Introduction
Narcolepsy is a common cause of chronic sleepiness and is often accompanied by symptoms that include odd mixtures of sleep and wakefulness. A patient of ours is an intelligent and highly motivated young woman who developed unrelenting sleepiness during law school. No matter how much she slept at night, she struggled to stay awake while studying and her grades began to slip. One night when dozing off, she was certain that she heard someone breaking into her apartment, but after a few minutes, she realized it was a vivid, dream-like hallucination. A few weeks later, while joking with a friend, she suddenly slumped face down on her desk; she was fully conscious but unable to move for approximately a minute. Her story is quite typical, and now, even with a variety of medications, her day-to-day life is much harder than it used to be.
As in this young woman, all individuals with narcolepsy experience persistent daytime sleepiness. They may feel rested upon awakening, but most of their day is disrupted by moderate to severe sleepiness that causes them to doze off at inappropriate times and interferes with their ability to remain attentive in school, at work, and when driving. In addition, people with narcolepsy usually have a variety of other symptoms including sleep paralysis (paralysis for approximately a minute upon awakening), hypnagogic hallucinations (vivid and sometimes frightening hallucinations at the beginning or end of sleep), and cataplexy (sudden episodes of emotionally triggered muscle weakness). Typically beginning in adolescence, narcolepsy is common, affecting ∼1 in 2000 people. Excessive daytime sleepiness is usually the first symptom, with cataplexy and other phenomena developing over the next few months and persisting for life.
For over 100 years, clinicians have recognized narcolepsy (Westphal, 1877; Gelineau, 1880; Schenck et al., 2007), but only in the last decade have neuroscientists been able to shed light on its true cause and underlying neurobiology. The goals of this review are to describe briefly the symptoms, etiology, and management of narcolepsy, and then review the underlying neurobiology and important directions for future research.
Etiology
Toward the end of World War I, an epidemic of encephalitis swept across Europe. In many patients, this caused crushing sleepiness, and the Austrian neurologist Constantin von Economo found that these patients usually had inflammation and injury to the posterior hypothalamus (von Economo, 1930). He went on to speculate that the sleepiness of narcolepsy might be caused by injury to this region, but for decades this hypothesis could not be tested as so little was understood about the cells and functions of the hypothalamus. In 1998, two labs independently discovered a pair of hypothalamic neuropeptides termed orexin-A and -B (or hypocretin 1 and 2) and their receptors (OX1 and OX2) (de Lecea et al., 1998; Sakurai et al., 1998). The orexins have since been demonstrated to play essential roles in maintaining wakefulness and regulating transitions between sleep and wake (Chemelli et al., 1999; Mochizuki et al., 2004; Adamantidis et al., 2007; Diniz Behn et al., 2010; Sasaki et al., 2011). The following year, another pair of research teams found compelling evidence that narcolepsy can be caused by a loss of orexin signaling. Masashi Yanagisawa's group produced an orexin ligand knock-out mouse with sleepiness and cataplexy strikingly similar to human narcolepsy (Chemelli et al., 1999). Simultaneously, Emmanuel Mignot's group demonstrated that canine narcolepsy resulted from a mutated orexin receptor (Lin et al., 1999). The definitive link between narcolepsy and orexin followed soon after when researchers demonstrated a lack of orexin peptides in the hypothalami and CSF of narcolepsy patients (Peyron et al., 2000; Thannickal et al., 2000; Mignot et al., 2002).
Further research has demonstrated that ∼90% of the orexin-producing neurons are lost in human narcolepsy with cataplexy. The endogenous opiate dynorphin and NARP (a protein involved in glutamate signaling) are also produced by the orexin neurons, and both of these markers are absent in the lateral hypothalamus of patients with narcolepsy (Blouin et al., 2005; Crocker et al., 2005). This cell loss seems highly selective, as neurons producing melanin-concentrating hormone, which are intermingled with the orexin neurons, seem completely unaffected (Peyron et al., 2000; Thannickal et al., 2000). Collectively, these studies provide strong evidence that some process selectively destroys the orexin neurons.
These studies focused on patients that have narcolepsy with cataplexy, yet much less is understood about the neuropathology of narcolepsy without cataplexy. This type of narcolepsy affects approximately half of all patients with narcolepsy, and the severity of symptoms is often less than in patients with cataplexy (Sasai et al., 2009). Though little is known about the underlying neuropathology, narcolepsy without cataplexy may simply be caused by less severe injury to the orexin neurons (Thannickal et al., 2009), resulting in mainly sleepiness and a small reduction in CSF orexin level (Mignot et al., 2002; Andlauer et al., 2012). Mild to moderate loss of the orexin neurons has also been demonstrated in Parkinson's disease (Fronczek et al., 2007, 2009; Thannickal et al., 2007) and traumatic brain injury (Baumann et al., 2009), disorders that often produce sleepiness but no cataplexy.
In addition to controlling sleep/wake states, the orexin neurons also regulate metabolism, feeding, reward, and autonomic tone (Aston-Jones et al., 2010; Cason et al., 2010; Dimitrova et al., 2011), resulting in additional symptoms. For example, weight gain is common at the onset of narcolepsy, especially in children, perhaps from a reduction in basal metabolic rate (Plazzi et al., 2006; Sonka et al., 2010). Mice lacking orexins have a decreased tendency for addiction (DiLeone et al., 2003; Smith and Aston-Jones, 2012), but whether this occurs in people with narcolepsy is not yet clear (Dimitrova et al., 2011). This review will focus on the primary symptoms of narcolepsy, sleepiness and cataplexy, though numerous reviews discuss other roles for the orexin system (Aston-Jones et al., 2008; Mieda and Sakurai, 2009; Sakurai and Mieda, 2011; Sinton, 2011; Nixon et al., 2012).
Autoimmune hypothesis
Human leukocyte antigens (HLA) are linked to many autoimmune diseases, and narcolepsy has the strongest known HLA association. HLA DQB1*0602 is found in ∼90% of patients with narcolepsy, and simply carrying this gene increases the risk of narcolepsy ∼200-fold (Mignot et al., 1993, 1994). This striking association has led many researchers to speculate that an autoimmune process kills the orexin neurons.
Several observations support the autoimmune hypothesis. Narcolepsy is linked to polymorphisms in the T-cell receptor α gene that may alter immune responses to some antigens (Hallmayer et al., 2009). Many patients also report that their narcolepsy began soon after strep throat or another infection, and levels of antibodies targeted at streptococcus bacteria are often elevated in the months after the onset of narcolepsy, suggesting that immune system activation may trigger an attack on the orexin neurons (Aran et al., 2010). In addition, antibodies against tribbles homolog 2, a protein found in many cell types including the orexin neurons, are sometimes elevated in narcolepsy (Cvetkovic-Lopes et al., 2010; Kawashima et al., 2010; Toyoda et al., 2010). Just recently, there was a 12-fold increase in new cases of narcolepsy in children (all with DQB1*0602) in Finland and Sweden inoculated with a brand of H1N1 influenza vaccine that contained a potent adjuvant intended to produce vigorous immune responses (Nohynek et al., 2012; Partinen et al., 2012). These observations suggest that in genetically susceptible individuals, an immunologic stimulus may trigger an immune response that also kills off the orexin neurons.
The autoimmune hypothesis still has several weaknesses. Researchers have not yet identified a key target antigen or humoral or cellular mechanisms that could attack the orexin neurons. MRI scans and analysis of CSF have not shown evidence of brain inflammation. Though some patients have improved with intravenous immune globulins (an immune system modulator) (Dauvilliers et al., 2004), the response is inconsistent, and case reports of other treatments such as high-dose corticosteroids have shown little benefit (Hecht et al., 2003). Though less likely, it is still possible that the orexin neurons are killed by a completely different mechanism such as selective neurodegeneration or a neurotropic virus. Clearly, much more needs to be done to determine whether an autoimmune process kills the orexin neurons, and if so, to discover how that process can be altered.
Treatment
Narcolepsy has generally been treated with a combination of stimulants for excessive daytime sleepiness and antidepressants for cataplexy (Table 1) (Black and Guilleminault, 2001; Mignot and Nishino, 2005). Monoamine neurotransmitters, especially dopamine, promote arousal, while some such as norepinephrine and serotonin suppress cataplexy. Stimulants (e.g., dextroamphetamine, methylphenidate) improve sleepiness by enhancing release and decreasing reuptake of dopamine and other monoamine neurotransmitters (Kuczenski and Segal, 1997; Nishino et al., 1998; Kanbayashi et al., 2000; Wisor et al., 2001; Leonard et al., 2004). Modafinil has some similarities with traditional stimulants and may be a more selective dopamine reuptake blocker (Scammell et al., 2000; Wisor and Eriksson, 2005; Golicki et al., 2010). Antidepressants, such as venlafaxine and clomipramine, are often effective at reducing cataplexy, likely by blocking reuptake of norepinephrine (Schachter and Parkes, 1980; Nishino and Mignot, 1997).
Table 1.
Medications that reduce sleepiness and cataplexy in narcolepsy
| Human | Dog | Mouse | |
|---|---|---|---|
| Antidepressants (e.g. venlafaxine, clomipramine) | ↓Cataplexy | ↓Cataplexy | ↓Cataplexy |
| Stimulants (e.g. methylphenidate, modafinil) | ↓Sleepiness | ↓Sleepiness | ↓Sleepiness |
| Gamma-hydroxybutyrate (e.g. sodium oxybate) | ↓Sleepiness | ND | ND |
| ↓Cataplexy | |||
| Histamine H3 receptor inverse agonist (e.g. tiprolisant) | ↓Sleepiness | ND | ↓Sleepiness |
| ↓Cataplexy | |||
| Orexin-A* | ND | ↓Sleepiness | ↓Sleepiness |
| ↓Cataplexy | ↓Cataplexy |
ND, Not determined.
*Given intracerebroventricularly or intravenously. Downward-pointing arrows indicate that the symptoms were reduced (Babcock et al., 1976; Schachter and Parkes, 1980; Shelton et al., 1995; Scammell and Matheson, 1998; Fujiki et al., 2003; Willie et al., 2003, 2005; Mieda et al., 2004; Lin et al., 2008; Golicki et al., 2010; Lammers et al., 2010).
Sodium oxybate, the sodium salt gamma hydroxybutyrate, is also quite effective for treating sleepiness and cataplexy (Boscolo-Berto et al., 2011). Sodium oxybate is given at bedtime and promotes deep non-REM sleep, probably through activation of GABAB receptors (Vienne et al., 2010). After several weeks, sleepiness and cataplexy often improve, but the mechanisms underlying this slow improvement are unknown.
Several drug companies are now developing histamine H3 receptor inverse agonists as a new method for increasing arousal. H3 receptors are inhibitory autoreceptors that reduce activity in neurons that make histamine and other wake-promoting monoamines (Parmentier et al., 2007). Thus, H3 inverse-agonists increase activity in monoamine neurons and reduce sleepiness in people, dogs, and mice with narcolepsy (Guo et al., 2009; Inocente et al., 2012). Clinical trials are now underway to establish the efficacy and safety of these drugs in treating sleepiness and cataplexy.
Since narcolepsy results from selective loss of the orexin neurons, restoration of orexin signaling should be a highly effective and targeted treatment. The orexin peptides are relatively large, and only a small amount of orexin-A can cross the blood–brain barrier (Kastin and Akerstrom, 1999). Injection of orexin-A into the lateral ventricles of narcoleptic mice improves wakefulness and reduces cataplexy (Mieda et al., 2004), and both intravenous and nasal delivery of orexin-A to nonhuman primates alleviate performance deficits after sleep deprivation (Deadwyler et al., 2007). These approaches may not be practical for most patients, but the results of these studies provide good proof of concept. As an alternative, orexin gene therapy has been used to induce expression of orexin peptides in a variety of neurons in or near the hypothalamus in mice and has resulted in reductions in both sleepiness and cataplexy (Liu et al., 2008, 2011). Still, it may be years before gene therapy is considered safe enough for use in human narcolepsy. Ideally, narcolepsy would be treated with a small molecule orexin agonist, and though pharmacologically challenging, this is now a goal for several labs. This would be a great accomplishment as narcolepsy is remarkably simple when compared with other neurological disorders: sleepiness, cataplexy, and the other symptoms are likely all due to a loss of orexin signaling. Thus, there is real hope that the symptoms of narcolepsy could be dramatically improved by restoring orexin signaling.
Animal models
Researchers have now studied several engineered and naturally occurring animal models of narcolepsy (Chen et al., 2009; Scammell et al., 2009). These models all have good face validity (they demonstrate sleepiness and cataplexy), predictive validity (medications for human narcolepsy reduce symptoms in animal models), and construct validity (lack of a functional orexin system). Dogs with autosomal recessive narcolepsy have a mutation in the gene coding for the orexin OX2 receptor, resulting in sleepiness and severe cataplexy elicited by social interaction and palatable food (Mitler et al., 1974, 1976; Mitler, 1975; Lin et al., 1999). In mice, sleepiness and varying degrees of cataplexy occur in models lacking the orexin neuropeptides, the orexin receptors, or the orexin neurons (Chemelli et al., 1999; Hara et al., 2001; Willie et al., 2003; Mochizuki et al., 2004, 2011). In general, mice fully lacking the orexin peptides or both receptors have more severe symptoms, while mice lacking either the OX1 or OX2 receptor have milder phenotypes (Willie et al., 2003). The great advantage of these mouse models is that they enable researchers to examine the fundamental neurobiology of narcolepsy.
Neurobiology of sleepiness
Excessive daytime sleepiness is a defining feature of narcolepsy, and researchers have considered several possible explanations for this frequently debilitating symptom. Prolonged periods of wakefulness increase homeostatic sleep drive, and it is possible that people with narcolepsy have higher sleep drive than normal. However, this seems unlikely as the total amounts of sleep in people and in mice with narcolepsy are essentially normal, as are their responses to sleep deprivation (Tafti et al., 1992; Besset et al., 1994; Mochizuki et al., 2004). Circadian timing signals help promote wakefulness during the day, and another explanation for excessive sleepiness is that these signals might be less effective in narcolepsy. This too seems unlikely, as the fundamental circadian rhythms of narcoleptic mice and people are close to normal (Dantz et al., 1994; Mochizuki et al., 2004). Many people with narcolepsy have fragmented sleep, suggesting a third explanation that poor sleep at night could cause sleepiness during the following day. In fact, sodium oxybate was first used in narcolepsy simply to improve sleep quality (Mamelak et al., 2004). However, although sodium oxybate immediately improves sleep, the improvements in daytime alertness may not become apparent until weeks later (Boscolo-Berto et al., 2011). In addition, many patients with narcolepsy feel well rested upon awakening, and daytime sleepiness in narcolepsy does not correlate with the quality of nighttime sleep (Sturzenegger and Bassetti, 2004).
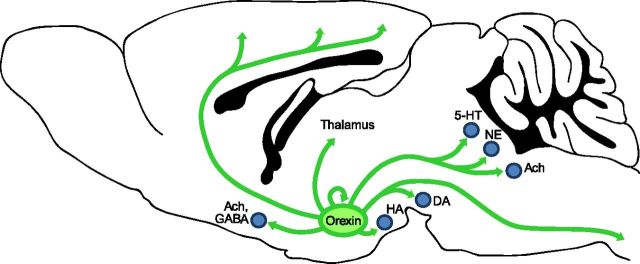
Sleep state instability, with low thresholds to transition between wake and sleep, may provide the best explanation for the sleepiness of narcolepsy (Mochizuki et al., 2004). The orexin neurons innervate and excite many of the key wake-promoting systems, including noradrenergic neurons of the locus ceruleus, serotonergic neurons of the dorsal raphe, histaminergic neurons of the tuberomammillary nucleus, and cholinergic neurons in the basal forebrain and pons (Fig. 1) (Peyron et al., 1998; Horvath et al., 1999; Eggermann et al., 2001). These regions not only powerfully promote wakefulness, they also inhibit sleep-promoting systems. In addition, the orexin neurons may be autoexcitatory (Li et al., 2002), and once they become active during the waking period they may remain active, leading to sustained excitation of the other wake-promoting systems. Conversely, in the absence of orexins, the wake-promoting neurons may not receive adequate or consistent excitatory drive, leading to reduced arousal, disinhibition of sleep-promoting pathways, and inappropriate transitions into sleep.
Figure 1.
Orexin neurons project throughout the brain to promote and maintain wakefulness. Orexin neurons in the lateral hypothalamus project to the major arousal-promoting nuclei, including neurons producing histamine (HA; tuberomammillary nucleus), norepinephrine (NE; e.g., locus ceruleus), serotonin (5-HT; e.g., dorsal raphe), dopamine (DA; e.g., ventral tegmental area), and acetylcholine (Ach; e.g., basal forebrain, pedunculopontine and laterodorsal tegmental nuclei). The orexin neurons provide direct, excitatory inputs to the cortex, thalamus, and spinal cord. In addition, the orexin neurons may be autoexcitatory.
Neurobiology of cataplexy
Cataplexy is sudden muscle weakness often triggered by strong emotions. The loss of muscle tone can be partial, affecting just the face and neck, or complete, resulting in full postural collapse. An episode of cataplexy usually lasts from a few seconds up to 1 or 2 min, and during this time consciousness is fully preserved. One of the most striking aspects of cataplexy is that it is often triggered by positive emotions, such as those associated with laughter or telling a joke (Overeem et al., 2011). Cataplexy may be a severe form of “feeling weak with laughter” or an atavistic expression of tonic immobility, a reflex akin to feigned death or “playing possum” (Overeem et al., 1999, 2002).
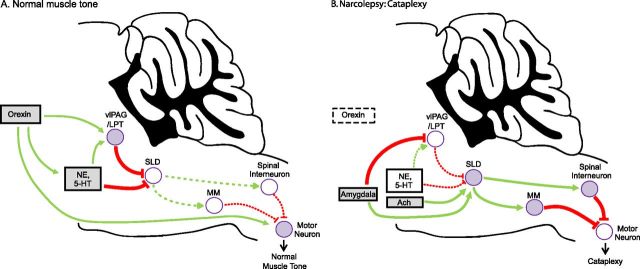
When an individual is in REM sleep, nearly all skeletal muscles (except those involved in respiration and eye movements) are paralyzed. This is called REM sleep atonia, and similar mechanisms may cause the muscle paralysis of cataplexy (Fig. 2). During REM sleep, motor neurons are strongly inhibited by GABAergic and glycinergic neurons in the spinal cord and medial medulla (Soja et al., 1987; Kodama et al., 2003; Brooks and Peever, 2008). These inhibitory premotor neurons are activated by glutamatergic neurons in the sublaterodorsal nucleus (SLD; just ventral to the locus ceruleus) (Boissard et al., 2002). Lesions of these pathways in animals can produce REM sleep without atonia, and similar injuries may cause REM sleep behavior disorder in humans, in which REM sleep paralysis fails, and patients act out their dreams (Lu et al., 2006; Boeve et al., 2007). Normally, during wakefulness, these atonia-producing pathways are held in check by norepinephrine, serotonin, and GABAergic neurons of the ventrolateral periaqueductal gray and adjacent lateral pontine tegmentum (vlPAG/LPT) (Boissard et al., 2002; Lu et al., 2006). During cataplexy, noradrenergic and serotonergic neuron activity is suppressed, permitting atonia, but the wake-promoting, histaminergic neurons of the tuberomammillary nucleus remain active, helping to preserve consciousness (Wu et al., 1999, 2004; John et al., 2004). In addition, the orexin peptides may prevent atonia by directly exciting neurons at multiple levels of this system, including those in the vlPAG/LPT, monoaminergic regions, and motor neurons (Horvath et al., 1999; Peever et al., 2003; Yamuy et al., 2004; Lu et al., 2006).
Figure 2.
Atonia pathways triggering cataplexy. A, Several pathways suppress atonia during normal wakefulness. Atonia is driven by neurons in the SLD that activate neurons in the spinal cord and medial medulla (MM) that inhibit motor neurons using GABA and glycine. During wakefulness, this atonia system is inhibited by neurons in the vlPAG/LPT and by monoaminergic neurons [e.g., norepinephrine (NE) and serotonin (5-HT)]. The orexin neurons are active during wake and they help maintain normal muscle tone by exciting neurons in the vlPAG/LPT, monoamine neurons, and motor neurons. B, In narcolepsy, loss of the orexin neurons plus strong, positive emotions can trigger cataplexy. Positive emotions may activate neurons in the amygdala that excite the SLD and inhibit the vlPAG/LPT. The SLD may also be activated by cholinergic inputs and a sudden withdrawal of monoamine tone. The SLD then excites neurons in the medial medulla and spinal cord that strongly hyperpolarize motor neurons, resulting in cataplexy. Normally, the many effects of the orexin system and a continued monoaminergic drive to the pons and directly to motor neurons would counter this triggering of atonia, but in the absence of orexins, these excitatory drives are lost and cataplexy occurs. Solid pathways from filled nuclei are active; dashed pathways from unfilled nuclei are inactive. Green pathways are excitatory; red pathways are inhibitory. Ach, Acetylcholine.
How might positive emotions activate these atonia pathways to produce cataplexy? The central nucleus of the amygdala sends excitatory projections to the SLD and inhibitory projections to the vlPAG/LPT (Boissard et al., 2003; Fung et al., 2011; Xi et al., 2011). Perhaps strong, positive emotions activate these limbic pathways, increasing the likelihood of atonia. In healthy individuals, this would be offset by the atonia-suppressing effects of the orexin peptides, resulting in no more than a fleeting sense of mild weakness. However, in people with narcolepsy, these emotional signals would be unopposed, resulting in sustained activation of the SLD and downstream pathways that lead to paralysis.
The models presented here provide thorough but simplistic explanations for the neural pathways that regulate sleepiness and cataplexy. They highlight the many important roles of the orexin system, but much more work is needed to sort out the details and essential elements.
Future directions
The orexin neurons innervate a variety of nuclei in the brain and spinal cord, but it remains unclear which of these pathways are necessary for stabilizing wakefulness and muscle tone. Recent studies using optogenetics and Designer Receptors Exclusively Activated by Designer Drugs have demonstrated the importance of the orexin system in promoting arousal (Adamantidis et al., 2007; Sasaki et al., 2011; Tsunematsu et al., 2011). Hopefully, the next generation of studies will map out the key targets, by stimulating or inhibiting orexin nerve terminals in key brain regions. Another approach will be to focally rescue orexin signaling. For example, we recently found that restoring orexin signaling to the tuberomammillary region can fully rescue the sleepiness of mice lacking the OX2 receptor (Mochizuki et al., 2011). This method nicely demonstrates which pathways are sufficient to rescue a behavior, and ongoing studies in other labs to focally disrupt orexin signaling should be able to define which pathways are necessary for regulating cataplexy and the other symptoms of narcolepsy.
Few studies have investigated the role of forebrain and limbic structures in regulating cataplexy. Just like in people with narcolepsy, emotional stimuli seem to trigger cataplexy in animal models: narcoleptic dogs have marked increases in cataplexy when playing or presented with highly palatable food (Baker et al., 1982; Siegel et al., 1989) and narcoleptic mice have increased cataplexy with social interaction, palatable food, or running wheels (España et al., 2007; Clark et al., 2009; Scammell et al., 2009). In addition, a population of amygdala neurons fire at increased rates during cataplexy in narcoleptic dogs (Gulyani et al., 2002), but no experiments have tested whether the amygdala or other limbic structures are necessary or sufficient for cataplexy. These studies would provide novel insights into the mechanisms that trigger cataplexy and would probably also provide useful information on limbic pathways that underlie positive affect.
The discovery of the orexin system sparked a surge of research that has substantially improved our understanding of narcolepsy, yet there remain many important and unanswered questions. How do positive emotions trigger cataplexy? Can we better understand the mechanisms of narcolepsy and the drugs used in its treatment to develop better and safer therapies? Can an effective orexin agonist be developed? Is narcolepsy an autoimmune disorder? If so, can we halt or reverse the process that kills the orexin neurons? These future studies will shed light not only on narcolepsy, but also on the many roles of the orexin system in normal brain function.
For additional information on narcolepsy, see Table 2.
Table 2.
Sources of additional information
| Narcolepsy Fact Sheet from NINDS: http://www.ninds.nih.gov/disorders/narcolepsy/detail_narcolepsy.htm |
| Narcolepsy Network: http://www.narcolepsynetwork.org/ |
| Wake Up Narcolepsy: http://www.wakeupnarcolepsy.org/ |
Footnotes
Editor's Note: Disease Focus articles provide brief overviews of a neural disease or syndrome, emphasizing potential links to basic neural mechanisms. They are presented in the hope of helping researchers identify clinical implications of their research. For more information, see http://www.jneurosci.org/misc/ifa_minireviews.dtl.
This work was supported by NIH Grants NS055367 and HL095491 (to T.E.S.) and the Natural Sciences and Engineering Research Council of Canada (C.R.B.).
T.S. has consulted for Jazz Pharmaceuticals.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andlauer O, Moore H, Hong S, Dauvilliers Y, Kanbayashi T, Nishino N, Han F, Silber M, Rico T, Einen M, Kornum B, Jennum P, Knudsen S, Nevsimalova S, Poli F, Plazzi G, Mignot E. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012 doi: 10.5665/sleep.2080. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran A, Einen M, Lin L, Plazzi G, Nishino S, Mignot E. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep. 2010;33:1457–1464. doi: 10.1093/sleep/33.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2008;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DA, Narver EL, Dement WC, Mitler MM. Effects of imipramine, chlorimipramine, and fluoxetine on cataplexy in dogs. Pharmacol Biochem Behav. 1976;5:599–602. doi: 10.1016/0091-3057(76)90298-7. [DOI] [PubMed] [Google Scholar]
- Baker TL, Foutz AS, McNerney V, Mitler MM, Dement WC. Canine model of narcolepsy: genetic and developmental determinants. Exp Neurol. 1982;75:729–742. doi: 10.1016/0014-4886(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Bassetti CL, Valko PO, Haybaeck J, Keller M, Clark E, Stocker R, Tolnay M, Scammell TE. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besset A, Tafti M, Nobile L, Billiard M. Homeostasis and narcolepsy. Sleep. 1994;17:S29–S34. [PubMed] [Google Scholar]
- Black J, Guilleminault C. Medications for the treatment of narcolepsy. Expert Opin Emerg Drugs. 2001;6:239–247. doi: 10.1517/14728214.6.2.239. [DOI] [PubMed] [Google Scholar]
- Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM, Siegel JM. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology. 2005;65:1189–1192. doi: 10.1212/01.wnl.0000175219.01544.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch EE, Ahlskog JE, Smith GE, Caselli RC, Tippman-Peikert M, Olson EJ, Lin SC, Young T, Wszolek Z, Schenck CH, Mahowald MW, Castillo PR, Del Tredici K, Braak H. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Boscolo-Berto R, Viel G, Montagnese S, Raduazzo DI, Ferrara SD, Dauvilliers Y. Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2011 doi: 10.1016/j.smrv.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Brooks PL, Peever JH. Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–3545. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen L, Brown RE, McKenna JT, McCarley RW. Animal models of narcolepsy. CNS Neurol Disord Drug Targets. 2009;8:296–308. doi: 10.2174/187152709788921717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EL, Baumann CR, Cano G, Scammell TE, Mochizuki T. Feeding-elicited cataplexy in orexin knockout mice. Neuroscience. 2009;161:970–977. doi: 10.1016/j.neuroscience.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, España RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovic-Lopes V, Bayer L, Dorsaz S, Maret S, Pradervand S, Dauvilliers Y, Lecendreux M, Lammers GJ, Donjacour CE, Du Pasquier RA, Pfister C, Petit B, Hor H, Mühlethaler M, Tafti M. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–719. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantz B, Edgar DM, Dement WC. Circadian rhythms in narcolepsy: studies on a 90 minute day. Electroencephalogr Clin Neurophysiol. 1994;90:24–35. doi: 10.1016/0013-4694(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Carlander B, Rivier F, Touchon J, Tafti M. Successful management of cataplexy with intravenous immunoglobulins at narcolepsy onset. Ann Neurol. 2004;56:905–908. doi: 10.1002/ana.20339. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, Fronczek R, Van der Ploeg J, Scammell T, Gautam S, Pascual-Leone A, Lammers GJ. Reward-seeking behavior in human narcolepsy. J Clin Sleep Med. 2011;7:293–300. doi: 10.5664/JCSM.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz Behn CG, Klerman EB, Mochizuki T, Lin SC, Scammell TE. Abnormal sleep/wake dynamics in orexin knockout mice. Sleep. 2010;33:297–306. doi: 10.1093/sleep/33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Mühlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- España RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–1425. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronczek R, Overeem S, Lee SY, Hegeman IM, van Pelt J, van Duinen SG, Lammers GJ, Swaab DF. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- Fronczek R, Baumann CR, Lammers GJ, Bassetti CL, Overeem S. Hypocretin/orexin disturbances in neurological disorders. Sleep Med Rev. 2009;13:9–22. doi: 10.1016/j.smrv.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Ripley B, Mignot E, Nishino S. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep. 2003;26:953–959. doi: 10.1093/sleep/26.8.953. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Xi M, Zhang J, Torterolo P, Sampogna S, Morales FR, Chase MH. Projection neurons from the central nucleus of the amygdala to the nucleus pontis oralis. J Neurosci Res. 2011;89:429–436. doi: 10.1002/jnr.22554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelineau J. De la narcolepsie. Gazette des hôpitaux. 1880;53:626–628. [Google Scholar]
- Golicki D, Bala MM, Niewada M, Wierzbicka A. Modafinil for narcolepsy: systematic review and meta-analysis. Med Sci Monit. 2010;16:RA177–RA186. [PubMed] [Google Scholar]
- Gulyani S, Wu MF, Nienhuis R, John J, Siegel JM. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience. 2002;112:355–365. doi: 10.1016/s0306-4522(02)00089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RX, Anaclet C, Roberts JC, Parmentier R, Zhang M, Guidon G, Buda C, Sastre JP, Feng JQ, Franco P, Brown SH, Upton N, Medhurst AD, Lin JS. Differential effects of acute and repeat dosing with the H3 antagonist GSK189254 on the sleep–wake cycle and narcoleptic episodes in Ox−/− mice. Br J Pharmacol. 2009;157:104–117. doi: 10.1111/j.1476-5381.2009.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, Mayer G, Plazzi G, Nevsimalova S, Bourgin P, Hong SC, Hong SS, Honda Y, Honda M, Högl B, Longstreth WT, Jr, Montplaisir J, Kemlink D, Einen M, Chen J, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–711. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hecht M, Lin L, Kushida CA, Umetsu DT, Taheri S, Einen M, Mignot E. Report of a case of immunosuppression with prednisone in an 8-year-old boy with an acute onset of hypocretin-deficiency narcolepsy. Sleep. 2003;26:809–810. doi: 10.1093/sleep/26.7.809. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Inocente CD, Arnulf I, Bastuji H, Thibault-Stoll A, Raoux AM, Reimão R, Lin JS, Franco P. Pitolisant, an inverse agonist of the histamine H3 receptor: an alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepiness. Clin Neuropharmacol. 2012;35:55–60. doi: 10.1097/WNF.0b013e318246879d. [DOI] [PubMed] [Google Scholar]
- John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbayashi T, Honda K, Kodama T, Mignot E, Nishino S. Implication of dopaminergic mechanisms in the wake-promoting effects of amphetamine: a study of D- and L-derivatives in canine narcolepsy. Neuroscience. 2000;99:651–659. doi: 10.1016/s0306-4522(00)00239-6. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–223. [PubMed] [Google Scholar]
- Kawashima M, Lin L, Tanaka S, Jennum P, Knudsen S, Nevsimalova S, Plazzi G, Mignot E. Anti-Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. Sleep. 2010;33:869–874. doi: 10.1093/sleep/33.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci. 2003;23:1548–1554. doi: 10.1523/JNEUROSCI.23-04-01548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Lammers GJ, Bassetti C, Billiard M, Black J, Broughton R, Dauvilliers Y, Ferini Strambi L, Garcia-Borreguero D, Goswami M, Högl B, Iranzo A, Jennum P, Khatami R, Lecendreux M, Mayer G, Mignot E, Montplaisir J, Nevsimalova S, Peraita-Adrados R, Plazzi G, et al. Sodium oxybate is an effective and safe treatment for narcolepsy. Sleep Med. 2010;11:105–106. doi: 10.1016/j.sleep.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Leonard BE, McCartan D, White J, King DJ. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum Psychopharmacol. 2004;19:151–180. doi: 10.1002/hup.579. [DOI] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Lin JS, Dauvilliers Y, Arnulf I, Bastuji H, Anaclet C, Parmentier R, Kocher L, Yanagisawa M, Lehert P, Ligneau X, Perrin D, Robert P, Roux M, Lecomte JM, Schwartz JC. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin−/− mice and patients. Neurobiol Dis. 2008;30:74–83. doi: 10.1016/j.nbd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu M, Thankachan S, Kaur S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, Neve R, Shiromani PJ. Orexin (hypocretin) gene transfer diminishes narcoleptic sleep behavior in mice. Eur J Neurosci. 2008;28:1382–1393. doi: 10.1111/j.1460-9568.2008.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, Sakurai T, Yanagisawa M, van den Pol AN, Shiromani PJ. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci. 2011;31:6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Black J, Montplaisir J, Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27:1327–1334. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
- Mieda M, Sakurai T. Integrative physiology of orexins and orexin receptors. CNS Neurol Disord Drug Targets. 2009;8:281–295. doi: 10.2174/187152709788921663. [DOI] [PubMed] [Google Scholar]
- Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E, Nishino S. Emerging therapies in narcolepsy-cataplexy. Sleep. 2005;28:754–763. doi: 10.1093/sleep/28.6.754. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lin X, Hesla PE, Dement WC, Guilleminault C, Grumet FC. A novel HLA DR17,DQ1 (DQA1-0102/DQB1-0602 positive) haplotype predisposing to narcolepsy in Caucasians. Sleep. 1993;16:764–765. doi: 10.1093/sleep/16.8.764. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lin X, Arrigoni J, Macaubas C, Olive F, Hallmayer J, Underhill P, Guilleminault C, Dement WC, Grumet FC. DQB1*0602 and DQA1*0102 (DQ1) are better markers than DR2 for narcolepsy in Caucasian and black Americans. Sleep. 1994;17:S60–S67. doi: 10.1093/sleep/17.suppl_8.s60. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- Mitler MM. Letter: animal model of narcolepsy-cataplexy. J Am Vet Med Assoc. 1975;166:643. [PubMed] [Google Scholar]
- Mitler MM, Boysen BG, Campbell L, Dement WC. Narcolepsy-cataplexy in a female dog. Exp Neurol. 1974;45:332–340. doi: 10.1016/0014-4886(74)90122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Soave O, Dement WC. Narcolepsy in seven dogs. J Am Vet Med Assoc. 1976;168:1036–1038. [PubMed] [Google Scholar]
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Arrigoni E, Marcus JN, Clark EL, Yamamoto M, Honer M, Borroni E, Lowell BB, Elmquist JK, Scammell TE. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci U S A. 2011;108:4471–4476. doi: 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mao J, Sampathkumaran R, Shelton J. Increased dopaminergic transmission mediates the wake-promoting effects of CNS stimulants. Sleep Res Online. 1998;1:49–61. [PubMed] [Google Scholar]
- Nixon JP, Kotz CM, Novak CM, Billington CJ, Teske JA. Neuropeptides controlling energy balance: orexins and neuromedins. Handb Exp Pharmacol. 2012:77–109. doi: 10.1007/978-3-642-24716-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsén P, Saarenpää-Heikkilä O, Kilpi T. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overeem S, Lammers GJ, van Dijk JG. Weak with laughter. Lancet. 1999;354:838. doi: 10.1016/s0140-6736(99)80023-3. [DOI] [PubMed] [Google Scholar]
- Overeem S, Lammers GJ, van Dijk JG. Cataplexy: ‘tonic immobility’ rather than ‘REM-sleep atonia’? Sleep Med. 2002;3:471–477. doi: 10.1016/s1389-9457(02)00037-0. [DOI] [PubMed] [Google Scholar]
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med. 2011;12:12–18. doi: 10.1016/j.sleep.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Parmentier R, Anaclet C, Guhennec C, Brousseau E, Bricout D, Giboulot T, Bozyczko-Coyne D, Spiegel K, Ohtsu H, Williams M, Lin JS. The brain H3-receptor as a novel therapeutic target for vigilance and sleep–wake disorders. Biochem Pharmacol. 2007;73:1157–1171. doi: 10.1016/j.bcp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, Hublin C, Linna M, Olsén P, Nokelainen P, Alén R, Wallden T, Espo M, Rusanen H, Olme J, Sätilä H, Arikka H, Kaipainen P, Julkunen I, Kirjavainen T. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever JH, Lai YY, Siegel JM. Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol. 2003;89:2591–2600. doi: 10.1152/jn.00968.2002. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Parmeggiani A, Mignot E, Lin L, Scano MC, Posar A, Bernardi F, Lodi R, Tonon C, Barbiroli B, Montagna P, Cicognani A. Narcolepsy-cataplexy associated with precocious puberty. Neurology. 2006;66:1577–1579. doi: 10.1212/01.wnl.0000216142.21375.71. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. 2011;32:451–462. doi: 10.1016/j.tips.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sasai T, Inoue Y, Komada Y, Sugiura T, Matsushima E. Comparison of clinical characteristics among narcolepsy with and without cataplexy and idiopathic hypersomnia without long sleep time, focusing on HLA-DRB1(*)1501/DQB1(*)0602 finding. Sleep Med. 2009;10:961–966. doi: 10.1016/j.sleep.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Matheson J. Modafinil: a novel stimulant for the treatment of narcolepsy. Expert Opin Investig Drugs. 1998;7:99–112. doi: 10.1517/13543784.7.1.99. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Willie JT, Guilleminault C, Siegel JM. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–116. [PMC free article] [PubMed] [Google Scholar]
- Schachter M, Parkes JD. Fluvoxamine and clomipramine in the treatment of cataplexy. J Neurol Neurosurg Psychiatry. 1980;43:171–174. doi: 10.1136/jnnp.43.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck CH, Bassetti CL, Arnulf I, Mignot E. English translations of the first clinical reports on narcolepsy and cataplexy by Westphal and Gelineau in the late 19th century, with commentary. J Clin Sleep Med. 2007;3:301–311. [PMC free article] [PubMed] [Google Scholar]
- Shelton J, Nishino S, Vaught J, Dement WC, Mignot E. Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogs. Sleep. 1995;18:817–826. [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS, Fahringer H, Cave G, Kilduff T, Dement WC. Heart rate and blood pressure changes during sleep-waking cycles and cataplexy in narcoleptic dogs. Am J Physiol. 1989;256:H111–H119. doi: 10.1152/ajpheart.1989.256.1.H111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton CM. Orexin/hypocretin plays a role in the response to physiological disequilibrium. Sleep Med Rev. 2011;15:197–207. doi: 10.1016/j.smrv.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin/hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35:798–804. doi: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soja PJ, Morales FR, Baranyi A, Chase MH. Effect of inhibitory amino acid antagonists on IPSPs induced in lumbar motoneurons upon stimulation of the nucleus reticularis gigantocellularis during active sleep. Brain Res. 1987;423:353–358. doi: 10.1016/0006-8993(87)90862-6. [DOI] [PubMed] [Google Scholar]
- Sonka K, Kemlink D, Busková J, Pretl M, Srůtková Z, Maurovich Horvat E, Vodicka P, Poláková V, Nevsímalová S. Obesity accompanies narcolepsy with cataplexy but not narcolepsy without cataplexy. Neuro Endocrinol Lett. 2010;31:631–634. [PubMed] [Google Scholar]
- Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- Tafti M, Rondouin G, Besset A, Billiard M. Sleep deprivation in narcoleptic subjects: effect on sleep stages and EEG power density. Electroencephalogr Clin Neurophysiol. 1992;83:339–349. doi: 10.1016/0013-4694(92)90069-t. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson's disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009;32:993–998. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Tanaka S, Miyagawa T, Honda Y, Tokunaga K, Honda M. Anti-Tribbles homolog 2 autoantibodies in Japanese patients with narcolepsy. Sleep. 2010;33:875–878. doi: 10.1093/sleep/33.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T, Kilduff TS, Boyden ES, Takahashi S, Tominaga M, Yamanaka A. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 2011;31:10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienne J, Bettler B, Franken P, Tafti M. Differential effects of GABAB receptor subtypes, {gamma}-hydroxybutyric acid, and baclofen on EEG activity and sleep regulation. J Neurosci. 2010;30:14194–14204. doi: 10.1523/JNEUROSCI.3145-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Economo C. Sleep as a problem of localization. J Nerv Mental Dis. 1930;71:247–259. [Google Scholar]
- Westphal C. Eigentümliche mit Einschlafen verbundene Anfälle. Archiv für Psychiatrie und Nervenkrankheiten. 1877;7:631–635. [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Willie JT, Renthal W, Chemelli RM, Miller MS, Scammell TE, Yanagisawa M, Sinton CM. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience. 2005;130:983–995. doi: 10.1016/j.neuroscience.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS. Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132:1027–1034. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, John J, Boehmer LN, Yau D, Nguyen GB, Siegel JM. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J Physiol. 2004;554:202–215. doi: 10.1113/jphysiol.2003.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi M, Fung SJ, Sampogna S, Chase MH. Excitatory projections from the amygdala to neurons in the nucleus pontis oralis in the rat: an intracellular study. Neuroscience. 2011;197:181–190. doi: 10.1016/j.neuroscience.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–5345. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]