Laboratory) and fluorescein isothiocyanate-labeled phalloidin (Invitrogen). Phalloidin, a toxin that binds actin, was used to stain tissue and enhancevisualization of the preparation. Fluorescent signals were imaged using a scanning confocal microscope. The z-series data were processed with Adobe Photoshop CS2.
3. Results
DMS dose response on gut motility and heart rate
Myosuppressins were first discovered based on their myoinhibitory effect on gut contractions and, subsequently, also found to decrease heart rate. The influence of DMS on the spontaneous contractions of adult D. melanogaster gut and pupal heart was measured to test our hypothesis that the peptide decreased muscle contractility.
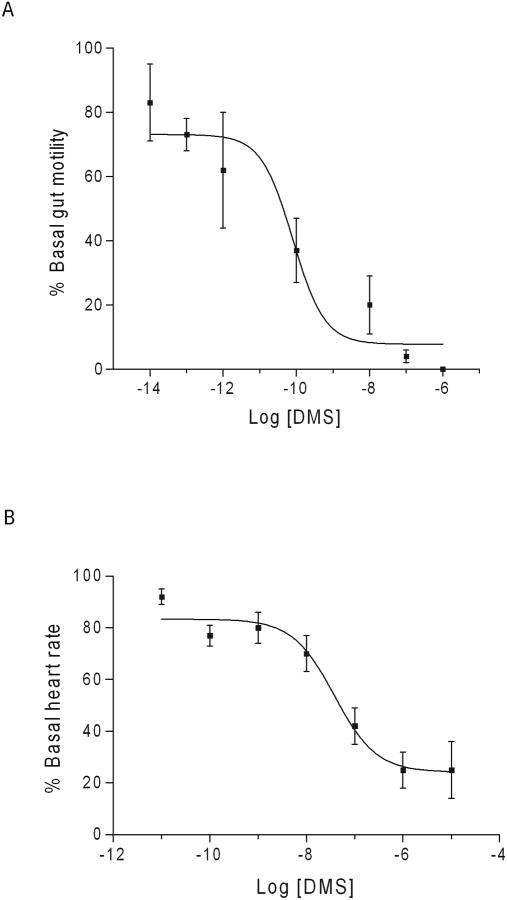
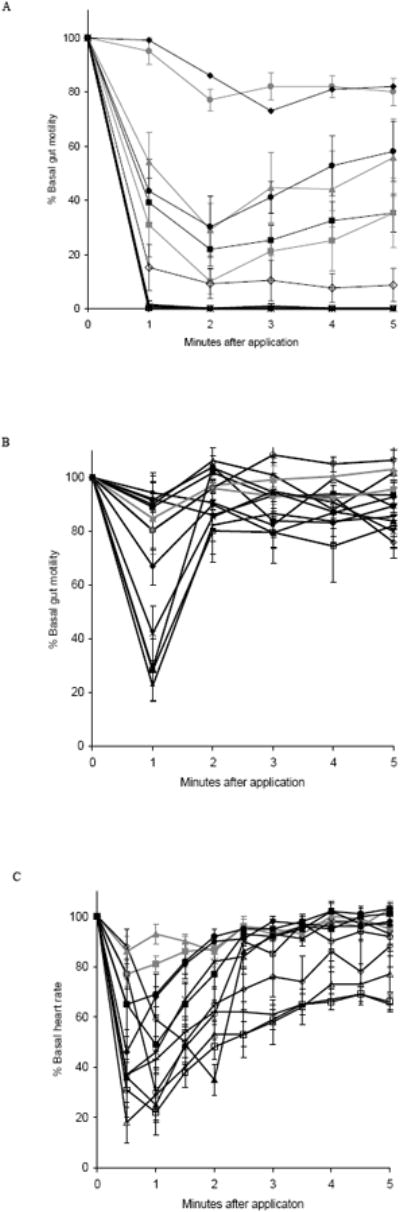
Dromyosuppressin was a potent inhibitory agent on adult gut contractions with an EC50 = 0.8 pM (Fig. 1A). DMS also decreased pupal heart contractions but with a decreased potency; EC50 = 30 nM (Fig. 1B). In addition to the magnitude of effectiveness, the response to the peptide over time was different in gut compared to heart (Fig. 2). Maximum effects typically occurred within 1 minute of peptide application. Saline, the carrier, did not significantly affect adult gut motility or pupal heart contractions (Fig. 2; Tables 1 and 2).
Fig. 1.
Dose-response curves of DMS on adult gut and pupal heart. The y-axis is DMS activity as percent basal (A) gut motility or (B) heart rate. The x-axis is log DMS concentration (molarity, M). Mean values ± S.E.M. of the maximal response are reported.
Fig. 2.
The effects of alanyl-substituted analogs are reported as mean values ± S.E.M. (A) Adult gut,
 DMS,
DMS,
 [A1]DMS,
[A1]DMS,
 [A2]DMS,
[A2]DMS,
 [A3]DMS,
[A3]DMS,
 [A6]DMS,
[A6]DMS,
 [A9]DMS,
[A9]DMS,
 [A4]DMS,
[A4]DMS,
 [A10]DMS,
[A10]DMS,
 [A5]DMS,
[A5]DMS,
 [A7]DMS,
[A7]DMS,
 DMS-OH,
DMS-OH,
 [A8]DMS,and
[A8]DMS,and
 saline, (B) larval gut, DMS,
saline, (B) larval gut, DMS,
 [A1]DMS,
[A1]DMS,
 [A6]DMS,
[A6]DMS,
 [A9]DMS,
[A9]DMS,
 [A8]DMS,
[A8]DMS,
 [A3]DMS,
[A3]DMS,
 [A10]DMS,
[A10]DMS,
 DMS-OH,
DMS-OH,
 [A5]DMS,
[A5]DMS,
 [A2]DMS,
[A2]DMS,
 [A4]DMS,
[A4]DMS,
 [A7]DMS, and
[A7]DMS, and
 saline and (C) pupal heart,
saline and (C) pupal heart,
 [A2]DMS,
[A2]DMS,
 [A1]DMS,
[A1]DMS,
 [A6]DMS,
[A6]DMS,
 DMS,
DMS,
 [A9]DMS,
[A9]DMS,
 [A8]DMS,
[A8]DMS,
 [A5]DMS,
[A5]DMS,
 [A7]DMS,
[A7]DMS,
 [A3]DMS,
[A3]DMS,
 [A10]DMS,
[A10]DMS,
 DMS-OH,
DMS-OH,
 [A4]DMS, and
[A4]DMS, and
 saline.
saline.
Table 2.
The effects of alanine-substituted analogs on adult, pupal, and larval heart are reported as mean values calculated as percent inhibition relative to the effect of DMS. The symbol † indicates p ≤ 0.00001.
| % Inhibition (heart) | |||||||
|---|---|---|---|---|---|---|---|
| Adult | p value | Pupal | p value | Larval | p value | ||
| DMS |
|
100 | 100 | 100 | |||
| [A1]DMS |
|
108 | 0.8 | 120 | 0.2 | 64 | 0.3 |
| [A2]DMS |
|
95 | 0.9 | 126 | 0.1 | 50 | 0.1 |
| [A3]DMS |
|
73 | 0.3 | 108 | 0.6 | 73 | 0.4 |
| [A4]DMS |
|
114 | 0.7 | 77 | 0.2 | 27 | 0.01 |
| [A5]DMS |
|
76 | 0.5 | 78 | 0.3 | 45 | 0.07 |
| [A6]DMS |
|
100 | 1 | 115 | 0.3 | 55 | 0.08 |
| [A7]DMS |
|
46 | 0.2 | 45 | 0.005 | 27 | 0.02 |
| [A8]DMS |
|
86 | 0.7 | 83 | 0.3 | 55 | 0.2 |
| [A9]DMS |
|
103 | 0.9 | 98 | 0.9 | 55 | 0.3 |
| [A10]DMS |
|
41 | 0.06 | 35 | 0.0002 | 77 | 0.6 |
| DMS-OH |
|
30 | 0.05 | 22 | † | 27 | 0.02 |
| Saline |
|
24 | 0.04 | 0 | † | 23 | 0.04 |
Structure-activity relationship elucidated for DMS on gut and heart
An aim of this study was to delineate SAR to identify amino acids critical for activity and an active core, the shortest length of residues not significantly different from the effect of DMS. A SAR provides data to identify antagonists for mechanistic and functional studies. Two sets of analogs, alanyl-substituted and N-terminal truncations, were chosen to provide independent but complementary SAR data. The importance of the C-terminal amide was examined by testing the free acid form of the peptide.
The influence of DMS was measured in two or more stages of the life cycle and gut and heart to gain insight into mechanisms underlying critical physiological processes on developmental- and tissue-specific levels. The effects of the analogs and saline were reported relative to DMS. Analogs were tested at 1uM because DMS is potent at this concentration (Fig. 1), thus, subtle responses, important in characterizing antagonists, would be detected.
Alanyl-substituted DMS analogs analyzed on gut and heart
Alanyl-substituted analogs were used to delineate SAR by replacing each residue, one by one, with alanine to investigate the contribution of the amino acid side chain to DMS activity in gut and heart (Fig. 2; Tables 1 and 2). Controls included the analysis of the un-substituted parent peptide and physiological saline, the carrier, without DMS or analog (Fig. 2, Tables 1 and 2).
In adult gut, the replacement of only one residue, leucine 8, (L8), produced an inactive analog, [A8] DMS. Substitution of all other residues resulted in analogs with reduced but prominent levels of activity at 70-100% inhibition (Fig. 2A; Table 1). The free acid analog retained activity to 71% inhibition compared to DMS. Application of saline was ineffective at 23% inhibition compared to DMS.
In larval gut, the alanine scan demonstrated numerous residues were important to activity. Substitution of D2, V3, D4, H5, F7, L8, and F10 produced analogs with significantly different responses compared to DMS at 32%, 26%, 10%, 14%, 10%, 42%, and 26% inhibition, respectively. The replacement of T1 and V6 generated analogs which mimicked DMS at 95% and 91% inhibition, respectively; [A9] DMS was effective at 74% inhibition (Fig. 2B; Table 1). The free acid analog and saline were significantly different from DMS at 19% and 8% inhibition, respectively.
To explore the tissue specificity of DMS SAR alanyl-substituted analogs were next tested on heart (Fig. 2C; Table 2). In adult heart, no individual residue was critical for the effect of DMS (Table 2). [A7] DMS and [A10] DMS did, however, show reduced effectiveness at 46% and 41% inhibition, respectively. Substitution of T1 and D4 resulted in enhanced activity at 108% and 114% inhibition. The free acid analog had a reduced effect compared to DMS at 30% inhibition. Saline was relatively ineffective on adult heart rate at 24% inhibition compared to DMS.
In pupal heart, replacement of F7 and F10 produced [A7] DMS and [A10] DMS with significantly different responses from DMS at 45% and 35% inhibition, respectively. Substitution of T1, D2, V3, and V6 generated analogs with enhanced activity at 120%, 126%, 108%, and 115% inhibition, respectively (Fig. 2C; Table 2). The free acid analog was significantly different from DMS at 22% inhibition. The effect of saline was significantly different from DMS at 0% inhibition.
In larval heart, replacement of only one residue, D4, resulted in a significantly different response from DMS at 27% inhibition (Table 2). The effect of no other analog was significantly different from DMS. Substitution of all other residues resulted in analogs with reduced but observable levels of activity at 27-77% inhibition. The free acid analog and saline were less active than DMS at 27% and 23% inhibition, respectively.
N-terminal DMS truncation analogs evaluated on gut and heart
The second approach to establish DMS SAR was to examine the peptide length required for DMS activity in gut and heart. N-terminal truncated analogs were generated by systematically removing a residue, one at a time. Analysis of N-terminal truncated analogs was the strategy taken because of the high degree of structure conservation in the C terminus of myosuppressins.
In adult gut, removing the first two amino acids produced [2-10] DMS and [3-10] DMS which did not significantly alter activity compared to DMS (Table 3). Truncation of the next residue, V3, decreased activity to 87% inhibition. Further trimming of the N terminus produced [5-10] DMS and [6-10] DMS which were significantly different from DMS at 49% and 54% inhibition, respectively. In larval gut, the influence of all N-terminal truncated analogs tested was significantly different from DMS (Table 3).
Table 3.
The effects of N-terminal truncated analogs on adult and larval gut are reported as mean values calculated as percent inhibition relative to the effect of DMS. The symbol † indicates p ≤ 0.00001.
| % Inhibition (gut) | ||||||
|---|---|---|---|---|---|---|
| Adult | p value | Larval | p value | |||
| DMS |
|
TDVDHVFLRFamide | 100 | 100 | ||
| [2-10]DMS |
|
DVDHVFLRFamide | 100 | 1 | 17 | † |
| [3-10]DMS |
|
VDHVFLRFamide | 100 | 1 | 29 | 0.0004 |
| [4-10]DMS |
|
DHVFLRFamide | 87 | 0.09 | 8 | † |
| [5-10]DMS |
|
HVFLRFamide | 49 | 0.0001 | 0 | † |
| [6-10]DMS |
|
VFLRFamide | 54 | † | 14 | † |
| Saline |
|
23 | † | 8 | † | |
In adult heart, the truncation of the first five residues produced analogs which were not significantly different from DMS (Table 4). However, the removal of an additional residue, V6, produced an analog which was significantly different from DMS at 14% inhibition.
Table 4.
The effects of N-terminal truncated analogs on adult, pupal, and larval heart are reported as mean values calculated as percent inhibition relative to the effect of DMS. The symbol † indicates p ≤ 0.00001.
| % Inhibition (heart) | |||||||
|---|---|---|---|---|---|---|---|
| Adult | p value | Pupal | p value | Larval | p value | ||
| DMS |
|
100 | 100 | 100 | |||
| [2-10]DMS |
|
86 | 0.7 | 126 | 0.1 | 127 | 0.4 |
| [3-10]DMS |
|
68 | 0.4 | 108 | 0.5 | 223 | 0.003 |
| [4-10]DMS |
|
81 | 0.5 | 137 | 0.08 | 150 | 0.3 |
| [5-10]DMS |
|
86 | 0.7 | 89 | 0.5 | 36 | 0.03 |
| [6-10]DMS |
|
65 | 0.4 | 103 | 0.8 | 14 | 0.01 |
| [7-10]DMS |
|
14 | 0.04 | 8 | † | 14 | 0.01 |
| Saline |
|
24 | 0.04 | 0 | † | 23 | 0.04 |
In pupal heart, [2-10] DMS, [3-10] DMS, [4-10] DMS, and [6-10] DMS were more effective than DMS at 126%, 108%, 137%, and 103% inhibition, respectively (Table 4). [5-10] DMS decreased activity at 89% inhibition compared to DMS. The sixth residue, V6, trimmed from the N terminus generated [7-10] DMS which was significantly different from DMS at 8% inhibition.
In larval heart, truncation of the first three residues produced [2-10] DMS, [3-10] DMS, and [4-10] DMS which were more effective than DMS at 127%, 223%, and 150% inhibition, respectively (Table 4). [5-10] DMS decreased activity at 36% inhibition compared to DMS. [6-10] DMS, and [7-10] DMS were each significantly different from DMS at 14% inhibition.
p-Benzoyl-phenylalanine-containing DMS analogs evaluated
To further investigate DMS SAR a modified amino acid replaced D2 and D4 in a tyrosyl-extended analog. In adult gut and pupal heart, Y-[Bpa2] DMS mimicked the un-substituted peptide (Fig. 3). In adult gut, the analog decreased motility with an EC50 = 0.01 pM (Fig. 3A). In pupal heart, the analog decreased rate with an EC50 = 0.01 nM (Fig. 3B). In adult heart, the analog was more effective than DMS with an EC50 = 10 uM (Fig. 3C).
Fig. 3.
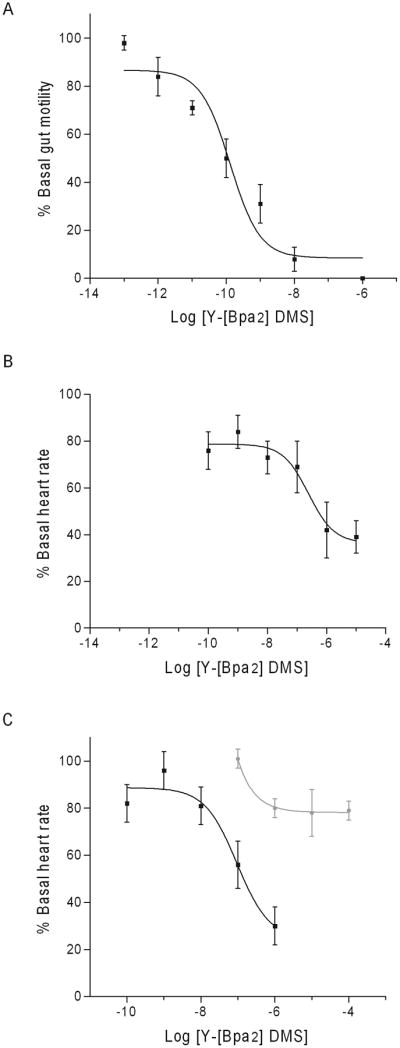
Dose-response curves of Y-[Bpa2]-DMS on (A) adult gut, (B) pupal heart, and (C) adult heart are reported as mean values ± S.E.M. of the maximal response. The y-axis is Y-[Bpa2]-DMS activity as percent basal (A) gut motility or (B and C) heart rate. The x-axis is log [Y-Bpa2]-DMS concentration (M). The effects of DMS on (C) adult heart are included (gray line).
In adult gut Y-[Bpa4] DMS decreased contractility to 53% inhibition, yet in heart the analog was inactive at 0% inhibition compared to DMS. In adult heart, applying Y-[Bpa4] DMS and peptide at equal concentration (10 nM) reduced DMS activity from 100% to 16% inhibition, which suggest the analog acted as an antagonist. In larval gut, Y-[Bpa4] DMS was active to 97% inhibition; however, Y-[Bpa2] DMS was inactive at 3% inhibition.
DMS analog delivered with protease inhibitors
A 1% protease inhibitor cocktail was delivered with an analog to investigate mechanisms. [6-10] DMS was used in this analysis because it decreased adult gut motility and pupal heart rate at 54% and 103% inhibition, respectively. In adult gut, DMS plus 1% protease inhibitor cocktail (DMS + PI) was significantly different from DMS alone, at 146% versus 100% inhibition, respectively (Table 5). Also, [6-10] DMS + PI was significantly different in adult gut from analog alone, 124% inhibition versus 78% inhibition (Table 5). Saline + PI was not significantly different from saline alone in adult gut (Table 5).
In pupal heart, DMS + PI was not significantly different from DMS at 86% versus 100% inhibition, respectively (Table 5). Also, [6-10] DMS + PI was not significantly different from [6-10] DMS at 100% versus 103% inhibition, respectively (Table 5). Saline + PI was not significantly different from saline alone (Table 5). Taken together, the inclusion of protease inhibitor cocktail may be interpreted to suggest that mechanisms associated with DMS are under developmental- or tissue-specific regulation.
Antisera detect DMS-specific immunoreactivity in gut and heart
Peptide-specific antisera [8, 11, 14] were used to identify cells and neuronal processes containing DMS immunoreactive material. Immunostaining provides complementary yet independent data on mechanisms involved in signaling. DMS was expressed in neurons from which immunoreactive fibers arborized impinging throughout the adult brain including the cerebrum, optic lobes, and subesophageal ganglion (Fig. 4A). Fibers were observed to project from DMS-stained cells to innervate the gut. The delivery of the peptide was consistent with the effect of DMS on gut motility, and indicative of the gut receiving neuronal input.
Fig. 4.
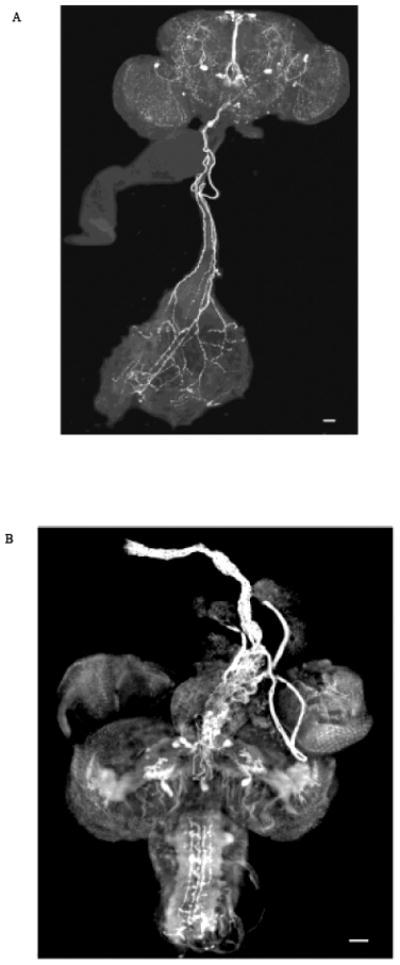
Staining of (A) adult CNS and gut and (B) pupal CNS and anterior dorsal vessel (aorta) identify immunoreactive material as fluorescent signal (white). DMS-specific antisera stained neurons in the (A) subesophageal ganglion and (B) protocerebrum which sent immunoreactive fibers to innervate the gut and aorta, respectively. The bars in the lower right-hand corner represent 50 μM.
Additionally, neurons in the superior protocerebrum were stained by DMS-specific antisera (Fig. 4B). Immunoreactive fibers projected from protocerebral neurons in the pupal brain to innervate the anterior dorsal vessel, the aorta. Thus, immunostaining data were consistent with cardiac function receiving input from unique cells in the brain providing further evidence that DMS activity is regulated on a tissue-specific level.
4. Discussion
Our results strongly support the conclusion DMS plays a role in muscle physiology, and its pleotropic effects on contractility involve mechanisms regulated on a developmental- and tissue-specific level. A detailed DMS SAR provided functional agonists and antagonists to gain insight into mechanisms regulating gut and heart contractions. DMS-specific antisera identified distinct brain cells from which immunoreactive fibers delivered the peptide to the gut and heart consistent with tissue-specific neuronal control of muscle contractility.
DMS influenced gut motility and heart rate dose dependently over a range of concentrations with physiological relevance, and analog activity was specific to adult, pupa, or larva and gut or heart. Individual single alanyl-substituted analogs were inactive, yet others were more potent than DMS (Tables 1 and 2). N-terminal truncated analogs showed increased activity (Tables 3 and 4) suggesting shorter peptide lengths may also be targets to increase potency. Previous studies of the relationship of structure to myosuppressin activity did not provide a complete profile of residues or peptide lengths required for activity in more than one stage of development or multiple tissues in one animal species [1, 9, 20]. Thus, there was no opportunity to investigate and compare mechanisms at different stages of the life cycle or in multiple tissues. The generation of analogs which act as developmental- or tissue-specific antagonists or analogs with increased potency may benefit understanding peptidergic control of muscle contractility.
The incorporation of a modified amino acid, Bpa, a phenylalanine analog, produced developmental- and tissue-specific antagonists and agonists with increased potency which was consistent with DMS SAR data obtained from alanyl-substituted and N-terminal truncation analogs. Analysis of the Bpa-containing analogs generated by replacing D2 and D4 supported other DMS SAR data; they indicated mechanisms were different in gut and heart, and adult and larva. Additionally, the position of the residue in the peptide sequence was critical for whether it played a role in activity or binding or both.
These results set the basis for an approach to probe myosuppressin receptor proteins because Bpa is a photoactivatable cross-linker [6] and the tyrosyl extension provides a site for labeling. Labeled Y-SchistoFLRFamide, a locust myosuppressin analog, suggested two receptors are associated with oviduct [19] which is in agreement with genome database mining that identified two DMSRs [4]. The existence of two GPCR proteins to which DMS binds is consistent with the presence of multiple signaling pathways which may contribute to the observed developmental- and tissue-specific mechanisms.
Analogs containing modified amino acids may also provide protection from peptidases. Peptide-based drugs delivered intravenously or orally are susceptible to proteolysis. An initialforay into reducing proteolysis employing inhibitors was consistent with the presence of mechanisms specific to development and unique to different tissues. However, the use of protease inhibitors to limit proteolysis is unattractive due to lack of specificity and cost, thus, resulting in harmful side effects. Development of a peptide-based drug typically incorporates modified residues or non-peptidyl moieties to address degradation in the blood stream or gastrointestinal tract.
An animal receives numerous physiological and environmental inputs which impact the requirements for nutrient absorption and circulation during its life cycle. Probing tissue with peptide-specific antisera provided an independent but complementary approach to explore mechanisms involved in myosuppressin control of muscle contractility relevant to gut and heart physiology. DMS-specific antisera stained brain cells from which immunoreactive processes projected to uniquely innervate the gastrointestinal tract or the cardiovascular system showing gut motility and heart rate received independent neural input. Previously, we probed the blowfly nervous system with myosuppressin antisera and found the peptide was delivered from the CNS to the heart [1]. Also, we found blowfly gut was innervated by myosuppressin immunoreactive processes which originated in the CNS [16]. Thus, our results are in agreement with and expand past studies to provide independent and complementary data to support the conclusion myosuppressin acts through developmental- and tissue-specific mechanisms under neural regulation to play a role in gut and cardiovascular physiology. Knowledge of the mechanisms underlying the pleotropic effects of myosuppressin on muscle contractility may pave the way to identify targets and lead to the development of strategies to address related diseases.
Highlights.
DMS decreased gut motility and cardiac contractility dose dependently.
Truncated and Y-[Bpa2] DMS analogs were increased agonists in pupal and adult heart.
Adult, pupal, and larval heart and gut active cores were developmental- and tissue specific.
Y-[Bpax] DMS activity and binding differed on a developmental- and tissue-specific level.
DMS-specific antisera stained unique cells and fibers that innervated gut or heart.
Acknowledgments
This research was supported by National Institutes of Health funding R21HL093627, Concept to Commercialization Funding from the University of Michigan Medical Innovation Center, and a University of Michigan Cardiovascular Center Innovative Grant to RN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angioy AM, Muroni P, Barbarossa IT, McCormick J, Nichols R. Evidence dromyosuppressin acts at posterior and anterior pacemakers to decrease the fast and the slow cardiac activity in the blowfly Protophormia terraenovae. Peptides. 2007;28:585–93. doi: 10.1016/j.peptides.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dockray GJ, Reeve JR, Jr, Shively J, Gayton RJ, Barnard CS. A novel active pentapeptide from chicken brain identified by antibodies to FMRFamide. Nature. 1983;305:328–30. doi: 10.1038/305328a0. [DOI] [PubMed] [Google Scholar]
- 3.Duttlinger A, Berry K, Nichols R. The different effects of three Drosophila melanogaster dFMRFamide-containing peptides on crop contractions suggest these structurally related peptides do not play redundant functions in gut. Peptides. 2002;23:1953–7. doi: 10.1016/s0196-9781(02)00179-1. [DOI] [PubMed] [Google Scholar]
- 4.Egerod K, Reynisson E, Hauser F, Cazzamali G, Williamson M, Grimmelikhuijzen CJ. Molecular cloning and functional expression of the first two specific insect myosuppressin receptors. Proc Natl Acad Sci USA. 2003;100:9808–13. doi: 10.1073/pnas.1632197100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman GM, Cook BJ, Nachman RJ. Primary structure and synthesis of a blocked myotropic neuropeptide isolated from the cockroach, Leucophaea maderae. Comp Biochem Physiol C. 1986;85:219–24. doi: 10.1016/0742-8413(86)90077-0. [DOI] [PubMed] [Google Scholar]
- 6.Kauer JC, Erickson-Viitanen S, Wolfe HR, Jr, DeGrado WF. p-Benzoyl-L-phenylalanine, a new photoreactive amino acid. Photolabeling of calmodulin with a synthetic calmodulin-binding peptide. J Biol Chem. 1986;261:10695–700. [PubMed] [Google Scholar]
- 7.Marciniak P, Audsley N, Kuczer M, Rosinski G. Identification of myotropic neuropeptides from the brain and corpus cardiacum-corpus allatum complex of the beetle, Zophobas atratus. J Insect Sci. 2010;10:1–19. doi: 10.1673/031.010.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormick J, Nichols R. Spatial and temporal expression identify dromyosuppressin as a brain-gut peptide in Drosophila melanogaster. J Comp Neurol. 1993;338:278–88. doi: 10.1002/cne.903380210. [DOI] [PubMed] [Google Scholar]
- 9.Nachman RJ, Holman GM, Hayes TK, Beier RC. Structure-activity relationships for inhibitory insect myosuppressins: contrast with the stimulatory sulfakinins. Peptides. 1993;14:665–70. doi: 10.1016/0196-9781(93)90095-x. [DOI] [PubMed] [Google Scholar]
- 10.Nichols R. Isolation and structural characterization of Drosophila TDVDHVFLRFamide and FMRFamide-containing neural peptides. J Mol Neurosci. 1992;3:213–8. doi: 10.1007/BF03380141. [DOI] [PubMed] [Google Scholar]
- 11.Nichols R, McCormick J, Lim I. Multiple antigenic peptides designed to structurally-related Drosophila peptides. Peptides. 1997;18:41–5. doi: 10.1016/s0196-9781(96)00279-3. [DOI] [PubMed] [Google Scholar]
- 12.Nichols R, McCormick J, Cohen M, Howe E, Jean C, Paisley K, et al. Differential processing of neuropeptides influences Drosophila heart rate. J Neurogenet. 1999;13:89–104. doi: 10.3109/01677069909083468. [DOI] [PubMed] [Google Scholar]
- 13.Nichols R, Demers LA, Larsen BM, Robinson D, Converso K, Russell MW, et al. Human RFamide-related peptide-1 diminishes cellular and integrated cardiac contractile performance. Peptides. 2010;31:2067–74. doi: 10.1016/j.peptides.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posnett DN, Tam JP. Multiple antigenic peptide method for producing antipeptide site-specific antibodies. In: Langone JJ, editor. Methods in Enzymology. San Diego: Academic Press; 1989. pp. 739–746. [DOI] [PubMed] [Google Scholar]
- 15.Price DA, Greenberg MJ. Structure of a molluscan neuropeptide. Science. 1977;197:670–1. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- 16.Richer S, Stoffolano JG, Jr, Yin CM, Nichols R. Innervation of dromyosuppressin (DMS) immunoreactive processes and effect of DMS and benzethonium chloride on the Phormia regina (Meigen) crop. J Comp Neurol. 2000;421:136–42. [PubMed] [Google Scholar]
- 17.Robb S, Packman LC, Evans PD. Isolation, primary structure and bioactivity of SchistoFLRF-amide, a FMRF-amide-like neuropeptide from the locust, Schistocerca gregaria. Biochem Biophys Res Comm. 1989;160:850–6. doi: 10.1016/0006-291x(89)92512-6. [DOI] [PubMed] [Google Scholar]
- 18.Stevens JS, Cashman CR, Smith CM, Beale KM, Towle DW, Christie AE, et al. The peptide hormone pQDLDHVFLRFamide (crustacean myosuppressin) modulates the Homarus americanus cardiac neuromuscular system at multiple sites. J Exp Biol. 2009;212:3961–76. doi: 10.1242/jeb.035741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Orchard I, Lange AB. Identification and characterization of two receptors for SchistoFLRFamide on locust oviduct. Peptides. 1994;15:875–82. doi: 10.1016/0196-9781(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Orchard I, Lange AB. Binding affinity and physiological activity of some HVFLRFamide analogues on the oviducts of the locust, Locusta migratoria. Regul Pept. 1995;57:339–46. doi: 10.1016/0167-0115(95)00047-F. [DOI] [PubMed] [Google Scholar]