Abstract
A method utilizing reversed-phase high-performance liquid chromatography has been developed for the purification to homogeneity of interleukin 2 (IL-2) isolated from a human T-cell leukemia. A final purification of 500,000-fold was obtained with a specific activity of pure IL-2 of 10(9) units/mg. The amino acid analysis of natural IL-2 is strikingly similar to the composition deduced from sequence analysis of a cDNA coding for human IL-2. Protein sequence analysis of CNBr-derived peptides yields data consistent with the sequence proposed from cloned cDNA. The availability of homogeneous IL-2 will allow accurate biological studies of its activity free from the contamination of the numerous lymphokine species that are known to be co-produced with IL-2 during the induction procedure.
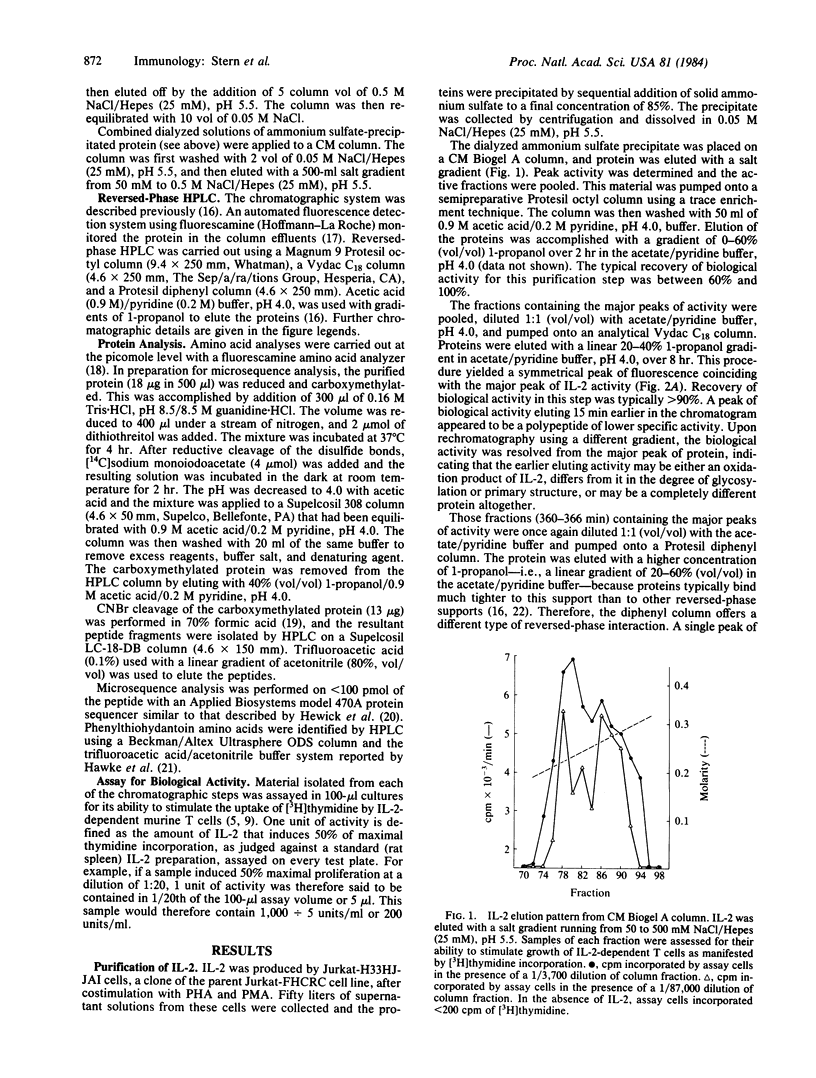
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. E., Gillis S., Smith K. A. Monoclonal cytolytic T-cell lines. J Exp Med. 1979 Jan 1;149(1):273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever M. A., Greenberg P. D., Fefer A., Gillis S. Augmentation of the anti-tumor therapeutic efficacy of long-term cultured T lymphocytes by in vivo administration of purified interleukin 2. J Exp Med. 1982 Apr 1;155(4):968–980. doi: 10.1084/jem.155.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabato G. Purification and initial characterization of rat interleukin 2. Proc Natl Acad Sci U S A. 1982 May;79(9):3020–3023. doi: 10.1073/pnas.79.9.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. J., Simon P. L., Koopman W. J., Fuller-Bonar J. Biochemical relationship of thymocyte mitogenic factor and factors enhancing humoral and cell-mediated immune responses. J Immunol. 1978 Oct;121(4):1353–1360. [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Frank M. B., Watson J., Mochizuki D., Gillis S. Biochemical and biologic characterization of lymphocyte regulatory molecules. VIII. Purification of interleukin 2 from a human T cell leukemia. J Immunol. 1981 Dec;127(6):2361–2365. [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Henney C. S. The biochemical and biological characterization of lymphocyte regulatory molecules. VI. Generation of a B cell hybridoma whose antibody product inhibits interleukin 2 activity. J Immunol. 1981 May;126(5):1978–1984. [PubMed] [Google Scholar]
- Gillis S., Scheid M., Watson J. Biochemical and biologic characterization of lymphocyte regulatory molecules. III. The isolation and phenotypic characterization of Interleukin-2 producing T cell lymphomas. J Immunol. 1980 Dec;125(6):2570–2578. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A., Watson J. Biochemical characterization of lymphocyte regulatory molecules. II. Purification of a class of rat and human lymphokines. J Immunol. 1980 Apr;124(4):1954–1962. [PubMed] [Google Scholar]
- Gillis S., Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980 Dec 1;152(6):1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Vassalli J. D., Reich E. Purification of murine T cell growth factor. A lymphocyte mitogen with helper activity. J Exp Med. 1981 Aug 1;154(2):422–431. doi: 10.1084/jem.154.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Henney C. S., Gillis S. In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J Immunol. 1983 Jan;130(1):222–227. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Kenny C., Moschera J. A., Stein S. Purification of human fibroblast interferon produced in the absence of serum by Cibacron Blue F3GA-Agarose and high-performance liquid chromatography. Methods Enzymol. 1981;78(Pt A):435–447. doi: 10.1016/0076-6879(81)78154-0. [DOI] [PubMed] [Google Scholar]
- Kuribayashi K., Gillis S., Kern D. E., Henney C. S. Murine NK cell cultures: effects of interleukin-2 and interferon on cell growth and cytotoxic reactivity. J Immunol. 1981 Jun;126(6):2321–2327. [PubMed] [Google Scholar]
- Lewis R. V., Fallon A., Stein S., Gibson K. D., Udenfriend S. Supports for reverse-phase high-performance liquid chromatography of large proteins. Anal Biochem. 1980 May 1;104(1):153–159. doi: 10.1016/0003-2697(80)90291-2. [DOI] [PubMed] [Google Scholar]
- Maizel A., Sahasrabuddhe C., Mehta S., Morgan J., Lachman L., Ford R. Biochemical separation of a human B cell mitogenic factor. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5998–6002. doi: 10.1073/pnas.79.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Kasahara T., Oppenheim J. J., Fauci A. S. B cell growth factor and T cell growth factor produced by mitogen-stimulated normal human peripheral blood T lymphocytes are distinct molecules. J Immunol. 1982 Dec;129(6):2486–2489. [PubMed] [Google Scholar]
- Robb R. J., Smith K. A. Heterogeneity of human T-cell growth factor(s) due to variable glycosylation. Mol Immunol. 1981 Dec;18(12):1087–1094. doi: 10.1016/0161-5890(81)90024-9. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S., Brink L. Amino acid analysis of proteins and peptides at the picomole level: the fluorescamine amino acid analyzer. Methods Enzymol. 1981;79(Pt B):20–25. doi: 10.1016/s0076-6879(81)79008-6. [DOI] [PubMed] [Google Scholar]
- Stein S., Böhlen P., Stone J., Dairman W., Udenfriend S. Amino acid analysis with fluorescamine at the picomole level. Arch Biochem Biophys. 1973 Mar;155(1):202–212. doi: 10.1016/s0003-9861(73)80022-0. [DOI] [PubMed] [Google Scholar]
- Stein S., Moschera J. High-performance liquid chromatography and picomole-level detection of peptides and proteins. Methods Enzymol. 1981;79(Pt B):7–16. doi: 10.1016/s0076-6879(81)79006-2. [DOI] [PubMed] [Google Scholar]
- Strausser J. L., Rosenberg S. A. In vitro growth of cytotoxic human lymphocytes. I. Growth of cells sensitized in vitro to alloantigens. J Immunol. 1978 Oct;121(4):1491–1495. [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Tilden A. B., Balch C. M. A comparison of PGE2 effects on human suppressor cell function and on interleukin 2 function. J Immunol. 1982 Dec;129(6):2469–2473. [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Wang C. Y., Mertelsmann R., Venuta S., Feldman S. P., Moore M. A. Purification of human interleukin 2 to apparent homogeneity and its molecular heterogeneity. J Exp Med. 1982 Aug 1;156(2):454–464. doi: 10.1084/jem.156.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]



