Abstract
Background
Fatty acid-binding protein 4 (FABP4/A-FABP/aP2), a lipid chaperone, is expressed in both adipocytes and macrophages. Recent studies have shown secretion of FABP4 from adipocytes and association of elevated serum FABP4 level with obesity, insulin resistance, and atherosclerosis. However, little is known about the role of FABP4 in essential hypertension.
Methods
We first examined serum FABP4 concentrations in 18 normotensives (NT) and 30 nontreated essential hypertensives (EHT). The EHT were divided into 18 insulin-sensitive EHT (EHT-S) and 12 insulin-resistant EHT (EHT-R) based on their insulin-sensitivity index, the M value, determined by the hyperinsulinemic–euglycemic clamp technique. In the second study, we determined FABP4 levels in 30 young NT men with or without a family history of hypertension (FH+ and FH–, respectively; n = 15 each).
Results
Serum FABP4 level was significantly higher in the EHT-R than in the NT, whereas elevation of FABP4 level in the EHT-S was not statistically significant. FABP4 level was positively correlated with age, body mass index (BMI), blood pressure, and triglycerides and negatively correlated with the M value. FABP4 level was an independent predictor of mean arterial pressure after adjustment of age, gender, and adiposity. The FH+ group had a significantly lower level of M value and higher level of FABP4 than did the FH– group, and FABP4 concentration was an independent determinant of the M value.
Conclusions
FABP4 contributes to blood pressure elevation and atherogenic metabolic phenotype in hypertensives, and the elevation of FABP4 is predisposed by a family history of hypertension.
Keywords: adipokine, blood pressure, essential hypertension, family history, fatty acid-binding protein, hypertension, insulin resistance
Intracellular lipid chaperones known as fatty acid-binding proteins (FABPs) are a group of molecules that coordinate lipid responses in cells.1,2 FABPs are abundantly expressed 14–15-kDa proteins that can reversibly bind hydrophobic ligands such as saturated and unsaturated long-chain fatty acids with high affinity.1,2 FABPs have been proposed to facilitate the transport of lipids to specific compartments in the cell, such as to the endoplasmic reticulum for signaling, trafficking, and membrane synthesis, to the mitochondria or peroxisome for oxidation, to cytosolic or other enzymes to regulate their activity, to the nucleus for lipid-mediated transcriptional regulation, and to lipid droplets for storage. One of the FABPs, fatty acid-binding protein 4 (FABP4), known as adipocyte FABP (A-FABP) or aP2, is expressed in both adipocytes and macrophages and plays important roles in the regulation of insulin sensitivity and the development of atherosclerosis.3,4,5,6,7,8,9
Adipose tissue is now known to secrete a variety of proteins called adipokines, such as tumor necrosis factor α, leptin, and adiponectin, which are implicated in a wide range of biological phenomena.10 Interestingly, recent studies have shown that FABP4 is secreted from adipocytes11 and that increased concentration of FABP4 is associated with obesity, insulin resistance, and carotid atherosclerosis.11,12,13,14 However, little is known about the relationship between FABP4 and essential hypertension. Since insulin resistance is involved in the pathogenesis of essential hypertension,15,16 we hypothesized that increase in serum FABP4 induces and/or reflects blood pressure elevation and atherogenic metabolic profiles in hypertensives. To investigate this hypothesis, we determined the relationships of FABP4 concentration with blood pressure, insulin sensitivity and metabolic profiles in hypertensives and NT with or without a family history of hypertension. The results of the present study supported our hypothesis.
Methods
This study registered in UMIN-CTR Clinical Trial (UMIN000007095) conformed to the principles outlined in the Declaration of Helsinki and was performed with the approval of the institutional ethical committee of our institution. Written informed consent was received from all of the subjects.
Study 1: Hypertensive patients and NT controls. Two groups of subjects were consecutively enrolled in study 1: mild to moderate essential hypertensives (EHT) and both age- and body mass index (BMI)-matched normotensives (NT). Subjects with any evidence of complications such as endocrine or metabolic disturbances, cerebrovascular or cardiovascular disease, and renal disease were excluded from enrollment. Medications that may affect insulin sensitivity were discontinued at least 2 weeks before the start of the study. Thirty EHT (male/female, 15/15; mean age: 47.9 ± 1.7 years) and 18 NT (male/female, 9/9; mean age: 48.1 ± 2.9 years) were finally enrolled. All of the subjects were hospitalized and were put on a regular diet (2,000 kcal/day) that included 310 g of carbohydrate, 50 g of fat, 80 g of protein, 120 mmol of sodium, and 75 mmol of potassium for more than 1 week. Insulin sensitivity was evaluated as the M value (metabolic clearance of glucose, mg/m2/min) by the hyperinsulinemic–euglycemic clamp technique. Mean – 1 s.d. of the M value in the NT was chosen as the cutoff point for insulin resistance. On the basis of this M value, the EHT were divided into two groups: one group of insulin-sensitive EHT (EHT-S) and one group of insulin-resistant EHT (EHT-R). Before the start of the clamp study, blood pressure was measured, and mean arterial pressure was calculated by 1/3 × (systolic blood pressure) + 2/3 × (diastolic blood pressure). Peripheral venous blood samples were also obtained for determination of FABP4, glucose, insulin, and lipid variables.
Study 2: Young NT men with or without a family history of hypertension. In study 2, young NT men were enrolled from university students as volunteers. Entry criteria were subjects who were aged between 18 and 28 years, who had a BMI of <30 kg/m2 and blood pressure of <140/90 mm Hg, and who were not taking any medications. Family history was ascertained by a self-report questionnaire sent to the parents and by self-records of the parents in clinic. Subjects with either parent being treated with antihypertensive medication for essential hypertension were classified as FH+, whereas those without such a history and whose blood pressure was <140/90 mm Hg at any recent health check on an annual basis were classified as FH–. Using self-records of the parents in clinic or annual health checkups, parental BP was evaluated on the basis of at least three measurements by a sphygmomanometer performed on different days. Subjects for whom a family history of hypertension was not certain and those with a family history of diabetes mellitus in any relatives were excluded. In accordance with the entry and exclusion criteria, we consecutively enrolled 15 FH+ and 15 FH– subjects. All of the subjects were hospitalized and underwent examinations as in study 1.
Hyperinsulinemic–euglycemic clamp technique. A 2-h hyperinsulinemic–euglycemic clamp was performed according to the method described by DeFronzo et al.17 After an overnight fast, a vein in a forearm was cannulated, and blood was continuously withdrawn at 2.0 ml/h through a catheter during the clamp for blood glucose monitoring. A contralateral antecubital vein was also cannulated with a plastic cannula for the infusion of insulin and glucose. Continuous insulin infusion, monitoring of glucose concentration, and infusion of various amounts of glucose in order to clamp glucose levels in the basal state were performed with a model STG-22 artificial endocrine pancreas (Nikkiso, Tokyo, Japan). The infusion rate of insulin (humalin R U-40; Shionogi Pharmaceutical, Osaka, Japan) was 40 mU/m2/min. During insulin infusion, euglycemia was maintained by infusion of a 20% glucose solution. The mean rate of glucose infusion for the last 30 min of the clamp was used as an index of insulin sensitivity (M value). The M value was expressed as mg of glucose per square meter of body surface area.
Biochemistry measurements. Serum FABP4 level was measured using a commercially available enzyme-linked immunosorbent assay kit (Biovendor R&D, Mordrice, Czech Republic). The accuracy, precision and reproducibility of this kit have been described previously.11 Fasting plasma glucose was determined by the glucose oxidase method. Fasting plasma insulin was measured by a radioimmunoassay method (Insulin RIA bead; Dianabot, Tokyo, Japan). Serum lipid profiles, including total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides, were estimated by enzymatic methods (Wako Chemicals, Osaka, Japan). Low-density lipoprotein (LDL) cholesterol level was calculated by the Friedewald equation.
Statistical analysis. Numeric variables are expressed as means ± s.e.m. The Mann–Whitney U-test was used for comparisons between two nonparametric unpaired variables. Group statistical comparisons were assessed by the χ2-test and one-way analysis of variance with post hoc Tukey–Kramer multiple comparisons. Before performing regression analyses, the distribution of each parameter was tested for its normality using the Shapiro–Wilk W-test, and non-normally distributed parameters were logarithmically transformed. Simple linear regression analysis was used to determine the correlation between two variables. In study 1, multiple linear regression analysis was performed to identify independent determinants of the FABP4 concentration or mean arterial pressure using the variables with a significant correlation in univariate regression analysis as independent predictors, showing the percentage of variance in the FABP4 concentration or mean arterial pressure that they explained (R2). In study 2, stepwise and multiple regression analyses were performed to identify independent determinants of the M value or FABP4 concentration in a forward direction with F for the entry set to 4 using the variables with a significant correlation in simple regression analysis as independent predictors. A P value of <0.05 was considered statistically significant. All data were analyzed by using JMP 9 for Macintosh (SAS Institute, Cary, NC).
Results
Study 1
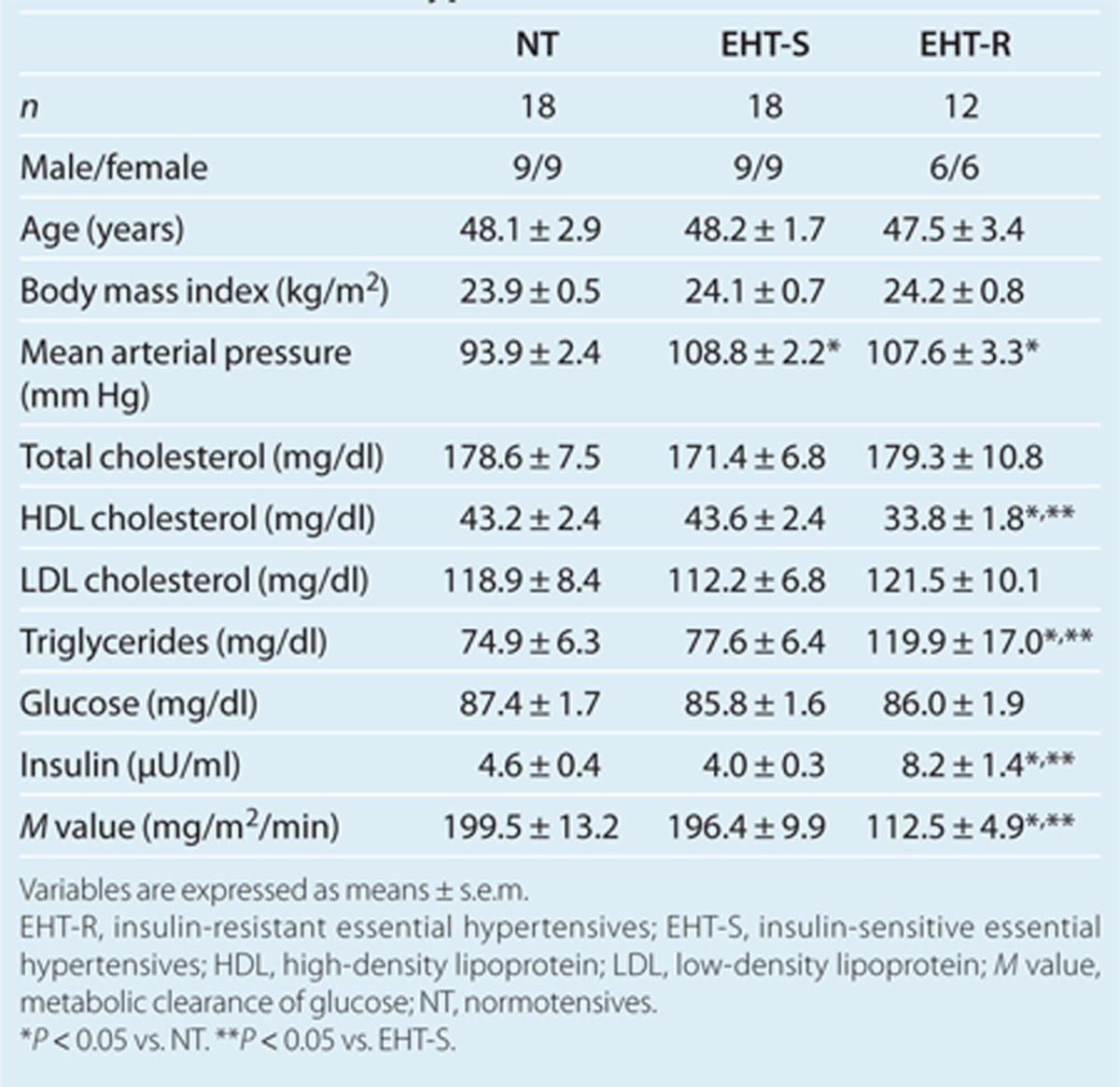
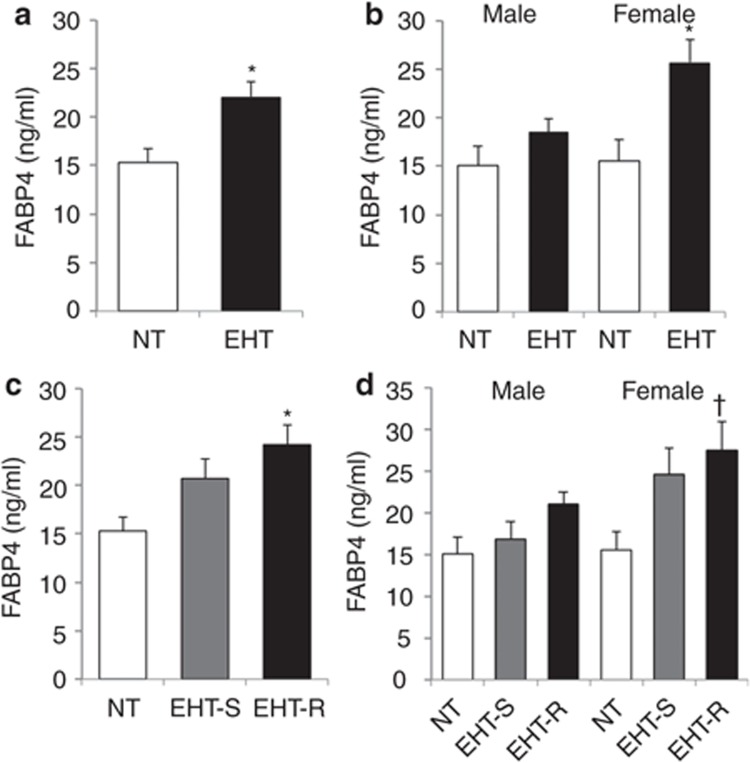
The EHT showed a significantly higher mean arterial pressure (108.3 ± 1.8 vs. 93.9 ± 2.4 mm Hg) and a lower M value (162.9 ± 9.8 vs. 199.5 ± 13.2 mg/m2/min) than did the NT. Using a cutoff point of mean – 1 s.d. of the M value in the NT (143.6 mg/m2/min), the EHT were divided into two groups: one group of 18 EHT-S (male/female, 9/9) and one group of 12 EHT-R (male/female, 6/6). As shown in Table 1, there was no significant intergroup difference in age, gender, or BMI. Mean arterial pressures in the EHT-S and EHT-R were comparable. The EHT-R had significantly higher levels of triglycerides and fasting insulin and lower levels of HDL cholesterol and M value than did the NT and EHT-S. The levels of fasting glucose, total cholesterol, and LDL cholesterol in the three groups were similar. No significant differences were found between glucose or lipid variables in the NT and EHT-S. In all of the subjects in study 1, FABP4 concentration was significantly higher in females than in males (21.9 ± 1.9 vs. 17.2 ± 1.2 ng/ml, P < 0.05). The EHT had a significantly higher level of serum FABP4 concentration than that in the NT (Figure 1a). When separated by gender, the significant difference was found in females, whereas there was a tendency of difference in males (Figure 1b). As shown in Figure 1c, serum FABP4 level in the EHT-R was significantly higher than that in the NT, whereas the difference in FABP4 level between the EHT-S and NT did not reach statistical significance (P = 0.06). Similar tendency was observed in both males and females (Figure 1d).
Table 1. Basal characteristics and biochemical variables of normotensives and hypertensives.
Figure 1.
FABP4 concentration in normotensives and essential hypertensives. Comparison of FABP4 concentration in normotensives (NT; n = 18: male/female, 9/9) and essential hypertensives (EHT; n = 30: male/female, 15/15) is shown in whole (a) subjects and the separated subjects by (b) gender. Comparison of FABP4 concentration in the NT (n =18: male/female, 9/9), insulin-sensitive EHT (EHT-S; n = 18: male/female, 9/9), and insulin-resistant EHT (EHT-R; n = 12: male/female, 6/6) is shown in whole (c) subjects and the separated subjects by (d) gender. Values are presented as means ± s.e.m. FABP4, fatty acid-binding protein 4. *P < 0.01 vs. NT, †P < 0.05 vs. NT.
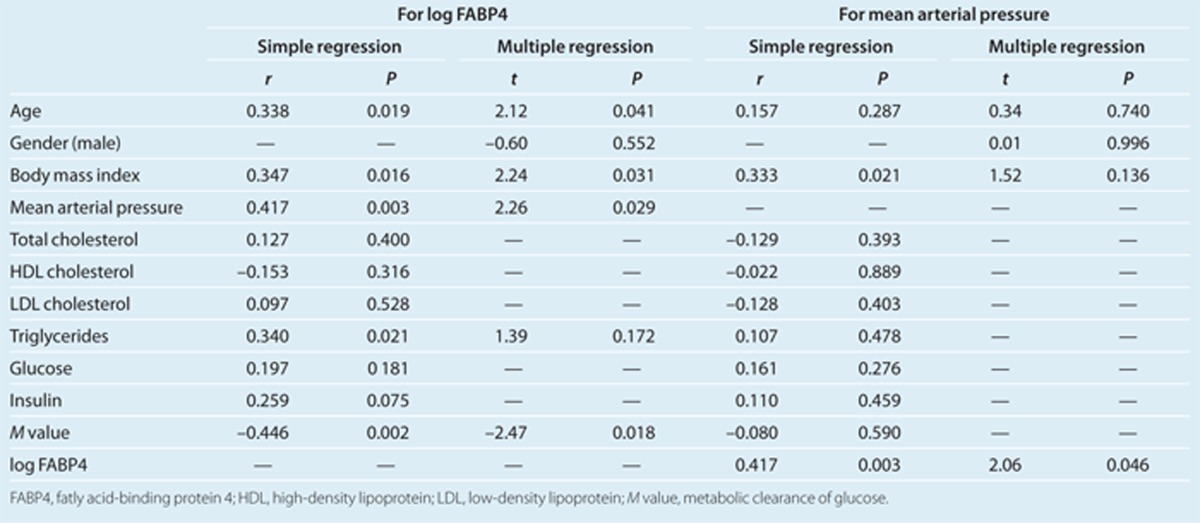
In all of the subjects in study 1, serum FABP4 level was positively correlated with age (r = 0.34, P < 0.05), BMI (r = 0.35, P < 0.05), mean arterial pressure (r = 0.42, P < 0.01), and triglycerides (r = 0.34, P < 0.05) and negatively correlated with the M value (r = –0.45, P < 0.01) (Table 2). Multiple regression analysis using the correlated parameters as well as gender showed that age, BMI, mean arterial pressure, and the M value were independent determinants of FABP4 concentration (Table 2), explaining a total of 50.7% of the variance in this measure (R2 = 0.507).
Table 2. Simple and multiple regression analyses for FABP4 or blood pressure in normotensives and hypertensives.
On the other hand, mean arterial pressure was positively correlated with BMI (r = 0.33, P < 0.05) and FABP4 level (r = 0.42, P < 0.01) (Table 2). Multiple regression analysis using the correlated parameters as well as age and gender showed that FABP4 concentration was the only independent predictor of mean arterial pressure (Table 2), explaining a total of 21.6% of the variance in this measure (R2 = 0.216).
Study 2
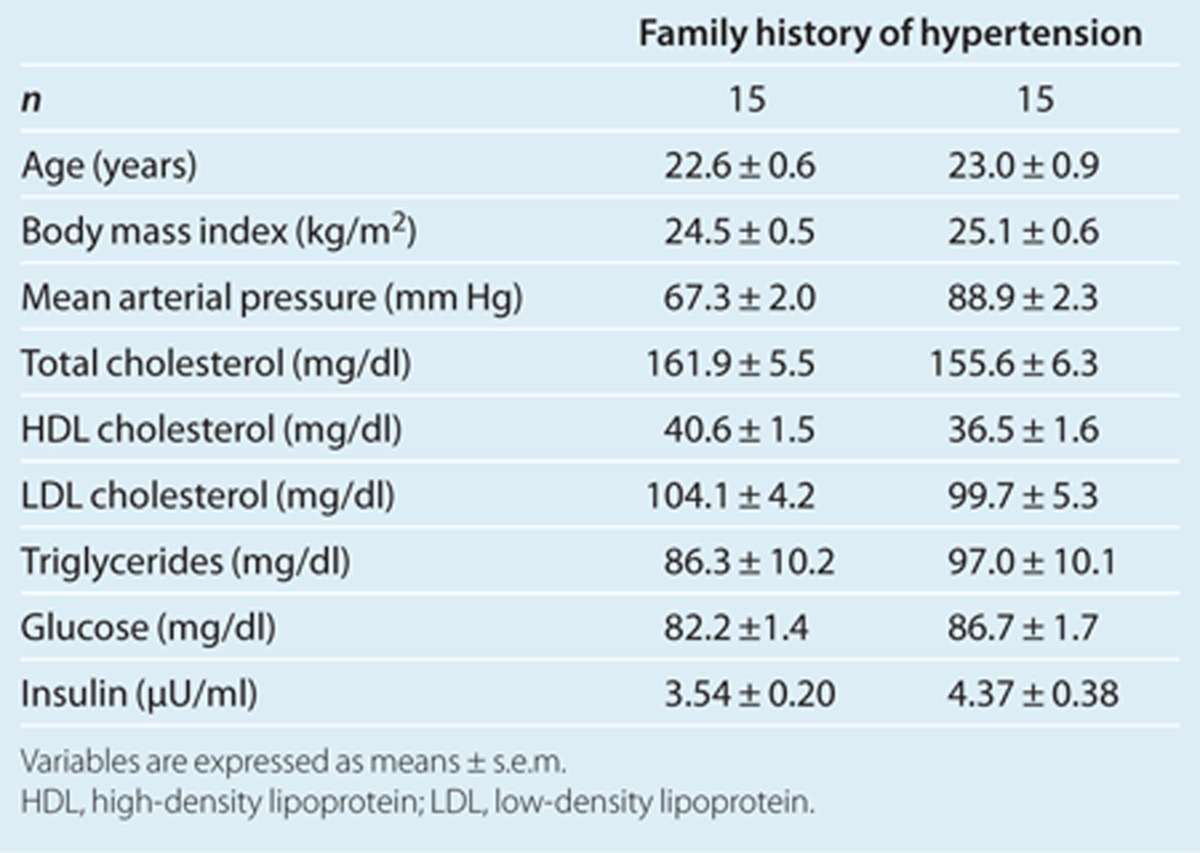
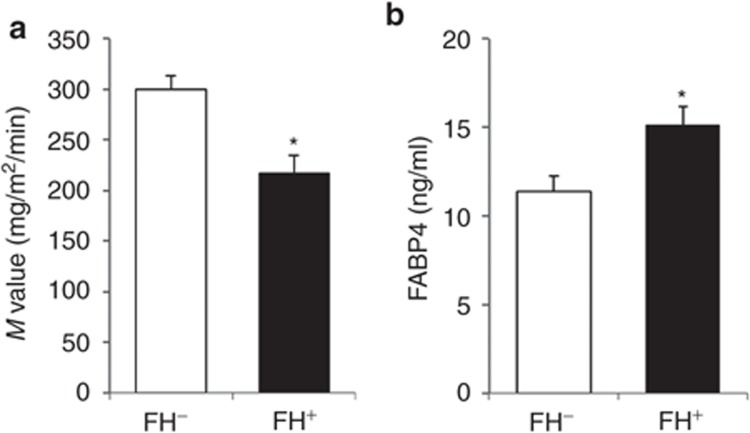
As shown in Table 3, the two groups, FH– and FH+, were well matched for age and BMI. There were no significant differences between mean arterial pressure or between levels of total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, and glucose in the two groups. The insulin level in the FH+ group tended to be higher than that in the FH– group (P = 0.06). The FH+ group had a significantly lower M value and higher level of FABP4 than did the FH– group (Figure 2a,b).
Table 3. Basal characteristics and biochemical variables of young normotensive males.
Figure 2.
The M value and FABP4 concentration in young normotensive males. Bar graphs show comparison of an insulin-sensitive index, the (a) M value, and comparison of (b) FABP4 concentration in young normotensive males with a negative history and positive history of hypertension: FH– (n = 15) and FH+ (n = 15), respectively. Values are presented as means ± s.e.m. M value, metabolic clearance of glucose; FABP4, fatty acid-binding protein 4. *P < 0.01.
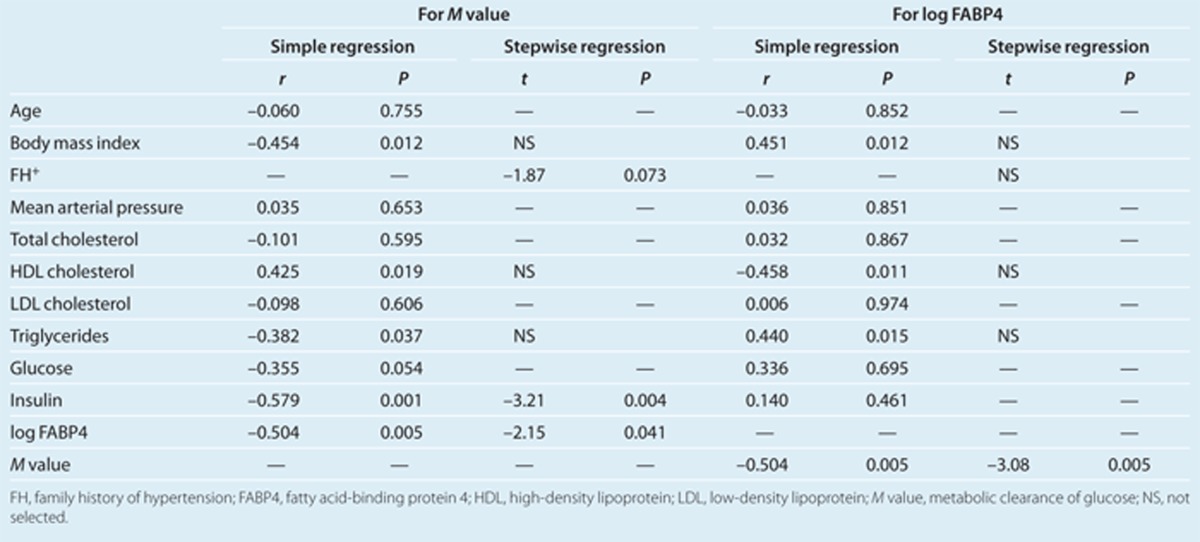
In a simple regression analysis, the M value was positively correlated with HDL cholesterol level (r = 0.43, P < 0.05) and negatively correlated with BMI (r = –0.45, P < 0.05), triglycerides (r = –0.38, P < 0.05), fasting insulin (r = –0.58, P < 0.01), and FABP4 concentration (r = –0.50, P < 0.01) in all of the study 2 subjects (Table 4). Stepwise regression analysis using the correlated parameters and family history of hypertension revealed that family history and levels of FABP4 and insulin were selected as independent predictor of the M value. A subsequent multiple regression analysis showed that FABP4 concentration and insulin level were independently correlated with the M value, explaining a total of 57.4% of the variance in this measure (R2 = 0.574) (Table 4).
Table 4. Simple and multiple regression analyses for M value or FABP4 in young normotensive males.
In addition to a negative correlation with the M value as stated above, FABP4 concentration showed a negative correlation with HDL cholesterol level (r = –0.46, P < 0.05) and positive correlations with BMI (r = 0.45, P < 0.05) and triglycerides (r = 0.44, P < 0.05) (Table 4). However, stepwise regression analysis using the correlated parameters revealed that the M value was the only independent predictor of FABP4 concentration.
Discussion
In the present study, we demonstrated that serum FABP4 level was significantly associated with BMI, blood pressure, insulin resistance, and dyslipidemia in patients with essential hypertension. Strikingly, FABP4 level was found to be an independent predictor of blood pressure after adjustment of age, gender, and BMI in study 1. Furthermore, in study 2, the M value and FABP concentration were independent predictors for each other in young NT men with or without a family history of hypertension, suggesting that elevation of FABP4 level mechanistically relates to insulin resistance even in the absence of hypertension. To the best of our knowledge, this is the first report on the impact of elevation of circulating FABP4 level on nontreated essential hypertension and the relationship between FABP4 concentration and insulin resistance associated with genetic predispositions for the development of essential hypertension.
Previous studies using animal models indicate that FABP4 plays a significant role in several aspects of metabolic syndrome, including insulin resistance, type 2 diabetes, and atherosclerosis, through its action at the interface of metabolic and inflammatory pathways in adipocytes and macrophages.1,2,3,4,5,6,7,8,9 It has also been demonstrated that chemical inhibition of FABP4 could be a therapeutic strategy against insulin resistance, diabetes mellitus, fatty liver disease, and atherosclerosis in experimental models.18 However, there have been no reports on the direct impact of FABP4 on blood pressure elevation in vivo in light of its effects on metabolism and inflammatory processes.
Interestingly, it has recently been shown that FABP4 is secreted from adipocytes,11 although there is no typical sequence of secretory signal peptides. We previously confirmed that FABP4 release from adipocytes was not an escape due to apoptosis or necrosis of adipocytes.9 Recent clinical studies have shown that elevation of serum FABP4 is associated with obesity, insulin resistance, and atherosclerosis.11,12,13,14 There are some reports regarding the relationship between FABP4 concentration and blood pressure.11,12,13,19,20 However, subjects with morbidities in addition to hypertension and subjects on medications that might have affected metabolic conditions including FABP4 concentration were recruited in those studies. In the present study, we carefully recruited patients with nontreated essential hypertension and found that a high concentration of FABP4 is related to blood pressure elevation and atherogenic metabolic profile. Similar impacts have been observed for reduction of serum level of adiponectin, an adipokine.21,22,23 Since it has been shown that serum FABP4 level is inversely correlated with serum adiponectin level,11,12 coordinated and integrated actions of both FABP4 and adiponectin might affect the ultimate pathophysiological condition of essential hypertension.
The impact of a family history of hypertension on blood pressure has been known for some time. Although the number of studies in which the effects of parental history of hypertension on blood pressure in offspring were examined is small, results of those studies indicate that parental history of hypertension induces blood pressure elevation from as early as the second decade of life until the eighth decade of life.24,25,26,27,28 Reduced insulin sensitivity, increased sympathetic activity, and inflammatory reactions reflected by increased circulating C-reactive protein level have been reported to be associated with a family history of hypertension.29,30,31 However, how the changes in insulin sensitivity and inflammatory reactions are induced in family members remains unclear. Close associations of FABP4 with a family history of hypertension (Figure 2) and insulin resistance (Table 4) and the role of FABP4 in inflammatory reactions1,2 suggest that FABP4 is a candidate of molecules linking genetic background of hypertension with metabolic phenotype. It has also been shown that subjects with a genetic variation of the FABP4 locus (T-87C) leading to decreased FABP4 expression in adipose tissue have lower levels of triglycerides as well as a significantly reduced risk for cardiovascular disease.32 The association between genetic variants and serum concentration of FABP4 needs to be addressed in the future.
The precise mechanisms underlying increased FABP4 levels in EHT subjects with insulin resistance and NT men with a family history of hypertension are unclear. The present study showed that FABP4 level is correlated with extent of insulin resistance independently of adiposity and dyslipidemia. A previous study has shown that the treatment with insulin increases FABP4 expression in adipose tissue in vivo.33 That study suggests that chronic hyperinsulinemia based on insulin resistance increases FABP4 expression in adipocytes, leading to increased secretion of FABP4 into systemic circulation. However, this explanation is not supported by the findings in study 2 that insulin level was not significantly different between the FH+ and FH– groups, whereas significant differences in the M value and FABP4 level were found. The subjects recruited in study 2 were young, and change in insulin sensitivity detected by the hyperinsulinemic–euglycemic clamp method in the FH+ group was not large enough to induce compensatory hyperinsulinemia. Together with laboratory findings that chemical inhibition of FABP4 increased insulin sensitivity,18 the present findings rather support the notion that FABP4 primarily influences insulin sensitivity.
It is still unknown whether association of elevated circulating FABP4 level with insulin resistance and hypertension is a result of direct physiological effects of FABP4 as an adipokine in vivo. To address this issue, effects of recombinant FABP4 on blood pressure as well as insulin resistance need to be clearly demonstrated. It is possible that circulating FABP4 modulates endothelial function and has an effect on vascular smooth muscle cell contraction, thereby modulating blood pressure. A previous report showed that FABP4 was expressed in endothelial cells in capillary and vein but not in artery.34 It was also reported that FABP4 was expressed in aortic endothelium of old atherosclerotic ApoE–/– mice35 and regenerated endothelial cells in artery after balloon injury.36 In addition, the relationship between circulating FABP4 levels and endothelial dysfunction assessed by a reactive hyperemia index using peripheral artery tonometry has been reported.37 Local expression of FABP4 in endothelium and/or circulating FABP4 might directly modulate endothelial function and blood pressure.
Determination of serum FABP4 might be a novel approach for identifying individuals at risk for hypertension and atherosclerotic events, which would enable earlier intervention. We have recently shown that serum FABP4 level predicts long-term cardiovascular outcomes in patients with end-stage renal disease at high risk for atherosclerotic cardiovascular events.20 If we could demonstrate a causative role of FABP4 in hypertension and atherosclerosis in humans by accumulating further evidence, FABP4 would be a novel target for prevention of atherosclerotic cardiovascular events.
A limitation of this study is the small number of subjects enrolled. Prospective studies using larger number of subjects are necessary for determining whether FABP4 level is indeed a major determinant of subsequent development of hypertension in subjects with a family history of hypertension. Serum FABP4 concentrations were sex-related, being higher in females than in males as previously reported.11,12,13 Although there was no intergroup difference in gender in study 1, we cannot exclude the possibility of a type II error and biased results of analyses due to the small number of subjects. Since we enrolled only male subjects to adjust for confounding factors in study 2, it remains unclear whether the results are applicable for females as well.
In conclusion, hyper-FABP4-emia is associated with insulin resistance in patients with essential hypertension. In addition, serum FABP4 level was significantly elevated in young NT men with a family history of hypertension, and the elevation was accompanied by reduced insulin sensitivity. The reduction in insulin sensitivity coupled with an increase in serum FABP4 concentration might precede the development of hypertension in the offspring of hypertensives. Phenotypes with earlier penetrance of increased FABP4 level may be especially useful in genetic analyses of insulin resistance and hypertension. The genetic basis of the association of increased serum FABP4 level and insulin resistance warrants further investigation.
Acknowledgments
M.F. has been supported by grants from Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Uehara Memorial Foundation, Mitsubishi Pharma Research Foundation, Naito Foundation Natural Science Scholarship, Takeda Science Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Kanae Foundation for the Promotion of Medical Science, Cardiovascular Research Foundation, Suzuken Memorial Foundation, Sumitomo Foundation, Tokyo Biochemical Research Foundation, Japan Diabetes Foundation, Ono Medical Research Foundation, Novartis Foundation (Japan) for the Promotion of Science, Akiyama Life Science Foundation, Terumo Life Science Foundation, Daiwa Securities Health Foundation, Suhara Memorial Foundation, Japan Foundation for Applied Enzymology, and Ichiro Kanehara Foundation. We are grateful to group members of our department, IZAYOI (Boston, MA), and G-PUC (Sapporo, Japan) for their scientific inputs and contribution.
The authors declared no conflict of interest.
References
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Ishimura S, Ota H, Miura T. Lipid chaperones and metabolic inflammation. Int J Inflam. 2011;2011:642612. doi: 10.4061/2011/642612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Uysal KT, Makowski L, Görgün CZ, Atsumi G, Parker RA, Brüning J, Hertzel AV, Bernlohr DA, Hotamisligil GS. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes. 2003;52:300–307. doi: 10.2337/diabetes.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev VR, Runner RP, Fan D, Ding L, Zhang Y, Tao H, Erbay E, Görgün CZ, Fazio S, Hotamisligil GS, Linton MF. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-?-regulated genes. Arterioscler Thromb Vasc Biol. 2011;31:1283–1290. doi: 10.1161/ATVBAHA.111.225839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker RA, Hotamisligil GS. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22:1686–1691. doi: 10.1161/01.atv.0000033090.81345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Fucho R, Görgün CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118:2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52:405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- Xu A, Tso AW, Cheung BM, Wang Y, Wat NM, Fong CH, Yeung DC, Janus ED, Sham PC, Lam KS. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115:1537–1543. doi: 10.1161/CIRCULATIONAHA.106.647503. [DOI] [PubMed] [Google Scholar]
- Tso AW, Xu A, Sham PC, Wat NM, Wang Y, Fong CH, Cheung BM, Janus ED, Lam KS. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30:2667–2672. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- Yeung DC, Xu A, Cheung CW, Wat NM, Yau MH, Fong CH, Chau MT, Lam KS. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1796–1802. doi: 10.1161/ATVBAHA.107.146274. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Iimura O. Insulin resistance and hypertension in Japanese. Hypertens Res. 1996;19 Suppl 1:S1–S8. doi: 10.1291/hypres.19.supplementi_s1. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Tuncman G, Görgün CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Doi M, Hirohata S, Kamikawa S, Usui S, Ogawa H, Sakane K, Izumi R, Ninomiya Y, Kusachi S. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid-binding protein in hypertensive patients. Heart Vessels. 2011;26:408–413. doi: 10.1007/s00380-010-0060-x. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Ishimura S, Ota H, Hayashi M, Nishitani T, Tanaka M, Yoshida H, Shimamoto K, Hotamisligil GS, Miura T. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS ONE. 2011;6:e27356. doi: 10.1371/journal.pone.0027356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003;16:72–75. doi: 10.1016/s0895-7061(02)03197-7. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Ura N, Higashiura K, Miyazaki Y, Murakami H, Hyakukoku M, Shimamoto K. Low adiponectin level in young normotensive men with a family history of essential hypertension. Hypertens Res. 2005;28:141–146. doi: 10.1291/hypres.28.141. [DOI] [PubMed] [Google Scholar]
- Burke V, Gracey MP, Beilin LJ, Milligan RA. Family history as a predictor of blood pressure in a longitudinal study of Australian children. J Hypertens. 1998;16:269–276. doi: 10.1097/00004872-199816030-00003. [DOI] [PubMed] [Google Scholar]
- Nelson MJ, Ragland DR, Syme SL. Longitudinal prediction of adult blood pressure from juvenile blood pressure levels. Am J Epidemiol. 1992;136:633–645. doi: 10.1093/oxfordjournals.aje.a116543. [DOI] [PubMed] [Google Scholar]
- Li R, Alpert BS, Walker SS, Somes GW. Longitudinal relationship of parental hypertension with body mass index, blood pressure, and cardiovascular reactivity in children. J Pediatr. 2007;150:498–502. doi: 10.1016/j.jpeds.2007.01.034. [DOI] [PubMed] [Google Scholar]
- van den Elzen AP, de Ridder MA, Grobbee DE, Hofman A, Witteman JC, Uiterwaal CS. Families and the natural history of blood pressure. A 27-year follow-up study. Am J Hypertens. 2004;17:936–940. doi: 10.1016/j.amjhyper.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Wang NY, Young JH, Meoni LA, Ford DE, Erlinger TP, Klag MJ. Blood pressure change and risk of hypertension associated with parental hypertension: the Johns Hopkins Precursors Study. Arch Intern Med. 2008;168:643–648. doi: 10.1001/archinte.168.6.643. [DOI] [PubMed] [Google Scholar]
- Goldstein IB, Shapiro D, Weiss RE. How family history and risk factors for hypertension relate to ambulatory blood pressure in healthy adults. J Hypertens. 2008;26:276–283. doi: 10.1097/HJH.0b013e3282f15c27. [DOI] [PubMed] [Google Scholar]
- Hausberg M, Sinkey CA, Mark AL, Hoffman RP, Anderson EA. Sympathetic nerve activity and insulin sensitivity in normotensive offspring of hypertensive parents. Am J Hypertens. 1998;11:1312–1320. doi: 10.1016/s0895-7061(98)00146-0. [DOI] [PubMed] [Google Scholar]
- Masuo K, Mikami H, Ogihara T, Tuck ML. Familial hypertension, insulin, sympathetic activity, and blood pressure elevation. Hypertension. 1998;32:96–100. doi: 10.1161/01.hyp.32.1.96. [DOI] [PubMed] [Google Scholar]
- Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, Hotamisligil GS. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci USA. 2006;103:6970–6975. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki SA, Abumrad NA. Expression of the adipocyte fatty acid-binding protein in streptozotocin-diabetes: effects of insulin deficiency and supplementation. J Lipid Res. 1993;34:1527–1534. [PubMed] [Google Scholar]
- Elmasri H, Karaaslan C, Teper Y, Ghelfi E, Weng M, Ince TA, Kozakewich H, Bischoff J, Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Li H, Xiao Y, Zhou Z, Xu A, Vanhoutte PM. Chronic administration of BMS309403 improves endothelial function in apolipoprotein E-deficient mice and in cultured human endothelial cells. Br J Pharmacol. 2011;162:1564–1576. doi: 10.1111/j.1476-5381.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Tse HF, Siu CW, Zhu SG, Man RY, Vanhoutte PM. Genomic changes in regenerated porcine coronary arterial endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2443–2449. doi: 10.1161/ATVBAHA.107.141705. [DOI] [PubMed] [Google Scholar]
- Aragonès G, Ferré R, Lázaro I, Cabré A, Plana N, Merino J, Heras M, Girona J, Masana L. Fatty acid-binding protein 4 is associated with endothelial dysfunction in patients with type 2 diabetes. Atherosclerosis. 2010;213:329–331. doi: 10.1016/j.atherosclerosis.2010.07.026. [DOI] [PubMed] [Google Scholar]