Abstract
Optical stimulation of neural tissue within the cochlea was described as a possible alternative to electrical stimulation. Most optical stimulation was performed with pulsed lasers operating with near-infrared (NIR) light and in thermal confinement. Under these conditions, the coexistence of laser-induced optoacoustic stimulation of the cochlea (“optophony”) has not been analyzed yet. This study demonstrates that pulsed 1850-nm laser light used for neural stimulation also results in sound pressure levels up to 62 dB peak-to-peak equivalent sound pressure level (SPL) in air. The sound field was confined to a small volume along the laser beam. In dry nitrogen, laser-induced acoustic events disappeared. Hydrophone measurements demonstrated pressure waves for laser fibers immersed in water. In hearing rats, laser-evoked signals were recorded from the cochlea without targeting neural tissue. The signals showed a two-domain response differing in amplitude and latency functions, as well as sensitivity to white-noise masking. The first component had characteristics of a cochlear microphonic potential, and the second component was characteristic for a compound action potential. The present data demonstrate that laser-evoked acoustic events can stimulate a hearing cochlea. Whenever optical stimulation is used, care must be taken to distinguish between such “optophony” and the true optoneural response.
Index Terms: Auditory system, hearing aids, infrared-radiation effects, optical-radiation effects, photoacoustic effects
I. Introduction
NEURAL stimulation with near-infrared (NIR) optical radiation has been suggested as an alternative to neural stimulation with electrical current. Such optical stimulation is possible in peripheral nerves [1]–[6] and in the cochlea [7]–[10]. The potential advantages of optical stimulation include the absence of physical contact between neural tissues and the stimulation source and the high spatial selectivity of optical stimulation compared to electrical stimulation. However, the method also has its limitations. Tissue between the radiation source and the neurons can be a confounding factor for stimulation. The tissue may scatter or absorb the radiation. Both will result in a need for increase of the radiation exposure to stimulate the target tissue. Scatter of the radiation will spread the stimulation, while absorption will result in heating and subsequent damage of the overlying structures.
The laser parameters recently used for optical stimulation include radiation wavelengths between 1850 and 2120 nm, pulse lengths between 5 µs and 10 ms, and radiant exposures up to 2 J/cm2. The laser–tissue interaction thus takes place in so-called “thermal confinement.” In thermal confinement, even at low energies used to stimulate the neurons, the fast heating of the small volume in front of the optical fiber results in expansion of this volume and in a stress-relaxation wave. For neural stimulation in the cochlea, it is important to determine whether this relaxation wave is audible and constitutes a confounding factor for neural-stimulation techniques. Although it has been well established that audible acoustic events appear at high stimulus energies [11], [12] or in the condition of stress confinement [13], acoustic events in the thermal confinement at low energies have not been investigated in vivo and their spatiotemporal characteristics have not been provided yet. For the potential use of neuronal optical stimulation in the cochlea, the awareness of generated pressure waves is highly important. Laser-induced pressure waves might interfere with auditory-nerve stimulation by stimulating remaining hair cells. On the other hand, laser-induced pressure waves could also serve as a new mechanism for sound stimulation in the hearing-impaired cochlea [14].
II. Material and Methods
A. Laser Stimulation
Optical stimulation was performed with a diode laser (Capella, Model R-1850, Aculight) with 1850-nm wavelength and variable pulse energies. The laser was operated at 33 or 100 Hz repetition rate. The radiation was coupled to an optical fiber (5 m length, NA 0.22 ± 0.02) with 200 µm core diameter (Ocean Optics, VIS/NIR, FL). The overall stability of the laser output was maintained by internal closed feedback loops that regulated the diode temperature (and thus, the emission wavelength) and output energy. Different output energies per pulse, 1 mm from tip of the optical fibers, were determined in air with an energy meter (Coherent J50LP-1A with 3Σ Coherent, FL). For comparability with previous reports all energies were related to the 200 µm core diameter of the used glass fiber. Dependent on the selected laser-setting radiant exposure ranged from 0.004 to 0.35 J/cm2.
During the experiments, the optical fiber was mounted on a three-axis micromanipulator (MM33, Narishige, Japan). The optical fiber was oriented onto the capsule of the middle cochlear turn (1 mm distance), next to the cochlea toward the inner wall of the bulla and perpendicular to the bulla opening (in air). Timing of the laser was controlled by custom written software (Audiology Laboratory, Otoconsult, Germany) from an external PC using a National Instruments 6259 M-Series MIO card.
B. In Vitro Experiments
1) Measurements in the Air
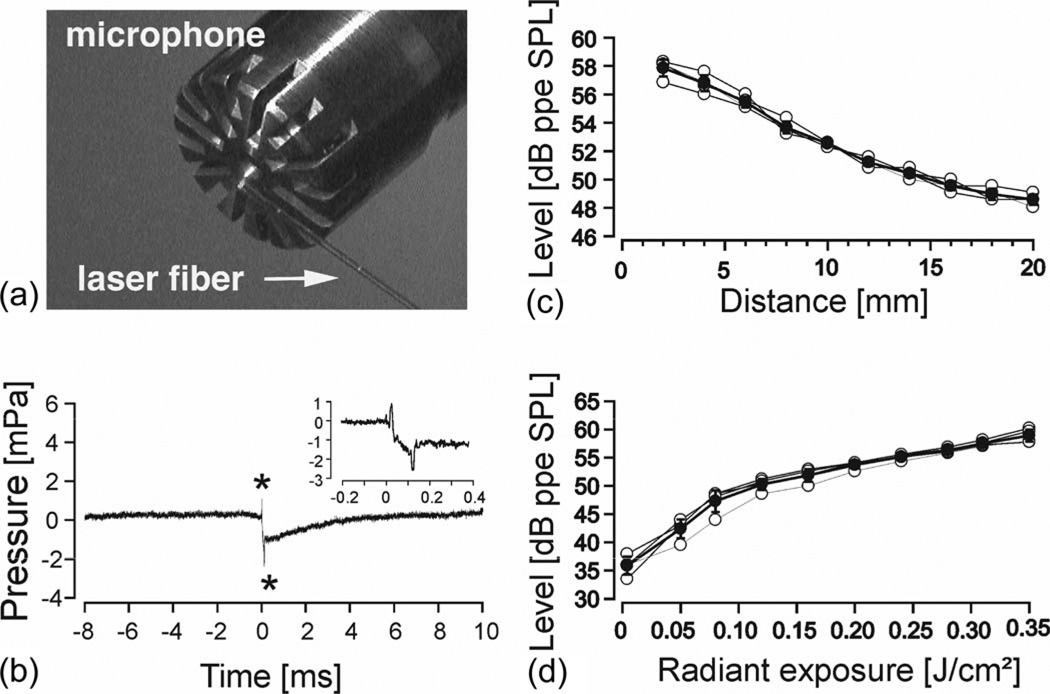
Sound-level measurements were conducted in a soundproof booth using 1/2 in (type 2669c), 1/4 in (type 2670), and 1/8 in (type 4138) microphones (Bruel & Kjær, Naerum, Denmark). Measured signals were amplified (Nexus-Conditioning Amplifier, Type 2690-S02, Bruel & Kjær, filter 20 Hz–100 kHz) and recorded with custom-written software (Audiology Laboratory). The measuring site was at the tip of the optical fiber. The fiber was oriented parallel to the membrane of the microphone with 2 mm distance between the tip and the center of the microphone membrane (see Fig. 1(a)). Laser pulse energies were varied and corresponding sound levels in front of the fiber were recorded. Afterward, the distance between microphone and laser fiber was increased in successive 2 mm steps, and a sound-level measurement (measured as peak-to-peak equivalent, ppe SPL) was conducted at each step (see Fig. 1(c)).
Fig. 1.
(a) 200 µm laser fiber relative to the recording microphone. Initially, the fiber tip was 2 mm away from the center of the microphone with its axis parallel to the microphone membrane. From there, it was removed in 2 mm steps. (b) Biphasic sound signal (asterisks) generated by a 100-µs laser pulse and 0.05 J/cm2 radiant exposure. The laser fiber is positioned at the starting point described in (a). Laser onset at 0 ms. Note: The time between the two pressure peaks is exactly 100 µs (see inset). The baseline returns back to 0 mPa within 5 ms. (c) Sound pressure levels [peak-to-peak equivalent] of the recorded signal with increasing distance between laser fiber and microphone (n = 4). The solid line represents mean ± standard deviation. Gray lines represent single measurements. λ = 1850 nm, 33 Hz, 100 µs pulsewidth and 0.35 J/cm2. (d) Sound pressure levels [peak-to-peak equivalent] of the recorded signal with different radiant exposure (n = 4). The laser fiber is positioned at the starting point described in (a). The solid line representsmean±standard deviation. Gray lines represent single measurements. λ = 1850 nm, 33 Hz, 100 µs pulsewidth.
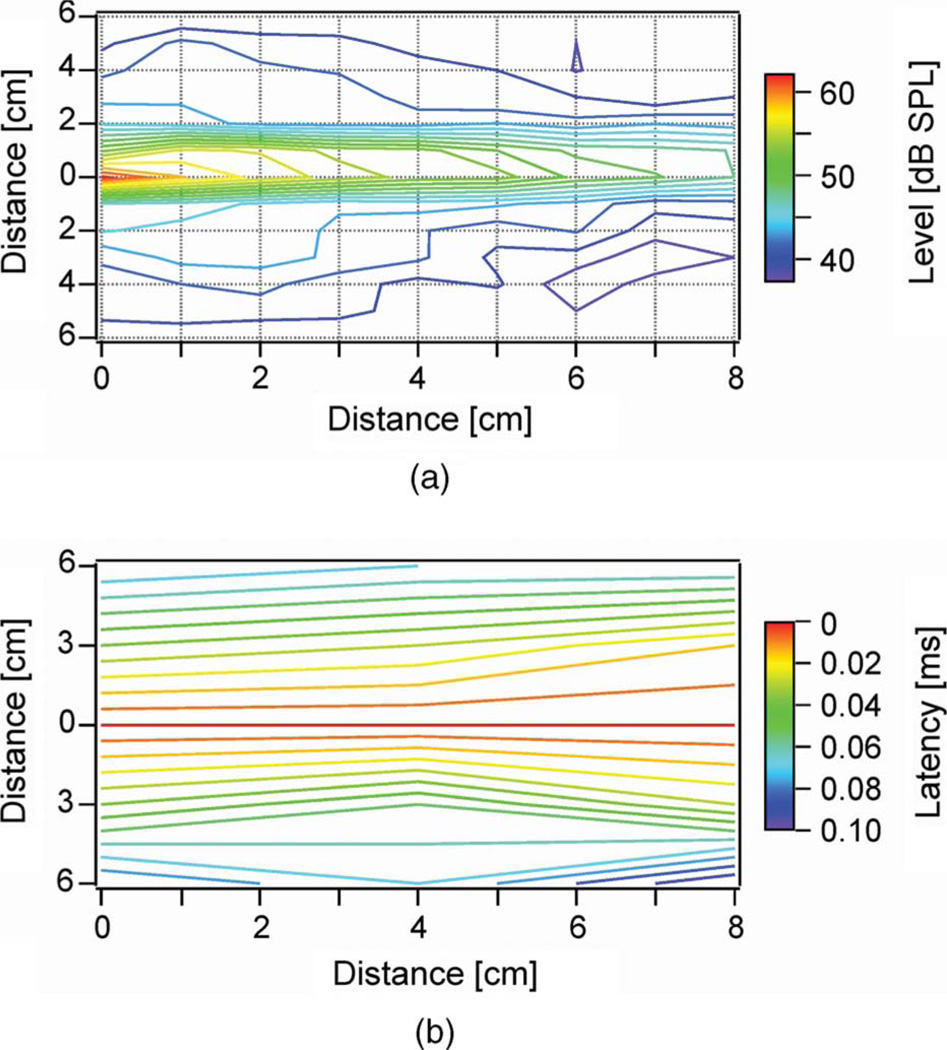
In a second set of experiments, the fiber tip was oriented toward the center of the microphone but did not touch the membrane of the microphone; it was oriented toward its casing. The laser fiber was thus perpendicular to the membrane of the microphone. The fiber tip was moved away from the microphone using 1 cm steps in each direction. At each position, sound measurements were performed to create a 2-D sound field map (see Fig. 2(a)).
Fig. 2.
(a) Sound field measured in front of the 200 µm diameter laser fiber. The fiber tip position was at 0 on the abscissa.A1/2-in microphone was retracted along the x-axis. Laser settings: λ = 1850 nm, 0.17 J/cm2 radiant exposure, 50.5 µs pulsewidth, and a 33 Hz repetition rate. (b) Latency differences of the laser-induced sound pressure waves depicted in (a). Note that almost no latency shift could be measured along the center beam pathway (at 0 cm on the ordinate) with increasing distance up to 8 cm. Perpendicular to the length of the center beam latency shifts could be noticed (3 cm perpendicular to the center beam at 0 cm distance latency differences were 0.06 ms).
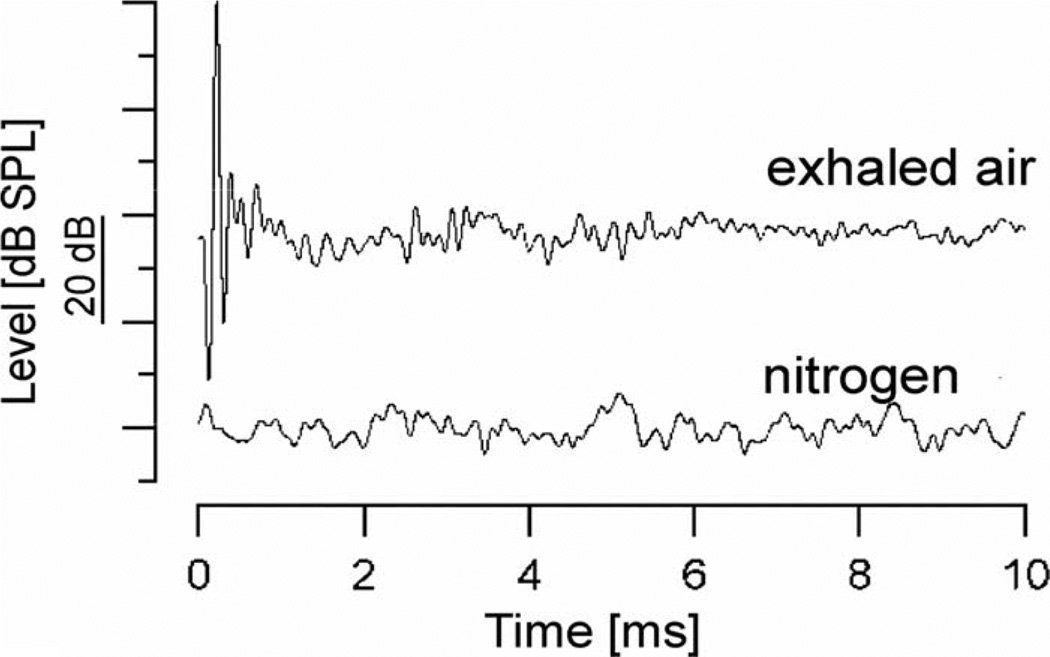
To verify that water vapor is the absorber for the optical radiation in air, qualitative measurements were conducted while air humidity was changed. For the measurements, a tube 5 cm in diameter and 30 cm in length was closed at both ends with wax paper. The optical fiber was inserted at one end of the tube through the wax paper. At the opposite end, a small hole (<1 mm) was made in the cover to allow constant gas flow through the chamber. Near the end of the optical fiber, a small insertion hole was drilled into the polyvinyl tube and a 1/8 in Bruel and Kjær microphone was inserted with the microphone’s membrane parallel to the optical path. Sound-pressure measurements were conducted for two conditions: 1) after the tube was ventilated for 2 min with dry nitrogen, and 2) after breathing for approximately 5 min into the tube. The two conditions are described as dry and humid.
2) Measurements in the Water
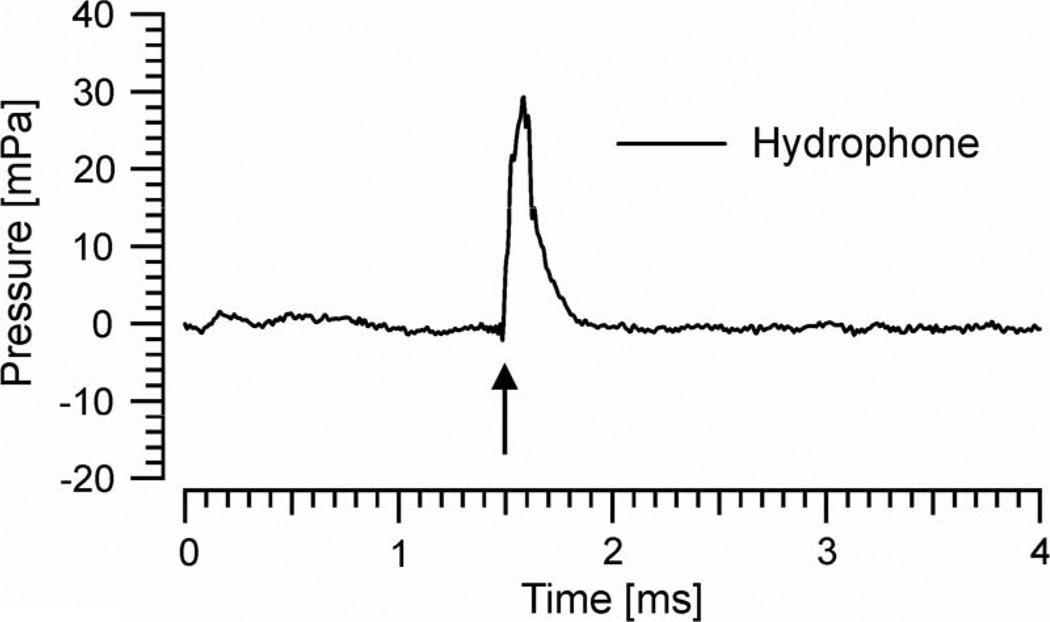
Sound-pressure measurements were also conducted in a swimming pool (>50 m3). A hydrophone (Reson TC4013, Slangerup, Denmark) was connected to a 24-bit soundcard (Hammerfall DSP Multiface, RME, Heimhausen, Germany) of a notebook to register pressure waves in the water. The hydrophone was >2 m away from the walls and the ground of the pool, as well as from the water surface. The tip of the optical fiber was oriented perpendicular to the hydrophone with 5 mm distance to its membrane.
C. In Vivo Experiments
In vivo experiments were conducted on six adult rats. All experimental procedures were approved by German state authorities and were in accordance with the EU and German guidelines for animal research.
1) Rat surgery and Monitoring of Cochlear Function
Adult Sprague–Dawley rats (age 1–3 month) were anesthetized with an initial intraperitoneal (i.p.) injection of 50 mg/kg ketamine and 10 mg/kg xylazine. The level of anesthesia was monitored by the respiratory rate and a paw-pinch withdrawal reflex. If needed, one-third of the initial dose was injected to maintain deep anesthesia. Heart rate, respiratory rate, and temperature were monitored. The body temperature was kept above 37 °C. A tracheotomy was performed and an endotracheal tube was placed to ventilate the animal. End-tidal CO2 concentrations were monitored throughout the experiment. Animal heads were secured in a headholder. To surgically access the cochlea, a retroauricular incision was made. Soft tissue and neck muscles were dissected to access the bulla. The bulla was surgically opened and the lateral wall was removed to access all turns of the cochlea including the round window niche. Care was taken not to damage the tympanic membrane and the stapedial artery.
Cochlear function was confirmed using click-evoked auditory brainstem responses (ABRs). For the measurements, two subcutaneous Ag/AgCl electrodes were inserted: one at the level of the interauricular line and the other retroauricularly. For stimulation, 50 µs condensation clicks (DT48, Beyerdynamic, Heilbronn, Germany) were applied through a plastic otoscopic conus positioned into the external meatus (closed system). Sound levels were monitored for calibration using a KE4 electric microphone (Sennheiser, Wedemark, Germany). Signals were amplified 80 dB and were bandpass-filtered 0.5–5 kHz using an amplifier (V2) and filter (F1, Otoconsult Company, Frankfurt, Germany). Responses to 100 click-stimulus presentations were averaged. Cochlear function was considered normal if the ABR threshold was below 40 dB SPL.
Subsequently, cochlear compound action potentials (CAPs) were recorded with a silver electrode (impedance < 5 kΩ), which was secured at the bony rim of the round window. A reference electrode was placed under the skin of the posterior neck. The signals were preamplified 40 dB using a V2 amplifier (Otoconsult Company), further amplified 20 dB and filtered (0.01–10 kHz; Amplifier F1, Otoconsult Company). The signals were digitized with a 100 kHz sampling rate. The response to 100 stimulus presentations were averaged and stored on the computer for offline analysis.
Laser-evoked CAPs (LCAP) were recorded afterward. In addition, they were acoustically masked with a noncoherent white-noise signal (HP Noise Generator 3722A, Great Britain) presented in a continuous fashion at different sound pressure levels via a calibrated DT48 loudspeaker (Beyerdynamic). The masker started 10 ms before the laser pulse and continued for the entire recording.
III. Results
A. In Vitro Experiments
Pulsed NIR laser light (pulse duration: 100 µs) reproducibly generated an acoustic event (see Fig. 1). In the time domain, two peaks of different polarity were recorded at the beginning and the end of the laser pulse, respectively (see Fig. 1(b)).With increasing distance between fiber tip and the microphone, a decrease in sound pressure was observed (see Fig. 1(c)). Increasing the radiant exposure lead to higher peak pressures (see Fig. 1(d)). Repetition rates between 2 and 33 Hz, and wavelengths between 1845 and 1855 nm revealed no measurable difference between the peak-pressure levels obtained 2 mm from the optical fiber tip (data not shown).
Next, a 2-D sound field was determined using a 1/2 in Bruel & Kjær microphone. The sound field was confined to a cylinder along the optical path of the fiber’s direction. Peak sound pressure levels reached a maximum of 62 dB ppe SPL close to the fiber’s tip. Even at a distance of 8 cm from the optical fiber’s beam, peak-pressure levels of 44 dB ppe SPL were detected (see Fig. 2(a)). In the center of the sound field, the same onset latency was measured along a distance of 4 cm in front of the fiber. However, perpendicular to the fiber’s tip, the delay increased with increasing distance, 3 cm away from the fiber resulting in a delay of 0.06 ms (see Fig. 2(b)).
To investigate whether water vapor might be the absorber for optical radiation in the air, sound-level measurements were conducted in a closed tube under humid and dry conditions. In the humid condition, which was generated by breathing into the tube, a similar sound event was recorded as in room air. However, when the humid air was replaced by dry nitrogen, no pressure waves could be elicited (see Fig. 3). This indicates that the absorption of the radiation by oxygen, carbon dioxide, or water, and the subsequent conversion of the radiation into heat generated the pressure. Based on the absorption characteristics of the NIR radiation, water is the most probable candidate [15].
Fig. 3.
Recorded sound pressure levels over time in front of a 200 µm diameter fiber positioned in a sealed tube filled with two different types of gases (nitrogen versus exhaled air). Note that no sound pressure could be measured in pure nitrogen gas. Laser settings were λ = 1850 nm, 33 Hz repetition rate, 100 µs pulse 0.35 J/cm2 radiant exposure.
To support this conclusion, acoustic measurements were also performed in water. For this purpose, hydrophone recordings were conducted in a large swimming pool (>50 m3). For the given experimental conditions, wave reflections from the pool walls are minimized and would occur >2 ms after the initial stimulus (thus would not interfere with the original event).With 0.35 J/cm2 radiant exposure, hydrophone recordings of 31 mPa or 89.9 dB ppe (N.B.: for water, the reference value of 1 µPa was used) were recorded at 5 mm distance from the fiber’s tip (see Fig. 4). In summary, pressure waves appear with the present laser settings also in fluids.
Fig. 4.
Hydrophone signal in mPa after immersing the 200 µm fiber in a large swimming pool. The tip of the fiber was ~5 mm away from the hydrophone, positioned perpendicular to the microphone axis (like in Fig. 1a). Laser onset indicated by arrow. Laser settings: λ = 1850 nm, 0.35 J/cm2 radiant exposure, 100 Hz, 100 µs pulsewidth.
B. In Vivo Experiments
Before initiation of laser stimulation in the cochlea, cochlear function was determined using click-evoked ABRs. The average ABR threshold was 35 ± 4 dB SPL (n = 6). Opening the bulla elevated the thresholds on average by 5 ± 3 dB SPL, and thus, affected the hearing threshold minimally.
1) Laser Stimulation of the Hearing Rat Cochlea
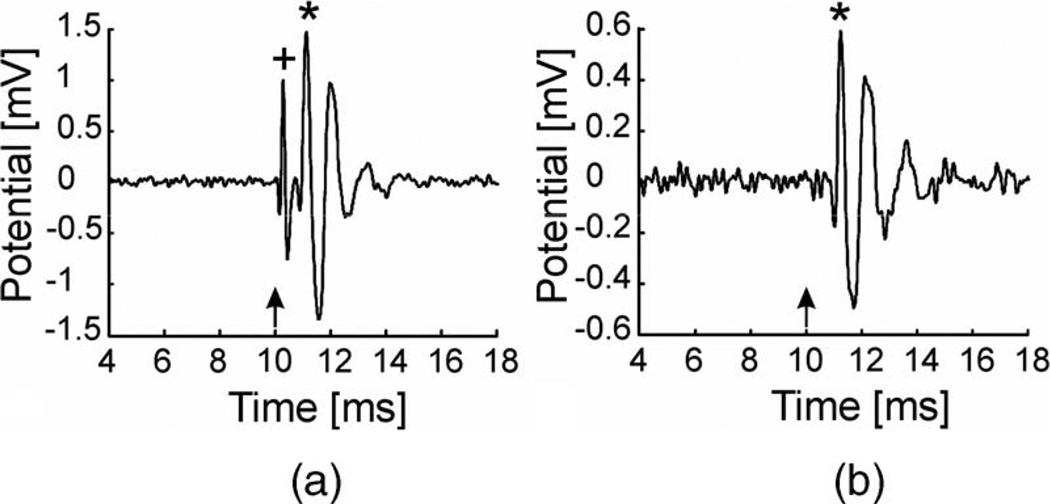
Laser-evoked CAPs (LCAPs) could be recorded with laser stimulation at the round window and along the cochlea. Depending on the orientation of the optical fiber, the response pattern recorded at the round window changed. When the optical fiber was oriented toward the round window or the cochlea 1 mm from the cochlear middle turn, a two-component signal was obtained (see Fig. 5(a)). The first component (peak) revealed a short latency and a small amplitude; the second component (peak) yielded a longer latency and larger amplitude (see Table I). To examine the effect of the optical fiber’s position on the recorded signal, different stimulation configurations were tested. While the laser beam was scanned over the cochlea or was located close to the cochlea, the two-component signal was elicited. Increasing the distance of the laser beam from the cochlea, both components decreased in amplitude so that only the second (larger) component remained when the laser beam was directed away from the cochlea (see Fig. 5(b)). Interestingly, the distance of the tip of the fiber from the cochlea was less important than the distance of the laser beam (see Section IV). In summary, the first response appeared only if the laser beam was very close to the cochlea; however, the second component could also be recorded in conditions where the laser beam was located further away.
Fig. 5.
(a) LCAP recorded with a 200 µm fiber oriented toward the middle cochlear turn. The distance between the fiber tip and the cochlea was 1 mm. Laser settings: λ = 1850 nm, 33 Hz, 100 µs pulsewidth 0.35 J/cm2 radiant exposure. Laser onset was at 10 ms (arrow). Note the two components of the CAP at 0.3 ms (+) and 1.14 ms (*) poststimulus. The amplitudes were 1.9 and 2.8 mVpp, respectively. (b) LCAP recorded with a 200 µm fiber oriented 2 mm next to the cochlea inside the bulla. Laser settings as above. Only the second component (*) at 1.26 ms latency showed up with a smaller amplitude of 1.9 mVpp. Note: Different ordinate ranges in (a) and (b).
TABLE I.
Latency Energy and Amplitude Energy Relations for the First and the Second LCAP Component (First Positive Wave Processed, n = 6)
| 1st component | 2nd component | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| laser fiber [Ø] |
radiant exposer [J/cm2] |
latency mean [ms] |
SD ± |
amplitude mean [µV] |
SD ± |
n | radiant exposer [J/cm2] |
latency mean [ms] |
SD ± |
amplitude mean [µV] |
SD ± |
n |
| 200 µm | 0,35 | 0,27 | 0,03 | 1085 | 772 | 6 | 0,35 | 1,12 | 0,08 | 1998 | 958 | 6 |
| 0,28 | 0,27 | 0,03 | 958 | 704 | 6 | 0,28 | 1,14 | 0,11 | 1996 | 979 | 6 | |
| 0,20 | 0,25 | 0,03 | 845 | 516 | 5 | 0,20 | 1,14 | 0,12 | 1990 | 1006 | 6 | |
| 0,12 | 0,26 | 0,03 | 637 | 309 | 4 | 0,12 | 1,20 | 0,11 | 1961 | 996 | 6 | |
| 0,05 | 0,29 | 0,02 | 238 | 54 | 3 | 0,05 | 1,32 | 0,15 | 1425 | 842 | 6 | |
| 0,004 | - | - | - | - | - | 0,004 | 2,11 | 0,49 | 249 | 24 | 2 | |
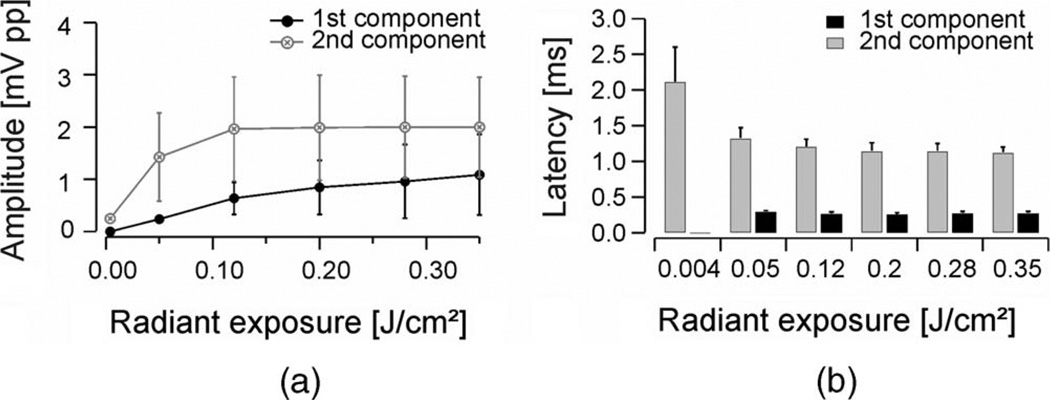
With the laser beam oriented toward the center of the cochlear middle turn, the radiant exposure was varied and the corresponding LCAP threshold was determined in six cochleae (see Fig. 6(a) and (b)). Amplitudes of the first component were up to ninefold smaller than those of the second component. The mutual relation of the amplitudes of the first and the second component in LCAP varied considerably. In no experiment, however, were the amplitudes of the second LCAP component smaller than those of the first one (see Table I). The threshold was determined for the first and the second LCAP component and was of 0.05 and 0.004 J/cm2, respectively. Amplitudes for the first component increased almost linearly with increasing laser radiant exposures (see Fig. 6(a)), as did the sound pressure levels measured with a microphone (see Fig. 1(d)) with radiant energies higher than 0.12 J/cm2. Amplitudes for the second component increased fast from their thresholds to reach a plateau at radiant exposures higher than 0.12 J/cm2 (see Fig. 6(a)).
Fig. 6.
(a) Peak-to-peak amplitudes of LCAP caused by laser stimuli at varying radiant exposures, fiber diameter 200 µm, λ = 1850 nm, 33 Hz, 100 µs pulsewidth. (b) Category plot of letencies of LCAPs recordings in respect to radiant exposure for the first and second component shown in (a).
Above threshold radiant exposures, average latencies for the first component did not change considerably and were in a range between 0.25 and 0.29 ms. Average latencies of the second component, however, revealed a systematic decrease from 2.1 ms at threshold radiant exposures of 0.004 J/cm2 to 1.12 ms at 0.35 J/cm2 (see Fig. 6(b)).
2) Masking Experiments
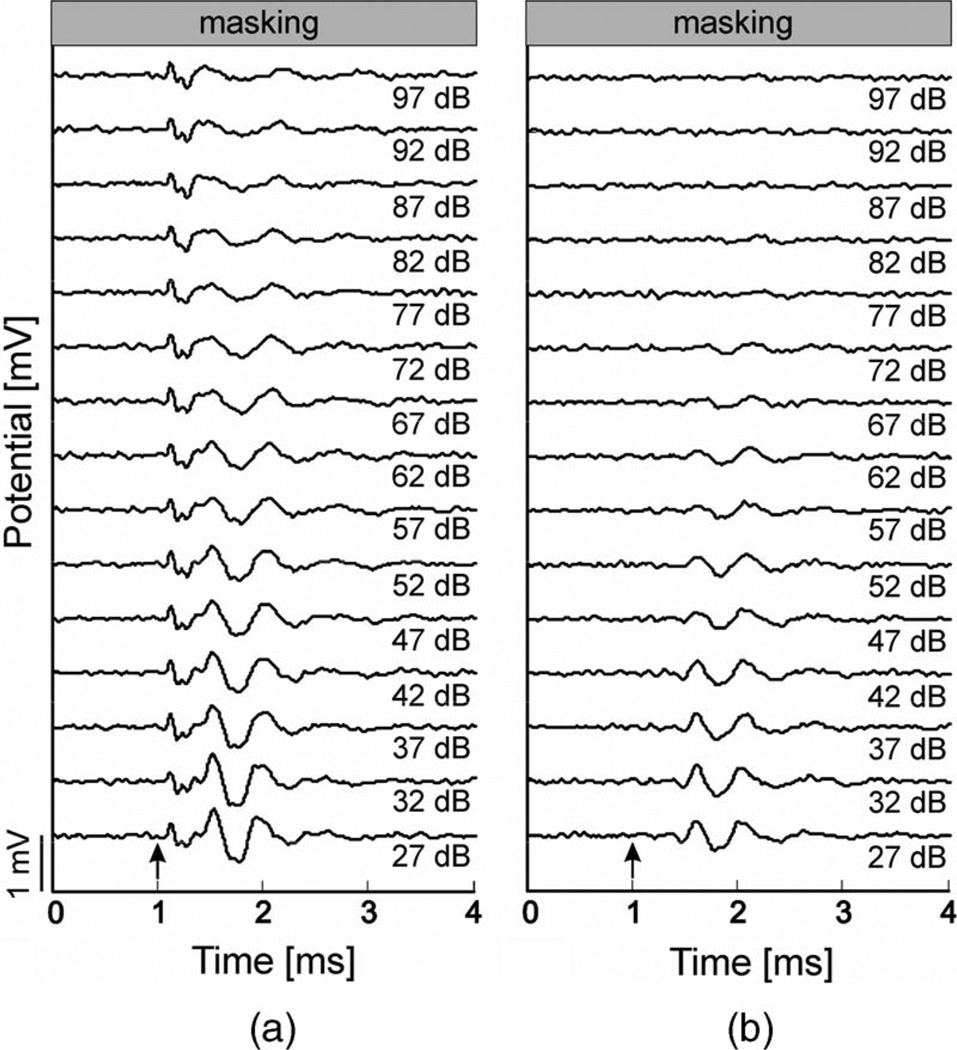
LCAPs were masked by simultaneous presentation of noncoherent white noise via a speaker. When the optical fiber was directed onto the cochlear middle turn (resulting in a two-component LCAP), the second component could be masked, whereas the early component decreased in amplitude (at maximum 10%) at high masking levels only (see Fig. 7(a)). The first component was thus difficult to mask by white noise. With the laser fiber at a greater distance to the cochlea, only the late component was recordable and could be completely masked by white noise (see Fig. 7(b)).
Fig. 7.
(a) Masking of laser-evoked LCAPs measured at the round window niche. Radiant exposure for stimulation of the middle cochlear turn was 0.28 J/cm2, 33 Hz, and 100 µs pulsewidth. Laser onset was at 1 ms (arrow). Noncoherent white noise was used for masking: 27–97 dB SPL with increasing levels at 5 dB steps. (b) Masking of LCAPs measured at the round window niche after orienting the 200 µm fiber next to the rat cochlea (laser setting and masking as in (a).
IV. Discussion
The present experiments revealed that pulsed NIR laser light with pulse lengths in the microseconds to milliseconds range generates an audible acoustic wave. Such an event is generated both in humid air as well as in water, and it can activate a hearing rodent cochlea. In addition to a response to direct neuronal stimulation, NIR lasers can also acoustically stimulate a hearing cochlea, generating an “optophonic” response.
A. Potential Mechanisms
The interactions between the incident radiation from a laser and the tissue can be described as photochemical, photomechanical, or photothermal. Therefore, radiation wavelength, pulse duration, radiant exposure of the laser, scattering, and absorption properties of the target tissue will determine which of the three mechanisms will dominate the laser–tissue interaction. For the NIR laser used in the present experiments, the photochemical interaction is unlikely.
When laser heating takes place in a time shorter or comparable to the time needed to initiate a collective motion of molecules within the absorbing target, this process is referred to as stress confinement and occurs for pulse duration under 1 µs.
For pulse durations longer than 1 µs but shorter than 200 ms, the optical energy delivered by a single pulse accumulates in the irradiated tissue before any of the heat can dissipate by conduction or convection and thermal confinement appears [16], [17]. The heating of a confined volume will result in an expansion of this volume, which can also be transmitted as an expansion wave.
All present experiments were performed in thermal confinement, comparable to previous studies using pulsed 1850-nm laser light [7]–[10]. As a result of the low-radiant exposures required for the optoneural cochlea stimulation (threshold 0.004 J/cm2), the generation of pressure waves was thought to be negligible. This study demonstrated that even with low-radiant exposure (0.004 J/cm2), laser-induced activation of the cochlea by sound waves takes place.
The question for this study was whether this expansion wave is audible. In general, photoacoustic (optoacoustic) effects are not a novel discovery. The description of a photoacoustic effect dates back to the year 1880 [18]. Optoacoustic sound generation by lasers is nowadays technically exploited with absorption in solids [19], liquids [20], and gases [21]. To our knowledge, this study is the first describing that the NIR-laser light used for direct neural stimulation at low-radiant exposures produces an audible pressure wave when operating in thermal confinement.
B. In Vitro Experiments—Pressure Waves in Air and Water
To test the pulsed 1850-nm laser for recordable sound phenomena, microphone recordings were performed in room air and water. In water, the absorption of 1850-nm laser light is reported with lλ=1850 nm ≈ 0.84 mm [15]. As the experiments presented here show, this absorption is sufficient to generate an acoustic pressure wave.
In air, the recorded pressure waves were most likely generated by the absorption of laser radiation in water vapor. With ambient conditions during the experiment being 21 °C and 35% relative humidity an absolute humidity of 6.46 g/m3 or 0.358 mmol/cm3 (molar mass of water 18.015 g/mol) can be calculated. Thus, with the absorption of water using the Lambert–Beer law, a length constant of 1/(ε′c) = lλ=1850 nm ≈ 13.0 cm results for these conditions. Hence, the laser absorption is not restricted to a small volume at the outlet but to an extended narrow cone in front of the fiber. The power density along the direction of the laser beam at a distance d from the fiber exit is given by
where r is the radius of the laser beam at a distance d from the optical fiber tip, ε′ the molar absorption coefficient of water and c the concentration. Assuming a linear relationship between sound pressure and power density, the data correspond qualitatively well, showing a “stripe-like” sound source with decreasing intensity along the laser beam (see Fig. 2(a)). However, in biological environments, the parameters might differ substantially from the above considerations (e.g., 44.2 g/m3 H2O at 100% relative humidity and 37 °C), leading to a less extended sound generation site and more spherical wavefront.
Whereas sound pressure waves in air reached up to 62 dB ppe SPL, well in the audible range, the latencies of the sound signals in front of the laser fiber were consistent with an axially extended sound source along the center beam path (see Fig. 2(b)). Peak pressures appeared simultaneously along the laser beam axis but were delayed for off-axis positions. For the sound propagation velocity in air (t = 20 °C) of 343 m/s, a delay of 0.089 ms would have been expected for a distance of 3 cm. In our experiment, the latency isocontour plot (see Fig. 2(b)) revealed a time delay of 0.06 ms. The difference can be explained by shortening the effective distance by the radius of the microphone (~0.63 cm) and the radial extension of the generation site. This result demonstrates that the pressure wave is generated along the laser beam and propagates sideways as a sound wave.
The cochlea is filled by endo- and perilymph, thus it is of interest to what extent laser-induced sound waves exist when the absorbing volume is significantly decreased by immersion in water. Measurements in a swimming pool showed that radiant exposures of 0.35 J/cm2 generated a sound pressure of 31 mPa in water. Within the cochlea, this 89.9 dB ppe (referred to water with reference value 1 µPa) is enough pressure to cause either a direct hair cell deflection or to set the basilar membrane into motion. Laser-induced pressure waves in water are well documented for experiments with high local absorption [22], [23].
Recently, 10 ns pulses of green laser light (532 nm) were used to vibrate circumscribed parts of the Guinea pig basilar membrane [14], [24] with the goal of restoring outer hair cell function. The green laser is not absorbed in water but in cellular structures and operates in stress confinement and not in thermal confinement as the laser used in this study. Regardless of the underlying optomechanical process, highly focused laser-induced pressure waves yield an interesting approach for stimulating the hearing impaired cochlea, but may have the disadvantage of acoustically generated artifacts in pure neural applications.
C. In Vivo Experiments—The “Optophonic” Response
With the present experiments, we have demonstrated that the pressure wave generated by low-level pulsed NIR-laser light is sufficient to evoke a cochlear response through acoustical stimulation of a hearing cochlea. This happened with laser pulses in the microsecond range, far away from the region of stress confinement. Measurements in hearing rats revealed a two-component LCAP when the optical fiber was close to the cochlea and a single LCAP when the fiber was further away from the cochlea. The single-component LCAP and the late component of the two-component LCAP revealed a mean latency of 1.34 ms and could be masked by acoustic white noise (see Fig. 7(a) and (b)), thus showing all characteristics of an acoustic CAP response (see Table I). In adult cats, CAP latencies are reported between 1.2 and 1.8 ms, depending on stimulus intensity [25]. This corresponds to the here presented LCAP mean latency of 1.34 ms. In rats, acoustic brainstem audiometry (ABR) wave I latencies (generated in the auditory nerve) were published that are close to the here determined CAP latency: wave I latencies in rats were 1.52–1.6 ms for sinusoidal stimulation at 8 and 6 kHz, respectively [26], and about 1.55 ms [27] for 60 dB SPL clicks.
This study analyzed laser-induced sound waves generated by low laser pulse energies. In the experiments, optophonic responses could be recorded with radiant exposure down to 0.004 J/cm2. Based on the present results, the second response of LCAP represents a cochlear response to the sound elicited by the laser. In analogy to the “electrophonic response” [28], it thus represents an “optophonic response”—a response of the hearing cochlea (hair cells) to the laser. In contrast to electric stimulation, however, this response is not due to direct hair cell stimulation, but due to an indirect acoustic event generated by the laser beam when it hits water molecules.
D. First LCAP Component
In addition to the second component, another response with a shorter latency could be recorded when the laser fiber targeted the cochlea or was positioned very close to the cochlea. This response was more difficult to mask and had a short latency (0.27 ms). Latencies of afferent axons responses have been above 0.8 ms [29], much longer than the present first component. One possible source of the signal would be a direct stimulation of primary afferent neurons through the cochlear wall (optoneural effect). The aforementioned latency of 0.27 ms, however, is much shorter than latencies obtained with direct electrical stimulation of the auditory nerve. For example, latencies of electrically evoked CAP with monophasic current pulses delivered to a monopolar intracochlear electrode (cathodic stimuli) have P2 latencies from 0.67 to 0.82 ms for cats and from 0.65 to 0.75 ms for Guinea pigs [30]. The short latency of the first LCAP component and its linear relation to radiant exposure makes a transcochlear optoneural stimulation in the present setup (stimulation from outside of the cochlea) improbable. In addition, the long pathway of the laser to the neurons, passing through water like perilymph (a good absorber) and bone, makes a direct stimulation unlikely. Another candidate for the early signal would be bone conduction versus air conduction. However, bone conduction has been reported with longer, not shorter, latencies than air conduction, and thus, yields no explanation for the short latency of the first component [31], [32]. Artifacts caused by laser light interactions with the electrode could be ruled out since the signal disappeared ~4 min postmortem in the euthanized animal.
A possible explanation for the early component is a cochlear microphonic potential (CM). CMs generated in hair cells have latencies of 0.2–0.5 ms [33], and are known to have a similar relation of amplitudes to sound pressure level. Dependent on sound frequency, a linearity remains up to a “limit of linearity,” above which the input–output function flattens [34]. In Guinea pigs, a linear increase of CM could be recorded with acoustic stimuli up to 80 dB SPL, and even at 100–110 dB SPL, a further increase in CM amplitude is reported [35].As the sound pressure level of the acoustic event increases in an almost linear fashion with radiant exposure above 0.08 J/cm2 (see Fig. 1(d)), the first component could represent CMs. When stimulating with laser pulses of longer duration (up to 2 ms) in vivo, signals of different polarity were revealed at the on- and offset of the laser pulse when masking the CAP with noise (data not shown). This result closely corresponds to the signal picked up by the microphone, again corresponding to the microphonic potentials. The difficulty to mask CM by white noise is easy to explain: masking CAPs acts by desynchronizing the action potentials of the auditory nerve. In microphonics, such desynchronization unlikely takes place; microphonics reflect the salient properties of the basilar membrane vibrations and not the properties of the auditory nerve fibers and the laser response can still be found in the average. Similarly, click-evoked CMs were difficult to mask with white noise (data not shown).
In conclusion, the laser beam not only generates a pressure wave in thermal confinement condition, the cochlea of an animal also responds to this pressure wave by generating a CM response and a CAP. Optophonic responses must be considered one component of the response of a hearing cochlea to pulsatile laser stimulation in microsecond range. Under conditions used for neural stimulation, optoacoustic effects are serious by-products. It is highly probable that previous reports represented a mixture of a true neural stimulation and optoacoustic effects. More effort in controlling the animals hearing conditions is required if the stimulated cochlea is only partially deaf.
E. Future Directions
The laser-induced neural stimulation of cochlear structures is an interesting alternative to electrical stimulation with cochlear implants. Since laser fibers are small and flexible, an insertion into the cochlea as with traditional cochlea implant electrodes could be possible. A previous study on the insertion force of laser fibers revealed similar forces as those produced with traditional CI electrodes [36]. Thus, research facilities in the auditory field should explore the potential of optoneural cochlea stimulation to restore hearing loss as an alternative to electrical stimulation. However, the acoustic component during neural laser stimulation could also yield a disturbing artifact that has to be overcome in a partially deaf cochlea. Considering patients with residual hearing only as candidates for optical cochlear implants, the acoustic by-product might be negligible. More studies in deafened cochleae are aim of a next study.
Combining the optoacoustic stimulation of cochlear structures (optophonic hearing) with direct laser stimulation of neural tissues within the cochlea (optoneural hearing) has a potential for a new generation of optically driven cochlear implants. Further studies need to analyze different laser wavelengths, pulsewidths, and repetition rates on multiple locations, in hearing and deafened cochleae, to reveal the optimal parameters for both optophonic and optoneural stimulation in the mammalian cochlea.
Acknowledgment
The authors thank D. Bystron and D. Kühne for excellent technical support and collaboration.
The work of C.-P. Richter was funded by the Federal funds from the National Institute of Deafness and Other Communication Disorders, Department of Health and Human Services, National Institutes of Health (NIH) under Contract HHSN260-2006-00006-C/Contract NIH N01-DC-6-0006. The work of I. Teudt and A. Kral was supported by Deutsche Forschungsgemeinschaft (SFB Transregio 37 Project A5). Pilot experiments for this study were performed in A. Kral’s laboratory at the Institute of Neurophysiology, University Clinics Hamburg-Eppendorf before moving the laboratory to Hannover.
Biographies

Ingo Ulrik Teudt was born in Hamburg, Germany, in 1976. He received the M.D. degree from the University Clinic Hamburg-Eppendorf, Eppendorf, Germany, in 2004.
Since 2010, he has been with the Department of Otolaryngology and the Institute of Audioneurotechnology (VIANNA),Medical School Hannover, Hannover, Germany. From 2004 to 2009, he was with the Department of Otolaryngology, University Clinic Hamburg-Eppendorf, where he received his doctoral thesis from the Institute of Molecular Biology in Prof. Beisiegels Laboratory in 2006. He was a Postdoctoral Researcher in Prof. Richters Laboratory, Department of Otolaryngology, Northwestern University, Chicago, IL, in 2006. His current research interests include the development and improvement of cochlear implant devices, and new strategies for cochlear stimulation using optoacoustic stimulation.

Hannes Maier was born in Frankfurt, Germany, May 2, 1958. He received the P.D. degree in experimental audiology/neurootology from the Hamburg University Medical School, Hamburg, Germany, and the Ph.D. degree in physics from the Max-Planck Institute for Biophysics, Frankfurt, Germany, in 1993.
Since 2011, he has been a Full-Time Research Scientist with the ENT Department and the Institute of Audioneurotechnology (VIANNA), Medical University Hannover, Hannover, Germany. From 2008 to 2010, he was Full-Time Consultant for research at Phonak Acoustic Implants SA, Lonay, Switzerland. He was a Scientist and the Head of Audiology in the Department of Otolaryngology, Hamburg University Medical School, from 1998 to 2008. He was a Project Manager/Postdoctoral Researcher in the Hearing Research Laboratory, Department of Otolaryngology, Tübingen University, Germany, until 1997. The author studied physics and mathematics at Gießen University, Germany, and the Université de Paris Sud, Paris, France, from 1981 to 1984. His current research interests include implantable hearing aids for direct, round window, and third window mechanical stimulation of the human cochlea.

Claus-Peter Richter was born in Erlangen, Germany. He received the Medical degree from the Johann Wolfgang Goethe-University, Frankfurt, Germany, the Masters degree in physics from the Max-Planck Institute for Biophysics, Frankfurt, and the Habilitation degree (“Privatdozent”) from the Department of Physiology, Johann Wolfgang Goethe-University.
Since 2008, he has been an Associate Professor of otolaryngology and is currently the Director of the Resident Research in the Department of Otolaryngology, Northwestern University, Chicago, IL. He has joint appointments in the Departments of Biomedical Engineering and Communication Sciences and Disorders, and is a Fellow of the Hugh Knowles Foundation. He joined the Laboratory of Dr. Dallos at the Northwestern University, in 1996, and became a Full-Time Faculty at the Northwestern University Medical School in 2002. His current research interests include the development and improvement of cochlear implant electrodes, the micromechanics of the mammalian cochlea, and the maturation of the mammalian inner ear. Recently, the research efforts of his laboratory are focused to develop cochlear implants that use optical radiation from pulsed lasers rather than electrical currents to stimulate auditory neurons.

Andrej Kral was born in Bratislava, Slovak Republic. In 1994 he received the Medical degree from the Comenius University, Bratislava, in 1994, and the Ph.D. degree in 1998 from the same university.
Since 2009, he has been a Full Professor of auditory neuroscience and the Director of the Institute of Audioneurotechnology and the Department of Experimental Otology, Medical University Hannover, Hannover, Germany. Since 2004, he has been an Adjunct Professor of cognition and neuroscience with the School of Behavioral and Brain Sciences, University of Texas at Dallas. He was appointed Associate Professor (“Privatdozent”) in the Department of Physiology, Johann Wolfgang Goethe-University, in 2002. In 2005, he was appointed Professor of neurophysiology with the University Clinics Hamburg-Eppendorf, heading the Laboratory of Auditory Neuroscience. His current research interests include auditory prostheses, cochlear implants, and maturation and plasticity of the central auditory system, as well as binaural cochlear implants and effects of deafness on the auditory system.
Footnotes
Color versions of one or more of the figures in this paper are available online at http://ieeexplore.ieee.org.
Contributor Information
Ingo Ulrik Teudt, Institute of Audioneurotechnology and the Department of Experimental Otology ENT-Clinics, Hannover School of Medicine, 30625 Hannover, Germany (teudt.ingo@mh-hannover.de).
Hannes Maier, Institute of Audioneurotechnology and the Department of Experimental Otology ENT-Clinics, Hannover School of Medicine, 30625 Hannover, Germany (maier.hannes@mh-hannover.de).
Claus-Peter Richter, Department of Otolaryngology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611 USA (cri529@northwestern.edu).
Andrej Kral, Institute of Audioneurotechnology and the Department of Experimental Otology ENT-Clinics, Hannover School of Medicine, 30625 Hannover, Germany (kral.andrej@mh-hannover.de).
References
- 1.Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A. Application of infrared light for in vivo neural. J. Biomed. Opt. 2005;vol. 10:064003-1–064003-6. doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- 2.Wells J, Kao C, Konrad P, Milner T, Kim J, Mahadevan-Jansen A, Jansen ED. Biophysical mechanisms of transient optical stimulation of peripheral nerve. J. Biophys. 2007;vol. 93:2567–2580. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells J, Konrad P, Kao C, Jansen ED, Mahadevan-Jansen A. Pulsed laser versus electrical energy for peripheral nerve stimulation. J. Neurosci. Methods. 2007;vol. 163:326–337. doi: 10.1016/j.jneumeth.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teudt IU, Nevel AE, Izzo AD, Walsh JT, Jr, Richter CP. Optical stimulation of the facial nerve: A new monitoring technique? Laryngoscope. 2007;vol. 117:1641–1647. doi: 10.1097/MLG.0b013e318074ec00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried NM, Lagoda GA, Scott NJ, Su LM, Burnett AL. Laser stimulation of the cavernous nerves in the rat prostate in vivo: Optimization of wavelength, pulse energy, and pulse repetition rate. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2008;vol. 2008:2777–2780. doi: 10.1109/IEMBS.2008.4649778. [DOI] [PubMed] [Google Scholar]
- 6.Fork RL. Laser stimulation of nerve cells in Aplysia. Science. 1971 Mar;vol. 171:907–908. doi: 10.1126/science.171.3974.907. [DOI] [PubMed] [Google Scholar]
- 7.Izzo AD, Suh E, Pathria J, Walsh JT, Whitlon DS, Richter CP. Selectivity of neural stimulation in the auditory system: A comparison of optic and electric stimuli. J. Biomed. Opt. 2007;vol. 12:021008-1–021008-7. doi: 10.1117/1.2714296. [DOI] [PubMed] [Google Scholar]
- 8.Izzo AD, Walsh JT, Jr, Jansen ED, Bendett M, Webb J, Ralph H, Richter CP. Optical parameter variability in laser nerve stimulation: A study of pulse duration, repetition rate, and wavelength. IEEE Trans. Biomed. Eng. 2007 Jun;vol. 54(no. 6):1108–1114. doi: 10.1109/TBME.2007.892925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter CP, Bayon R, Izzo AD, Otting M, Suh E, Goyal S, Hotaling J, Walsh JT., Jr Optical stimulation of auditory neurons: Effects of acute and chronic deafening. Hear Res. 2008;vol. 242:42–51. doi: 10.1016/j.heares.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajguru SM, Matic AI, Robinson AM, Fishman AJ, Moreno LE, Bradley A, Vujanovic I, Breen J, Wells JD, Bendett M, Richter C-P. Optical cochlear implants: Evaluation of surgical approach and laser parameters in cats. Hear Res. 2010 Jul;vol. 269:102–111. doi: 10.1016/j.heares.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attenborough K, Qin Q. Aspects of Laser-Generated Acoustic Shock Waves in Air. Hull, U.K: Univ. Hull, Acoustics Res. Centre; 2003. [Google Scholar]
- 12.Qin Q, Attenborough K. Characteristics and application of laser-generated acoustic shock waves in air. Appl. Acoust. 2004;vol. 65:325–340. [Google Scholar]
- 13.van Leeuwen TG. Pulsed laser tissue interaction. In: Welch AJ, Van Gemert MJ, editors. Optical-Response of Laser-irradiated Tissue. New York, Berlin: Springer; 1995. pp. 712–714. [Google Scholar]
- 14.Wenzel GI, Balster S, Zhang K, Lim HH, Reich U, Massow O, Lubatschowski H, Ertmer W, Lenarz T, Reuter G. Green laser light activates the inner ear. J. Biomed. Opt. 2009;vol. 14:044007-1–044007-6. doi: 10.1117/1.3174389. [DOI] [PubMed] [Google Scholar]
- 15.Hale GM, Guerry MR. Optical constants of water in the 200 nm to 200 mm wavelength region. Appl. Opt. 1973;vol. 12:555–563. doi: 10.1364/AO.12.000555. [DOI] [PubMed] [Google Scholar]
- 16.Jacques SL. Laser–tissue interactions. Photochemical, photothermal, and photomechanical. Surg. Clin. North Amer. 1992 Jun;vol. 72:531–558. doi: 10.1016/s0039-6109(16)45731-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhigilei LV, Garrison BJ. Microscopic mechanisms of laser ablation of organic solids in the thermal and stress confinement irradiation regimes. J. Appl. Phys. 2000;vol. 88:1281–1298. [Google Scholar]
- 18.Bell AG. On the production and reproduction of sound by light: The photophone. Proc. Amer. Assoc. Adv. Sci. 1880;vol. 29:115–136. [Google Scholar]
- 19.Pan Y, Perton M, Audoin B, Rossignol C. Acoustic waves generated by a laser point pulse in a transversely isotropic cylinder. J. Acoust. Soc. Amer. 2006;vol. 119:243–250. doi: 10.1121/1.2139648. [DOI] [PubMed] [Google Scholar]
- 20.Antonelli L, Blackmon F. Experimental demonstration of remote, passive acousto-optic sensing. J. Acoust. Soc. Amer. 2004;vol. 116:3393–3403. doi: 10.1121/1.1811475. [DOI] [PubMed] [Google Scholar]
- 21.Yonak SH, Dowling DR. Gas-phase generation of photoacoustic sound in an open environment. J. Acoust. Soc. Amer. 2003;vol. 114:3167–3178. doi: 10.1121/1.1628250. [DOI] [PubMed] [Google Scholar]
- 22.Blackmon F, Antonelli L. Remote, aerial, trans-layer, linear and nonlinear downlink underwater acoustic communication. Proc. Oceans. 2006 Sep;:1–7. [Google Scholar]
- 23.Sodha MS, Rai V, Verma MP, Konar S, Maheshwari KP. Underwater optical generation of sound: Oblique incidence. Pramana. 1993 Jul;vol. 41:1–7. [Google Scholar]
- 24.Zhang K, Wenzel G, Balster S, Lim H, Lubatschowski H, Lenarz T, Ertmer W, Reuter G. Optoacoustic induced vibrations within the inner ear. Opt. Express. 2009;vol. 17:23037–23043. doi: 10.1364/OE.17.023037. [DOI] [PubMed] [Google Scholar]
- 25.Moore DR. Development of the cat peripheral auditory system: Input–output functions of cochlear potentials. Brain Res. 1981;vol. 219:29–44. doi: 10.1016/0006-8993(81)90265-1. [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Tokuriki M, Shibamori Y, Saito T, Nojyo Y. Cochlear nerve demyelination causes prolongation of wave I latency in ABR of the myelin deficient (md) rat. Hear Res. 2004;vol. 191:119–124. doi: 10.1016/j.heares.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Popelar J, Groh D, Pelanova J, Canlon B, Syka J. Age-related changes in cochlear and brainstem auditory functions in Fischer 344 rats. Neurobiol. Aging. 2006;vol. 27:490–500. doi: 10.1016/j.neurobiolaging.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Moxon E. Ph.D. dissertation. Cambridge, MIT: MIT; 1971. Neural and mechanical responses to electric stimulation of the cat’s inner ear. [Google Scholar]
- 29.Ruggero MA, Rich NC. Timing of spike initiation in cochlear afferents: Dependence on site of innervation. J. Neurophysiol. 1987 Aug;vol. 58:379–403. doi: 10.1152/jn.1987.58.2.379. [DOI] [PubMed] [Google Scholar]
- 30.Miller CA, Abbas PJ, Rubinstein JT, Robinson BK, Matsuoka AJ, Woodworth G. Electrically evoked compound action potentials of guinea pig and cat: Responses to monopolar, monophasic stimulation. Hear Res. 1998;vol. 119:142–154. doi: 10.1016/s0378-5955(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 31.Sohmer H, Freeman S. The latency of auditory nerve brainstem evoked responses to air- and bone-conducted stimuli. Hear Res. 2001;vol. 160:111–113. doi: 10.1016/s0378-5955(01)00337-9. [DOI] [PubMed] [Google Scholar]
- 32.Mauldin L, Jerger J. Auditory brain stem evoked responses to bone-conducted signals. Arch Otolaryngol. 1979;vol. 105:656–661. doi: 10.1001/archotol.1979.00790230026006. [DOI] [PubMed] [Google Scholar]
- 33.Møller AR. Effect of click spectrum and polarity on round window N1N2 response in the rat. Audiology. 1986 Jan;vol. 25:29–43. [PubMed] [Google Scholar]
- 34.Tasaki I, Davis H, Legouix J-P. The space-time patterns of the cochlear microphonic in Guinea Pig. J. Acoust. Soc. Amer. 1952 Jul;vol. 24:502–519. [Google Scholar]
- 35.Zidanic M, Brownell WE. Fine structure of the intracochlear potential field. II. Tone-evoked waveforms and cochlear microphonics. J. Neurophysiol. 1992 Jan;vol. 67:108–124. doi: 10.1152/jn.1992.67.1.108. [DOI] [PubMed] [Google Scholar]
- 36.Balster S, Wenzel GI, Zhang K, Lim HH, Ertmer W, Lenarz T, Reuter G. Insertion-Force and -Depth of Laser Fibers Into a Cochlea Model. Baltimore, MD: ARO; pp. 7371–7371. [Google Scholar]