Abstract
Rationale
Upon discontinuation of chronic opioid treatment, withdrawal typically peaks in 1–3 days and decreases markedly within 1 week; however, persistent physiological changes have been reported long after other signs have waned.
Objective
The goal of this study was to compare the discriminative stimulus, directly observable signs, and physiological effects of withdrawal in morphine-treated monkeys.
Materials and methods
Monkeys received 5.6 mg/kg/12 h morphine and discriminated 0.0178 mg/kg naltrexone while responding under a fixed-ratio 5 schedule of stimulus–shock termination. Drug discrimination, behavioral observation, and telemetry were used to monitor the emergence of withdrawal, as well as any persistent changes, following discontinuation of morphine treatment.
Results
Naltrexone dose (0.001–0.032 mg/kg, s.c.) was positively related with indices of withdrawal. In the discrimination study, monkeys responded on the naltrexone lever 1–5 days following discontinuation of treatment; thereafter, they responded exclusively on the saline lever. After discontinuation of morphine, the frequency of observable signs peaked within 2–3 days and most were not significantly increased after 5 days. In contrast, increased heart rate and body temperature persisted for 14 days, returning to values obtained prior to discontinuation by 21 days.
Conclusions
To the extent that discriminative stimulus effects of withdrawal in nonhumans are predictive of subjective reports of withdrawal in humans, these data indicate that effective treatments for opioid dependence must address not only the short-term subjective components of withdrawal but also, and perhaps more importantly, lingering behavioral and physiological effects that might contribute to relapse long after chronic drug use is discontinued.
Keywords: Behavioral observations, Drug discrimination, Morphine, Naltrexone, Physical dependence, Rhesus monkey, Telemetry, Withdrawal
Abuse of both illicit and prescription opioids remains a serious public health problem. From 1995 to 2005, admission rates for primary abuse of opioids increased from 15% to 17% of the total number of admissions for substance abuse treatment, and in 2005, only alcohol accounted for more admissions (Treatment Episode Data Set 2006; http://www.oas.samhsa.gov). In 2005, heroin was the third most frequent illicit drug reported in emergency room visits (DAWNReport 2006; http://www.DAWNinfo. samhsa.gov). There is also increasing concern about the abuse of prescription opioids such as oxycodone. From 1995 to 2005, admission rates for treatment of prescription opioid abuse increased to 300%, and opioids were the second most frequent drugs reported in emergency room visits (DAWNReport 2006; http://www.DAWNinfo. samhsa.gov).
Even when treatment of opioid abuse appears to be effective, relapse rates are high with, for example, 34% of patients returning to heroin use within 3 days and 60% within 90 days of discontinuing use (Gossop et al. 2002). Return to drug use likely involves a number of factors, including avoidance of withdrawal (Light and Torrance 1929; Kleber et al. 1980). Signs (e.g., tremors, vomiting, lacrimation, and sweating) are apparent 1 day after the termination of opioids and typically peak by 3 days; however, other withdrawal-induced physiological changes persist for much longer periods. For example, discontinuation of opioid use produces hyperthermia and tachycardia that persist for several weeks (Himmelsbach 1942; Martin and Jasinski 1969). There is a paucity of data on the long-term physiological effects of opioid withdrawal, particularly in humans, perhaps due to the impracticality of such studies (i.e., noncompliance). Although withdrawal signs in monkeys (Brandt and France 1998) and rats (Wikler and Pescor 1967) also peak within 3 days, long-lasting physiological changes have not been reported in nonhumans (e.g., Chan et al. 1999). One goal of this study was to establish conditions under which the lingering withdrawal signs observed in humans could be studied in monkeys.
In addition to physiological assessment and direct observation, drug discrimination procedures can be used to study opioid dependence and withdrawal. The naltrexone discriminative stimulus in morphine-treated subjects can serve as an indicator of withdrawal (Gellert and Holtzman 1979) with opioid-treated animals responding predominately on the naltrexone lever after receiving an opioid antagonist or after discontinuation of treatment (Gellert and Holtzman 1979; France and Woods 1987, 1989). In monkeys, doses of antagonists that produce naltrexone-lever responding also produce observable withdrawal signs such as salivation and increased respiration (Paronis and Woods 1997; Sell et al. 2005). Both naltrexone-lever responding and observable signs can be attenuated by morphine (McMahon et al. 2004).
Although observable withdrawal signs can covary with the discriminative stimulus effects of opioid antagonists, there are conditions under which these indices of withdrawal can be dissociated. For example, clonidine, an α2-adrenergic receptor agonist, attenuates autonomic signs of withdrawal (Jasinski et al. 1985; Sell et al. 2005) but does not alter naltrexone-lever responding (Sell and France 2002), indicating that clonidine would not be practical for treating the subjective effects of opioid withdrawal and might increase relapse (Walsh et al. 2003). The current study used drug discrimination, behavioral observation, and telemetry procedures to further investigate whether discriminative stimulus effects that are related to opioid withdrawal can be dissociated from other indices of withdrawal. In addition, this study was part of an effort to develop a novel procedure for examining opioid withdrawal that would adequately reflect both short- and long-term effects reported in humans. Telemetry devices are uniquely suited for this task because persistent withdrawal signs in humans include hyperthermia and tachycardia, which are easily measured with these devices, and because continuous monitoring of these signs might detect changes that would not be observed when signs were measured intermittently.
Materials and methods
Subjects
Three female adult rhesus monkeys (Macaca mulatta; subjects Ch, Re and Cl) were individually housed under a 14/10-h light/dark cycle in stainless-steel cages with unlimited access to water. Monkeys were maintained at no less than 95% of their free-feeding weight (6.7–7.9 kg) with monkey chow (Harlan Teklad, High Protein Monkey Diet, Madison, WI, USA), fresh fruit, peanuts, and granola bars. Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee (The University of Texas Health Science Center at San Antonio) and the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
Surgery
Monkeys were anesthetized with 1 mL ketamine (100 mg/mL), intubated, and maintained on anesthesia with 2.5% isoflurane to implant telemetry devices (30 g, 20 cc, disk-shaped, model CTA-D70, Data Science International, Arden Hills, MN, USA) in the right flank. Muscle, subcutaneous tissue incision sites, and skin were closed with absorbable, 3–0 Vicryl suture silk (Ethicon, Somerville, NJ, USA). Penicillin B&G (40,000 IU/kg) and meloxicam were given postoperatively. Monkeys were allowed to recover for at least 1 week.
Apparatus
For drug discrimination, monkeys were seated in primate chairs (Primate Products, Miami, FL, USA) placed in ventilated, sound-attenuating chambers. Response panels were equipped with three stimulus lights arranged horizontally with response levers under the outer lights. Feet were placed in shoes containing brass electrodes to which a brief (3 mA, 250 ms) electric stimulus could be delivered from a remote a.c. generator (Coulbourn Instruments, Whitehall, PA, USA). Experiments were controlled and data were recorded through an interface connected to a computer (Med Associates, St. Albans, VT, USA).
When activated, telemetry devices transmitted radio wave signals to RMC-1 receivers that were attached to the cage. Heart rate (beats per minute), body temperature (°C), and activity (counts per minute) were collected for 5-min periods every 10 min and were recorded by a computer (Dataquest®A.R.T. system, Data Science International, Arden Hills, MN, USA).
Behavioral procedures
Monkeys received morphine twice daily (5.6 mg/kg s.c. at 0630 and 1830 hours) with sessions beginning 3 h after the morning injection (1030 hours), and they were weighed before sessions. Monkeys were previously trained to respond under a fixed-ratio 5 schedule of stimulus–shock termination (SST) while discriminating 0.0178 mg/kg naltrexone (France and Woods 1989). Sessions were divided into two to six cycles with each cycle comprising a 10-min timeout, during which chambers were dark and lever presses had no programmed consequence, and a 5-min response period, during which red lights above levers were illuminated and electric stimuli were scheduled to occur every 15 s. When monkeys responded five consecutive times on the correct lever, lights were extinguished and the SST schedule was postponed for 30 s. The correct lever was determined by the injection (saline or naltrexone) administered during the first minute of the cycle. The right lever was designated correct after naltrexone for two monkeys and after saline for the third monkey. Responses on the incorrect lever reset the response requirement on the correct lever. Response periods ended after 5 min or delivery of four electric stimuli, whichever occurred first.
For training sessions, zero to six saline injections preceded naltrexone, which was followed by one sham (noninjection) cycle during which responding on the naltrexone-appropriate lever postponed the SST schedule or monkeys received saline followed by one to five sham cycles. Test sessions were conducted every third session as long as the following criteria were satisfied during intervening training sessions: ≥80% of the total responses on the correct lever and <5 responses on the incorrect lever before completion of the first fixed ratio on the correct lever. During the test sessions, five consecutive responses on either lever postponed the SST schedule. Naltrexone dose-effect curves were determined by administering vehicle during the first cycle and increasing doses of naltrexone in subsequent cycles up to a dose that produced >80% naltrexone-lever responding. When treatment was discontinued, saline replaced morphine twice daily for 21 days; monkeys also received saline during the first of two daily test cycles.
Behavioral observations
Monkeys were observed in their home cages immediately before (1015 hours) and 30 min after (1100 hours) receiving vehicle or naltrexone with one injection administered each day in a mixed order among monkeys. When morphine treatment was discontinued, monkeys were observed immediately before (1015 hours) and after (1100 hours) discrimination sessions. Signs were recorded by a nonblinded observer who was familiar with the behavior of individual monkeys. A list of withdrawal signs (Table 1) comprised those previously reported as a result of μ-opioid withdrawal in primates (Katz 1986; Sell et al. 2005; Li et al. 2007; Light and Torrance 1929; Kleber et al. 1980), including signs that were observed in preliminary studies with the same monkeys (e.g., unusual tongue movement). During precipitated withdrawal studies, signs were recorded as present or absent every 15 s for 2 min, yielding a maximum possible frequency score of 8. When morphine treatment was suspended, signs were recorded every 30 s for 2 min during the two observation periods; thus, results from these periods were combined to obtain a maximum frequency score of 8.
Table 1.
Behavioral signs used to score μ-opioid withdrawal
| Behavioral signs |
|---|
| Body tremor: shaking or trembling |
| Twitch: sudden movement |
| Wet-dog shake: movement of head very rapidly from side to side |
| Lying down in cage |
| Scratching |
| Grimace |
| Unusual tongue movement: tongue rolled inside mouth from cheek to cheek |
| Protrusion of tongue |
| Salivation |
| Vocalization |
| Yawning |
| Eye-closing |
| Emesis |
Telemetry procedure
Heart rate, body temperature, and activity were measured in the home cage using telemetry devices. On days when naltrexone was studied, drug discrimination sessions were not conducted. Instead, telemetry devices recorded continuously for 12 h beginning immediately after the morning injection of morphine (0630–1830 hours); monkeys received vehicle or naltrexone in the home cage 3 h later (1030 hours). Prior to discontinuation of morphine treatment, heart rate, body temperature, and activity data were collected continuously 24 h/day for at least 5 days during which monkeys received their normal daily dose of morphine. When treatment was discontinued, telemetry devices recorded continuously except when monkeys were removed from the cage to be weighed and for daily discrimination sessions (1015–1115 hours).
Drugs
The compounds used in this study were morphine sulfate, naltrexone hydrochloride (The Research Technology Branch, National Institute on Drug Abuse, Rockville, MD, USA), and ketamine hydrochloride (Burns Veterinary Supply, Farmers Branch, TX, USA). All solutions were administered subcutaneously in a volume of 0.1 to 1.0 mL. Morphine and naltrexone were dissolved in sterile water, and ketamine was purchased as a commercially prepared solution.
Data analyses
Precipitated withdrawal
The percentage of responses occurring on the naltrexone lever (±SEM) was plotted as a function of naltrexone dose. For individual monkeys, the mean of five naltrexone dose-effect curves was determined, and those means were then averaged among three monkeys. For behavioral signs, the frequency with which a sign occurred was averaged among monkeys (±SEM) and plotted as a function of naltrexone dose. Signs that were observed in all monkeys, including tongue protrusion, unusual tongue movement, and twitch, were analyzed separately with one-factor (dose) repeated-measures analysis of variance (ANOVA). Heart rate, body temperature, and activity were analyzed separately with two-factor (dose, time) repeated-measures ANOVA. Significant dose and time interactions were further analyzed at each time point with one-factor (dose) repeated-measures ANOVA followed by Dunnett's test (p<0.05).
Morphine discontinuation
The percentage of responses occurring on the naltrexone lever (±SEM) during the two daily test cycles after discontinuation of morphine was averaged and plotted as a function of time. For behavioral signs, the frequency of a sign was averaged among monkeys (±SEM) and plotted as a function of time since the last morphine injection (presented as average frequency scores over a 3-day period). Signs that occurred in all monkeys, including vocalization, unusual tongue movement, wet-dog shakes, and tongue protrusion, were analyzed separately with one-factor (days since last injection) repeated-measures ANOVA. Telemetry data were averaged across 3 h and plotted as a function of time since the last morphine injection. Heart rate, body temperature, and activity were analyzed separately with two-factor (time since last injection, hour of day) repeated-measures ANOVA. Significant interactions were further analyzed at each time point with one-factor (hour of day) repeated-measures ANOVA followed by Dunnett's test (p<0.05). Due to technical difficulties, data from one monkey were not collected 14 days after discontinuing morphine treatment; therefore, a separate analysis was conducted for the two remaining monkeys for each dependent measure.
Results
Precipitated withdrawal
Administration of vehicle, 0.001, or 0.0032 mg/kg naltrexone produced little or no responding on the naltrexone lever; however, a dose of 0.01 mg/kg naltrexone produced 94.5±5.5% naltrexone-lever responding (Fig. 1, upper panel). Response rate was not markedly altered by vehicle or naltrexone (Fig. 1, bottom panel).
Fig. 1.
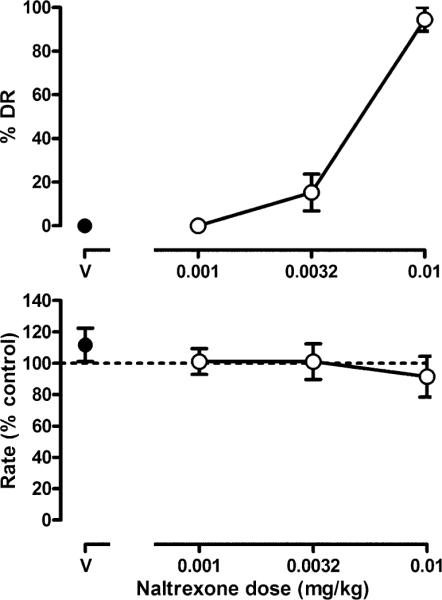
Discriminative stimulus effects of naltrexone in three morphine-treated monkeys discriminating 0.0178 mg/kg naltrexone while responding under a fixed-ratio 5 schedule of SST. Saline was administered on the first cycle. Data shown are from five determinations of naltrexone dose–effect curves for each monkey (mean±SEM). The SEM does not appear if it was less than the radius of the data point. Ordinate: percentage of responses occurring on the naltrexone lever (%DR drug responding, top) and mean response rate expressed as percentage of control rate (saline training days, bottom). Abscissa: naltrexone dose in milligrams per kilogram of body weight (V vehicle)
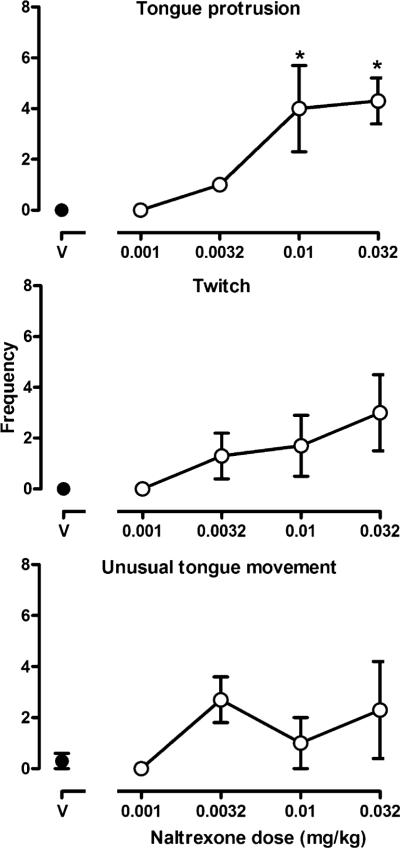
Naltrexone produced directly observable signs in monkeys receiving morphine daily. When vehicle was administered, these behavioral signs were rarely observed (Fig. 2, points above V); however, naltrexone dose-dependently increased tongue protrusion (F(4,8)=6.23, p<0.05; Fig. 2, top panel) and twitch (Fig. 2, middle panel), although twitch approached significance only with the largest dose of naltrexone (F(4,8)=2.37, p=0.06). There was a trend for naltrexone to increase unusual tongue movements (Fig. 2, bottom panel), but the effect was neither dose-related nor statistically significant. Other signs that were observed in some but not all monkeys were wetdog shakes and yawning (data not shown).
Fig. 2.
Selected behaviors observed every 15 s during a 2-min observation period. This occurred 30 min after the administration of vehicle (V) or naltrexone. Each data point represents the average (±SEM) number of times that a sign was observed (maximum possible score=8). One-factor repeated-measures ANOVA was followed by Dunnett's test to determine which points were significantly (*p<0.05) different from control (points above V). Ordinate: frequency of signs. See Fig. 1 for other details
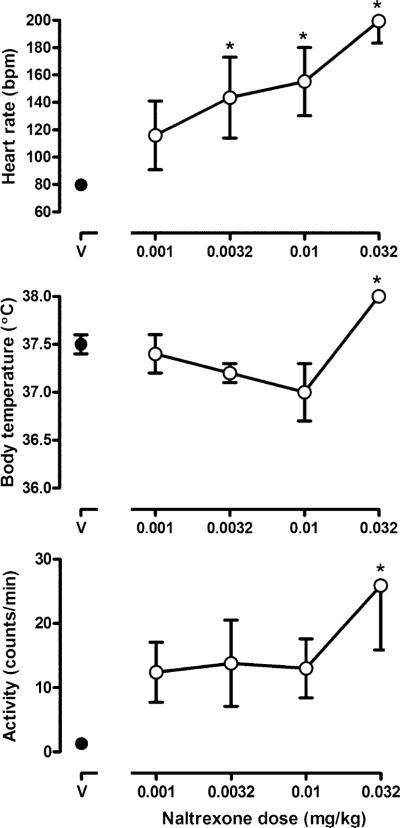
Naltrexone also increased heart rate, body temperature, and activity (Fig. 3). At the time of peak effect (20 min post naltrexone injection), naltrexone dose-dependently increased heart rate over the control value of 79.8±3.6 bpm with doses of 0.0032, 0.01, and 0.032 mg/kg increasing heart rate to 143.4±29.6, 155.2±24.9 and 199.4±16.0 bpm, respectively. There was an interaction between naltrexone dose and time since administration (F(72,144)=5.78, p<0.05). Increases were no longer apparent 30 min after 0.0032 mg/kg naltrexone or 1 h after 0.01 mg/kg naltrexone, although heart rate remained significantly elevated for more than 2 h after 0.032 mg/kg naltrexone (data not shown). In addition, a significant interaction occurred between dose and time such that 0.032 mg/kg naltrexone significantly increased body temperature from 20 to 40 min after administration. Although not significant, there was a trend for body temperature to decrease after the administration of a smaller dose of naltrexone. Finally, naltrexone markedly changed activity in all monkeys. Activity was significantly increased from 40 to 90 min after 0.032 mg/kg naltrexone (F(72,144)=1.45, p<0.05). Body weight was not altered by any dose of naltrexone (data not shown).
Fig. 3.
Heart rate (upper panel), body temperature (middle panel), and activity (bottom panel) after administration of vehicle (V) or naltrexone. Each symbol represents the average (±SEM) values for heart rate and body temperature at the time of the peak effect (20 min after injection) and for activity (40 min after injection). Two-factor repeated-measures ANOVA was followed by Dunnett's test to determine which points were significantly (*p<0.05) different from control (points above V). Ordinate: upper heart rate (beats per minute); middle body temperature (°C); bottom activity (counts per minute). See Fig. 1 for other details
Morphine discontinuation
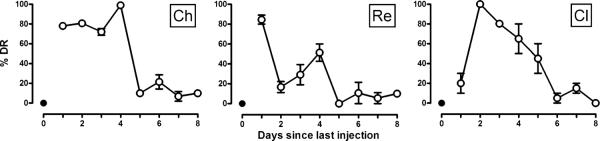
Naltrexone-lever responding peaked 1–3 days following discontinuation of morphine treatment, although the time course for these effects differed among monkeys (Fig. 4). When morphine was administered 3 h and saline was administered immediately before sessions, monkeys responded on the saline lever. Monkeys Ch and Re responded predominately on the naltrexone lever 1 day after the last dose of morphine; one monkey (Ch) responded on the naltrexone lever for 4 days whereas the other monkey (Re) responded predominately on the saline lever 2 days after discontinuation of morphine treatment. Monkey Cl responded predominately on the saline lever 1 day after the last dose of morphine and responded predominately on the naltrexone lever 2 days after morphine. There was a time-related decrease in naltrexone-lever responding in monkey Cl. By 6 days after the last dose of morphine, all three monkeys responded predominately on the saline lever and continued to respond on the saline lever until the conclusion of the study 15 days later. There was no significant change in response rate after discontinuing treatment (data not shown).
Fig. 4.
Discriminative stimulus effects after morphine discontinuation in three monkeys (represented by different panels for each subject). Twice daily injections of morphine were replaced with saline. Each data point represents the average (±SEM) percentage of responses occurring on the naltrexone lever for two cycles after receiving a saline injection. Abscissa: days since last injection. See Fig. 1 for other details
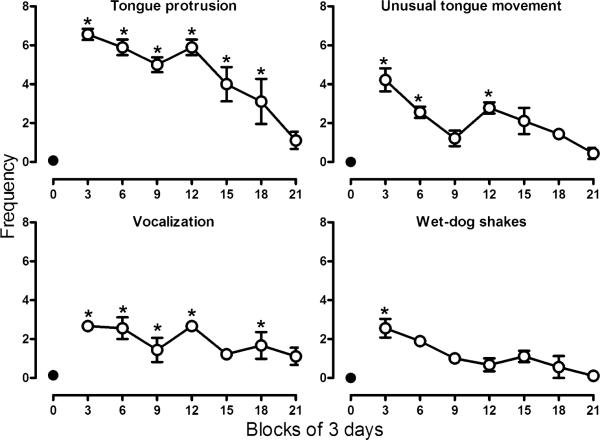
Behavioral signs were observed in all monkeys when morphine treatment was discontinued with the peak frequency occurring within 3 days after the last injection of morphine (Fig. 5). During treatment, signs such as tongue protrusion, unusual tongue movement, vocalization, and wet-dog shakes were rarely observed. Scores for all behavioral signs increased following discontinuation of treatment. Tongue protrusions were observed most often and were significantly increased for up to 18 days (F(7,14)= 7.40, p<0.05). Unusual tongue movements peaked during the first 3 days after discontinuation of treatment (F(7,14)= 2.92, p<0.05). Vocalizations (F(7,14)=2.79, p<0.05) and wet-dog shakes (F(7,14)=2.26, p<0.05) were also significantly increased after discontinuation of morphine treatment. Behavioral signs were no longer significantly increased 21 days after the last dose of morphine.
Fig. 5.
Selected behaviors observed every 30 s during 2-min periods both before and after discrimination sessions, prior to (day 0–control), and after morphine discontinuation. Each data point represents the average (±SEM) number of times that a sign was observed (maximum possible score=8). For clarity, each point summarizes data over 3 days for each monkey (±SEM). Abscissa: days since last injection. See Fig. 2 for other details
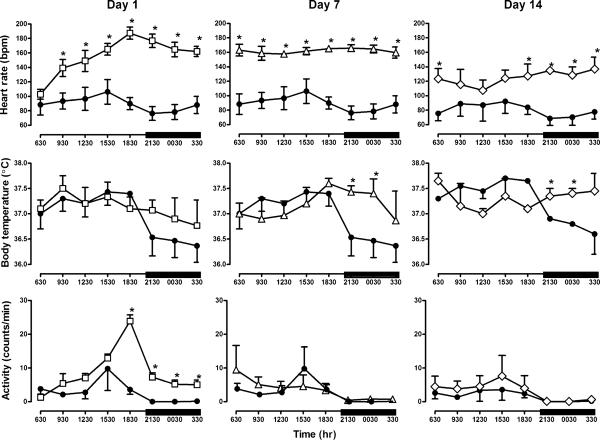
Telemetry recordings revealed a circadian pattern for physiological signs during morphine treatment with increased heart rate, temperature, and activity during the light period compared to the dark period (Fig. 6). Discontinuation of morphine treatment markedly disrupted this circadian pattern. For example, heart rate during the light period was significantly increased and there was a significant interaction between time since morphine administration and hour of day (F(21,42)=13.59, p<0.05). This effect was first observed 15 h after the last morphine injection; heart rate remained increased throughout the dark period and up to 14 days after discontinuation of morphine treatment (F(7,7)=3.5, p=0.06). Heart rate was not significantly elevated after 21 days (data not shown). Although body temperature during the light period was not significantly altered after discontinuing morphine treatment, there was a significant interaction between time of day and time since the last morphine injection; body temperature during the dark period began to increase 1 day after discontinuing morphine treatment and was significantly elevated 7 days after the last dose of morphine (2130–0230 hours; F(21,42)=2.53, p<0.05). Body temperature remained significantly elevated 14 days after discontinuation of morphine treatment (F(7,7)=4.36, p<0.05) and was no longer elevated after 21 days. Activity was significantly increased (F(21,42)=3.54, p<0.05) during both the light and dark period (1830–0530 hours) during the first day that morphine treatment was discontinued. Activity was not increased 7 days after the last morphine injection.
Fig. 6.
Heart rate (open squares), body temperature (open triangles), and overall activity (open diamonds) prior to and 1, 7, and 14 days after discontinuation of morphine treatment during the period when lights were on in the housing room and when the lights were off in the housing room (dark bars). Closed circles represent values obtained prior to discontinuing morphine treatment (and are the same for days 1 and 7) and open symbols represent values obtained after morphine treatment was discontinued. For clarity, each point summarizes data over 3 h for each monkey (±SEM). Two-factor repeated-measures ANOVA was followed by Dunnett's test to determine which points were significantly (*p<0.05) different from control (closed circles). For day 14, only data from two monkeys were included in both the control data and the data obtained after morphine treatment was discontinued and are analyzed separately from days 1 and 7. Ordinate: upper heart rate (beats per minute); middle body temperature (°C); bottom activity (counts per minute). Abscissa: time of day represented every 3 h
When monkeys received 5.6 mg/kg/12 h of morphine, body weights were 7.0, 6.9, and 7.9 kg for monkeys Ch, Re, and Cl, respectively; weights decreased during the 21 days following discontinuation of morphine treatment to 6.4, 6.3, and 7.6 kg, respectively. These decreases were reversed when morphine treatment resumed. To avoid adverse effects that can be produced by μ-opioid receptor agonists (e.g., respiratory depression), treatment began again with a small dose of morphine and the dose increased gradually: 1.0 mg/kg/12 h for 3 days, 3.2 mg/kg/12 h for 3 days, 5.6 mg/kg/12 h. Body weights for monkeys Ch and Re returned to 7.0 and 6.9 kg after receiving 3.2 mg/kg/ 12 h of morphine for 3 days and 1 day, respectively. Body weight for monkey Cl remained decreased until 5.6 mg/kg/ 12 h of morphine was administered for 1 week.
Discussion
Dependence developed in monkeys treated with 5.6 mg/kg/ 12 h of morphine, as evidenced by the emergence of withdrawal when naltrexone was administered or when morphine treatment was discontinued. This study examined three different expressions of withdrawal using three different procedures. Similar signs were evident when withdrawal was precipitated by naltrexone compared to signs observed following discontinuation of morphine treatment. In addition to the systematic comparison of the emergence of withdrawal, this study monitored signs until they were no longer evident and demonstrated that some, but not all, withdrawal signs persisted for more than 2 weeks after morphine treatment was discontinued. Such long-lasting effects might contribute to relapse even after many withdrawal signs are no longer evident.
Opioid withdrawal has been well-studied in humans and other species, and the withdrawal signs that were measured in the current study are well-known in rhesus monkeys. The use of drug discrimination to study opioid withdrawal was demonstrated in morphine-treated rats discriminating naltrexone; stimulus control by naltrexone was reliable, reproducible, and correlated with decreases in body weight, which is an indicator of withdrawal in rats (Gellert and Holtzman 1979). Likewise, naltrexone-lever responding and withdrawal signs occur following naltrexone administration in rhesus monkeys treated chronically with the μ-opioid agonist l-α-acetylmethadol (LAAM; Sell and France 2002; Sell et al. 2005). In the current study, naltrexone precipitated behavioral and physiological withdrawal signs at doses that occasioned naltrexone-lever responding. When morphine treatment was discontinued, signs, including decreased body weight, emerged concurrently with responding on the naltrexone lever. The presence of directly observable signs of withdrawal when morphine treatment was discontinued, along with the positive relationship between naltrexone dose and behavioral and physiological effects, contributes to the notion that monkeys in this study were discriminating withdrawal-related stimuli, rather than discriminating the absence of morphine. Despite the relationship between responding on the naltrexone lever and other withdrawal signs, the discriminative stimulus effects of naltrexone can be dissociated from other withdrawal signs under some conditions (McMahon et al. 2004; Sell and France 2002; Sell et al. 2005). For example, withdrawal signs do not emerge upon discontinuation of drug treatment in monkeys receiving smaller daily doses of morphine (3.2 mg/kg/day), although the naltrexone discriminative stimulus under those conditions (France and Woods 1989) is qualitatively similar to that observed in the current study in which monkeys received 5.6 mg/kg/12 h of morphine. Together, these data suggest that naltrexone-lever responding measures a unique component of withdrawal that might be most closely related to the subjective experience of withdrawal (Preston and Bigelow 1998).
Directly observable withdrawal signs were similar regardless of whether withdrawal was precipitated by naltrexone or occurred following discontinuation of morphine treatment, and signs that were observed in the current study were consistent with other studies in rhesus monkeys. In monkeys receiving 3 mg/kg/6 h of morphine, the frequency of withdrawal signs, such as vocalization and grimacing, increased following either naltrexone administration or discontinuation of morphine treatment, although the number and severity of withdrawal signs were greater in those monkeys (Gmerek and Woods 1985; Gmerek et al. 1987) compared with monkeys in the current study that received 5.6 mg/kg/12 h of morphine. These characteristic withdrawal signs typically peak within 1–3 days after discontinuation of treatment.
Using telemetry devices, naltrexone was shown to increase heart rate, body temperature, and activity in a dose-dependent manner. A previous study used telemetry to examine the physiological effects of discontinuing morphine treatment and found that heart rate was markedly increased for up to 2 days after termination of morphine treatment in rats (Chan et al. 1999). In addition, heart rate increased significantly during withdrawal from opioids in humans (Newlin et al. 1992), and hyperthermia has been reported in opioid-withdrawn humans (Himmelsbach 1942; Martin and Jasinski 1969). In the current study, heart rate, body temperature, and activity were elevated 1 day after the last dose of morphine; disturbances in normal activity occurred largely during the dark period when monkeys are typically sleeping, suggesting a disruption in circadian patterns. Although one possible explanation for the significant increases in heart rate and body temperature following discontinuation of morphine treatment might be the increases in overall activity of monkeys in their home cages, changes in heart rate and body temperature do not necessary occur at the same time of day or for the same number of days after the last dose of morphine, suggesting that these measures are independent. Moreover, despite similarities in withdrawal signs observed in the current study and those observed in humans, there are some differences among studies. For example, hypothermia has been reported in morphine-treated monkeys following either administration of an opioid antagonist or termination of treatment (Holtzman and Villarreal 1969, 1971). Differences in temperature responses to opioid withdrawal in the same species might be due to procedural differences (e.g., monkey handling method) among studies (current study; Holtzman and Villarreal 1969, 1971).
One goal of this study was to examine differences in the duration of withdrawal signs following discontinuation of morphine treatment. Although the emergence of withdrawal was similar across various signs with peak naltrexone-lever responding, directly observable signs, and physiological signs occurring within 1–3 days, the dissipation of these effects differed markedly. Monkeys responded on the naltrexone lever for up to 5 days after discontinuation of morphine treatment and on the saline lever thereafter; this result is consistent with that demonstrated previously in opioid-dependent, naltrexone-discriminating monkeys (France and Woods 1989; Brandt and France 1998). In contrast, one directly observable sign, tongue protrusion, was still evident 2 weeks after the last dose of morphine. In addition, heart rate remained significantly elevated for at least 14 days and returned to values similar to those obtained before discontinuation of morphine treatment by 21 days. Body temperature was elevated the first day after discontinuing morphine treatment during the dark period, reached significance in 7 days, and returned to prediscontinuation values by day 21. Although overall activity was only increased the first day after discontinuing morphine treatment and, therefore, cannot account for the persistent elevated heart rate and body temperature, perturbations in circadian patterns for heart rate and body temperature persisted for weeks. Thus, although many opioid withdrawal signs are no longer evident 1 week after termination of chronic treatment, some signs persist much longer. These signs that were observed in monkeys are similar to those reported in humans, and these lingering effects might contribute to relapse long after opioid agonist use or treatment ends.
Administration of naltrexone or discontinuation of morphine treatment in rhesus monkeys produced discriminative stimulus effects and directly observable withdrawal signs that were, for the most part, short-lived. In contrast, heart rate and body temperature were markedly elevated for at least 14 days. Overall, there were marked disruptions to circadian patterns for heart rate, body temperature, and activity, particularly during the dark period, suggesting disruptions in sleeping patterns. The withdrawal syndrome observed in rhesus monkeys under these conditions is similar to withdrawal in humans (Beswick et al. 2003), demonstrating both short- and long-term effects and suggesting that this approach might especially useful for further investigations of opioid withdrawal. In addition, withdrawal might increase opioid self-administration. For example, during withdrawal, choice of heroin over food increases in monkeys (Negus 2006), and rats respond under conditions that previously resulted in heroin self-administration for up to 4 weeks after discontinuation of heroin (Zhou et al. 2004). Although people in treatment programs for opioid dependence report diminished subjective effects of withdrawal after several days, other manifestations of withdrawal persist for much longer and could contribute to relapse, highlighting the need for future treatments for opioid dependence and withdrawal to focus not only on the short-term, subjective aspects of withdrawal but also on the lingering physiological effects of withdrawal.
Acknowledgements
The authors would like to thank Christopher Cruz, Blake Harrington, Maria Hernandez, Dawn Logan, and Ryan Luna for their expert technical assistance.
This project was supported by USPHS Grants DA05018 and DA17918 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Beswick T, Best D, Rees S, Bearn J, Gossop M, Strang J. Major disruptions of sleep during treatment of the opiate withdrawal syndrome: differences between methadone and lofexidine detoxification treatments. Addict Biol. 2003;8:49–57. doi: 10.1080/1355621031000069882. [DOI] [PubMed] [Google Scholar]
- Brandt MR, France CP. Chronic l-alpha acetylmethadol in rhesus monkeys: discriminative stimulus and other behavioral measures of dependence and withdrawal. J Pharmacol Exp Ther. 1998;287:1029–1037. [PubMed] [Google Scholar]
- Chan R, Irvine R, White J. Cardiovascular changes during morphine administration and spontaneous withdrawal in the rat. Eur J Pharmacol. 1999;368:25–33. doi: 10.1016/s0014-2999(98)00984-4. [DOI] [PubMed] [Google Scholar]
- France CP, Woods JH. Morphine, saline and naltrexone discrimination in morphine-treated pigeons. J Pharmacol Exp Ther. 1987;242:195–202. [PubMed] [Google Scholar]
- France CP, Woods JH. Discriminative stimulus effects of naltrexone in morphine-treated rhesus monkeys. J Pharmacol Exp Ther. 1989;250:937–943. [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Discriminative stimulus effects of naltrexone in the morphine-dependent rat. J Pharmacol Exp Ther. 1979;211:596–605. [PubMed] [Google Scholar]
- Gmerek DE, Woods JH. Effects of b-funaltrexamine in normal and morphine-dependent rhesus monkeys: observation studies. J Pharmacol Exp Ther. 1985;235:296–301. [PubMed] [Google Scholar]
- Gmerek DE, Dykstra LA, Woods JH. Kappa opioids in rhesus monkeys. III. Dependence associated with chronic administration. J Pharmacol Exp Ther. 1987;242:428–436. [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97:1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- Himmelsbach CK. Clinical studies of drug dependence. Physical dependence, withdrawal and recovery. Arch Intern Med. 1942;69:766–772. [Google Scholar]
- Holtzman SG, Villarreal JE. Morphine dependence and body temperature in rhesus monkeys. J Pharmacol Exp Ther. 1969;166:125–133. [PubMed] [Google Scholar]
- Holtzman SG, Villarreal JE. Pharmacologic analysis of the hypothermic responses of the morphine-dependent rhesus monkey. J Pharmacol Exp Ther. 1971;177:317–325. [PubMed] [Google Scholar]
- Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42:1063–1066. doi: 10.1001/archpsyc.1985.01790340041006. [DOI] [PubMed] [Google Scholar]
- Katz JL. Effects of clonidine and morphine on opioid withdrawal in rhesus monkeys. Pscyhopharmacology. 1986;88:392–397. doi: 10.1007/BF00180844. [DOI] [PubMed] [Google Scholar]
- Kleber HD, Gold MS, Riordan CE. The use of clonidine in detoxification from opiates. Bull Narc. 1980;32:1–9. [PubMed] [Google Scholar]
- Li JX, Becker GL, Traynor JR, Gong ZH, France CP. Thienorphine: receptor binding and behavioral effects in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:227–236. doi: 10.1124/jpet.106.113290. [DOI] [PubMed] [Google Scholar]
- Light AB, Torrance EG. The effects of abrupt withdrawal followed by re-administration of morphine in human addicts, with special reference to composition of the blood, the circulation and the metabolism. Arch Intern Med. 1929;44:3–16. [Google Scholar]
- Martin WR, Jasinski DR. Physiological parameters of morphine dependence in man-tolerance, early abstinence, protracted abstinence. J Psychiatr Res. 1969;7:9–17. doi: 10.1016/0022-3956(69)90007-7. [DOI] [PubMed] [Google Scholar]
- McMahon L, Sell SL, France CP. Cocaine and other indirect-acting monoamine agonists differentially attenuate a naltrexone discriminative stimulus in morphine-treated rhesus monkeys. J Pharmacol Exp Ther. 2004;308:111–119. doi: 10.1124/jpet.103.058917. [DOI] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Wong CJ, Cheskin LJ. Cardiovascular responses to naloxone challenge in opiate-dependent individuals. Pharmacol Biochem Behav. 1992;43:357–360. doi: 10.1016/0091-3057(92)90162-9. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Woods JH. Ventilation in morphine-maintained rhesus monkeys. I: effects of naltrexone and abstinence-associated withdrawal. J Pharmacol Exp Ther. 1997;282:348–354. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Opioid discrimination in humans: discriminative and subjective effects of progressively lower training dose. Behav Pharmacol. 1998;9:533–543. doi: 10.1097/00008877-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Sell SL, France CP. Cocaine and amphetamine attenuate the discriminative stimulus effects of naltrexone in opioid-dependent rhesus monkeys. J Pharmacol Exp Ther. 2002;301:1103–1110. doi: 10.1124/jpet.301.3.1103. [DOI] [PubMed] [Google Scholar]
- Sell SL, McMahon LR, Koek W, France CP. Monoaminergic drugs and directly observable signs of LAAM-withdrawal in rhesus monkeys. Behav Pharmacol. 2005;16:53–58. doi: 10.1097/00008877-200502000-00006. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Bigelow GE. Evaluation of the effects of lofexidine and clonidine on naloxone-precipitated withdrawal in opioid-dependent humans. Addiction. 2003;98:427–439. doi: 10.1046/j.1360-0443.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and “relapse” in morphine-addicted rats. Psychopharmacologia. 1967;10:255–284. doi: 10.1007/BF00401386. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang F, Tang S, Liu H, Lai M, Yang G. Low dose of heroin inhibits drug-seeking elicited by cues after prolonged withdrawal from heroin self-administration in rats. NeuroReport. 2004;15:727–730. doi: 10.1097/00001756-200403220-00031. [DOI] [PubMed] [Google Scholar]