Abstract
We previously isolated an analog to chlorophyll-related compounds, pheophytin a, from the marine brown alga Sargassum fulvellum and demonstrated that it is a neurodifferentiation compound. In the current study, we investigated the effects of the pheophytin a analog vitamin B12 on PC12 cell differentiation. In the presence of a low level of nerve growth factor (10 ng ml−1), vitamin B12 demonstrated neurite outgrowth-promoting activity in PC12 cells. The effect was dose-dependent in the range of 6–100 μM. In the absence of nerve growth factor, vitamin B12 did not promote differentiation. To investigate the mechanism for this effect, we conducted differentiation assays and western blot analysis with signal transduction inhibitors and found that vitamin B12 did not promote PC12 cell differentiation in the presence of K252a or U0126 inhibitors. These results suggest that vitamin B12 stimulates PC12 cell differentiation through enhancement of the mitogen-activated protein kinase signal transduction pathway, which is also induced by nerve growth factor. Thus, vitamin B12 may be a good candidate for treatment of neurodegenerative diseases such as Alzheimer’s disease.
Keywords: PC12 cells, Neurodifferentiation, Vitamin B12, Nerve growth factor, Mitogen-activated protein kinase, Signal pathway
Introduction
Neurodegenerative disease is caused by changes in the central nervous system including loss of specific nerve cells and cellular accumulation of fibers (DiProspero et al. 2004; Iseri et al. 2006; Raynaud and Marcilhac 2006). Neurodegenerative disease has been attributed to disordered metabolite control of proteins resulting from protein misfolding (Papassotiropoulos et al. 2006). Protein misfolding is the primary cause of Alzheimer’s disease (AD), Parkinson’s disease, Huntington’s disease, Creutzfeldt-Jakob disease, cystic fibrosis, Gaucher’s disease, and many other degenerative and neurodegenerative disorders (Chaudhuri and Paul 2006; Papassotiropoulos et al. 2006).
With regard to AD, cognitive impairment is one of important signs and the rate of onset of the disease increases with age (Law et al. 2001; Jicha et al. 2006). AD neuropathology is characterized by extracellular deposition of β-amyloid plaques and intracellular deposition of neurofibrillary tangles in the cerebral cortex; development of neurofibrillary tangles has been linked to hyperphosphorylation of tau protein (Kozikowski et al. 2006; Terry 2006). In the nerve cells, β-amyloid neurotoxicity and microtubule destabilization due to tau hyperphosphorylation cause cell death (Chen et al. 2005; Maezawa et al. 2006). The neuronal death and loss of synapses results in perturbation of memory, speech, logical thought, and ultimately death (Selkoe 2004).
There is urgent need for new therapies for AD. Among the possible candidates for AD therapy, nerve growth factor (NGF) is significant. NGF elicits neuronal cell differentiation and promotes neuron survival (Greene 1978). Discovery of a novel neurodifferentiation compound capable of promoting NGF activity, even at a low level might provide the basis for new treatment for neurodegenerative disease. With this pursuit in mind, we have screened a variety of natural compounds with the goal of identifying those capable of stimulating neurite outgrowth-promoting activity in PC12 cells.
Previously, we reported the isolation and characterization of two compounds, from the marine brown alga, Sargassum macrocarpum that promote neurite outgrowth in PC12 cells; sargaquinoic acid and sargachromenol (Tsang et al. 2001; Kamei and Tsang 2003; Tsang and Kamei 2004; Tsang et al. 2005). Both compounds stimulate mitogen-activated protein kinase (MAPK) and protein kinase A (PKA) signal transduction pathways in PC12 cells. Furthermore, we previously showed that two analogs of sargaquinoic acid, vitamin K1 and vitamin K2, stimulate the differentiation of PC12D cells (Tsang and Kamei 2002). We also identified pheophytin a, a chlorophyll-related compound from the marine brown alga Sargassum fulvellum that has the neurite outgrowth-promoting activity similar to that of sargaquinoic acid (Ina et al. in press).
In the present study, we sought to determine whether vitamin B12, an analog of pheophytin a, has neurite outgrowth-promoting activity. Here we describe this compound’s neurite outgrowth-promoting activity and signal transduction pathway activation in PC12 cells.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM, low glucose) and horse serum (HS) were purchased from Gibco RBL (Rockville, MD). Fetal bovine serum (FBS) was purchased from Biosource (Rockville, MD). Mouse NGF 2.5S (male mouse submaxillary glands, 2.5S NGF), K252a, and okadaic acid were purchased from Alomone Labs (Jerusalem, Israel). Anti-active MAPK family sampler antibody was purchased from Promega (Madison, WI), and U0126 was purchased from Calbiochem (San Diego, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell differentiation assay
PC12 cells were maintained in DMEM supplemented with 10% HS, 10% FBS, 100 U ml−1 penicillin G, 200 μg ml−1 streptomycin, and 25 μg ml−1 ampicillin in a water-saturated atmosphere of 5% CO2 and incubated at 37 °C. A 48-well plate was coated with poly-d-lysine (10 μg ml−1). For the bioassay of neurite outgrowth-promoting activity, PC12 cells were seeded at a density of 5 × 103 cells per well in plates containing complete medium and incubated for 24 h. The medium was then changed to DMEM supplemented with 1% HS, 1% FBS, 100 U ml−1 penicillin G, 200 μg ml−1 streptomycin, and 25 μg ml−1 ampicillin containing NGF and cyanocobalamin (vitamin B12). Vitamin B12 was dissolved in sterile water and sterilized by filter sterilization. Vitamin B12 samples (0.2–100 μM) in less than 1.6% v/v of test medium were added to the wells. After 48-h incubation, differentiated cells with neurites longer than twice the cell body diameter were identified under a microscope (200× magnification). The neurite outgrowth-promoting activity was calculated by dividing differentiated cell numbers by total cell numbers in a field of vision.
To investigate the mechanism for the effects of vitamin B12 on PC12 cell differentiation, additional assays were performed with the addition of protein kinase inhibitors. PC12 cells were seeded in 48-well plates and incubated for 24 h. The medium was replaced with the test medium containing a protein kinase inhibitor (K252a or U0126), and cells were incubated for 1 h. U0126 and K252a were dissolved in DMSO and DMEM, respectively, before being added to the medium. Each inhibitor was further diluted with DMEM to the working concentration, and an aliquot of the working concentration was added to the cell cultures in less than 1.6% v/v of medium. NGF and vitamin B12 were added to the medium and incubated for 48 h. Differentiated cells were then counted as described above.
Western blot analysis
PC12 cells were plated at a density of 1 × 106 cells per 35-mm culture dish and cultured for 24 h. After incubation, the medium was replaced with DMEM and cells were cultured for 6 h. The DMEM was then replaced with the test medium contain NGF and/or vitamin B12. After incubation for 5 min, the cells were washed with ice-cold Tris-HCl buffer (pH 7.4) and lysed by addition of 300 μl lysis buffer (10 mM HEPES [pH 7.5], 1 mM EDTA, 10% sodium dodecyl sulfate [SDS], 320 nM okadaic acid, 20 mM p-nitrophenyl phosphate, 10 nM calyculin A, 10 mM NaF, 1 mM sodium orthovanadate, 5 μM potassium bisperoxo(1,10-phenanthroline)oxovanadate(V), 1 mM p-aminophenyl methanesulfonyl fluoride, 10 μg ml−1 pepstatin A, 10 μg ml−1 antipain, 10 μg ml−1 leupeptin, 10 μg ml−1 chymostatin, and 10 μg ml−1 phosphoramidon) to the culture dishes. Cell lysates were sonicated at 30 W for 1 min (250/450 Sonifier, Branson Co., Danbury, CT), and the sonicated sample was centrifuged at 25,000 × g for 15 min. The sample proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Advantec) based on the method described by Li et al. (2002).
Western blots were performed according to the Promega protocol. Actin and protein kinases from the MAPK family were detected using a chemiluminescence detection kit (Supersignal west pico chemiluminescent substrate, Pierce, Rockford, IL), their respective antibodies, and peroxidase-conjugated goat anti-rabbit IgG (whole molecule). Protein concentrations of the cell lysates were determined with the BCA protein assay kit (Pierce) using bovine serum albumin as the standard. The density of the band corresponding to MAPK was analyzed using a Gel-Pro analyzer (Media Cybernetics, MD). Data are expressed relative to the internal standard.
Statistical analysis of the data
Each data point represents the mean ± standard deviation (n = 4). The Students t-test was used to evaluate the statistical significance of differences between treatments. P < 0.05 was considered to be statistically significant.
Results
Effect of vitamin B12 on PC12 cell differentiation and changes in PC12 cell morphology
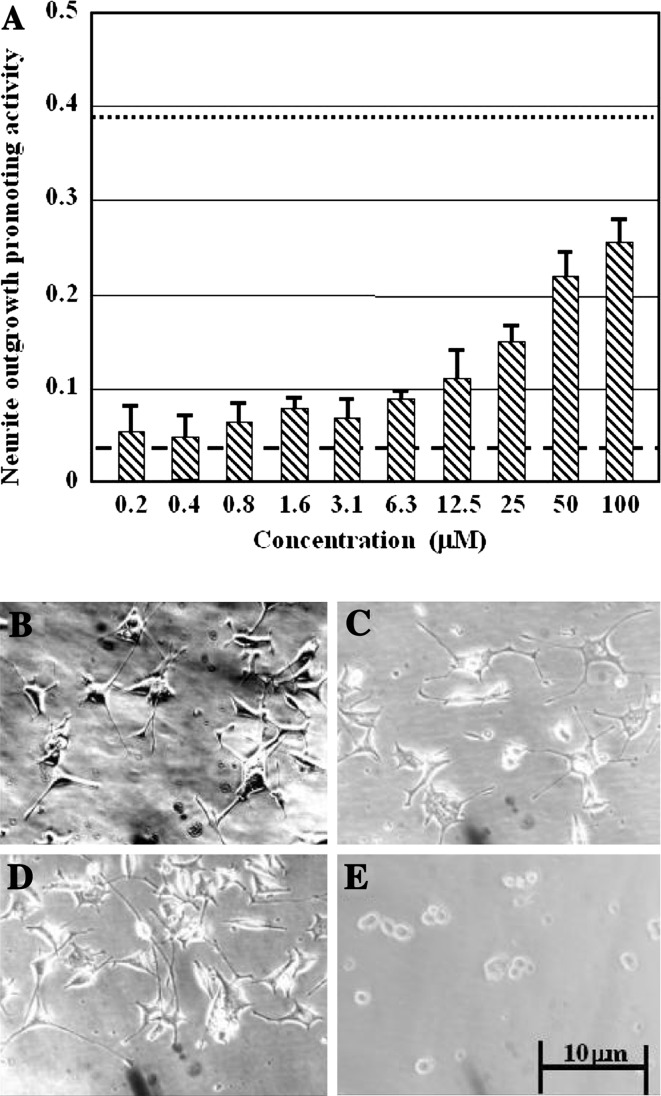
Vitamin B12 exhibited neurite outgrowth-promoting activity in PC12 cells in the presence of only a low level of NGF (10 ng ml−1). The effect of vitamin B12 on the differentiation of PC12 cells was dose-dependent at concentrations of 6–100 μM (Fig. 1A). In the absence of NGF, vitamin B12 did not stimulate PC12 cell differentiation. Even at the highest concentration tested, the neurite outgrowth-promoting activity of vitamin B12 was less than that of the positive control (50 ng ml−1). Vitamin B12 stimulated PC12 cell differentiation (Fig. 1B). In the presence of 10 ng ml−1 NGF and vitamin B12 (100 μM), neurite outgrowth was greater than with the negative control (Fig. 1C) but somewhat less than with the positive control, 50 ng ml−1 NGF (Fig. 1D). However, treatment with vitamin B12 alone did not induce the differentiation of PC12 cells (Fig. 1E), suggesting that vitamin B12 enhanced NGF activity in PC12 cells.
Fig. 1.
Dose-dependent effects of vitamin B12 on the differentiation of PC12 cells and the cell morphology after treated with vitamin B12. (A) dose-dependent effects of vitamin B12 on the differentiation of PC12 cells. ······, positive control (50 ng ml−1 NGF); ----, negative control (10 ng ml−1 NGF). (B) photomicrographs of PC12 cells following treatment with vitamin B12 (100 μM) and NGF (10 ng ml−1); (C) negative control (10 ng ml−1 NGF); (D) positive control (50 ng ml−1 NGF); (E), treatment with vitamin B12 (100 μM) only. Bar shows 10 μm
Inhibition of PC12 cell differentiation by protein kinase inhibitors
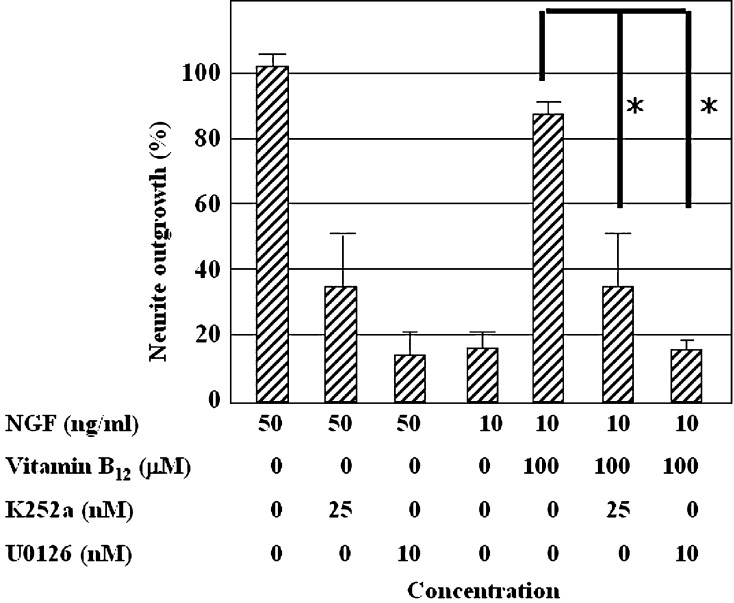
We used two protein kinase inhibitors, K252a and U0126, to investigate the mechanism for the effects of vitamin B12 on neurite outgrowth of PC12 cells. The ATP analog K252a, a cell permeable inhibitor of calmodulin kinase and phosphorylase kinase, inhibited the neurite outgrowth of PC12 cells treated with vitamin B12 (100 μM) and NGF (10 ng ml−1) (Fig. 2). U0126 is a selective and strong MAPK kinase 1 and 2 (MEK1, MEK2) inhibitor. The neurite outgrowth of PC12 cells treated with vitamin B12 were inhibited by U0126 (Fig. 2).
Fig. 2.
Effects of specific inhibitors on the induction of PC12 cell differentiation by vitamin B12. The neurite outgrowth-promoting activity of vitamin B12 is shown as a percentage of the activity of NGF (50 ng ml−1), the positive control
Activation of extracellular signal-regulated kinase 1/2 by vitamin B12
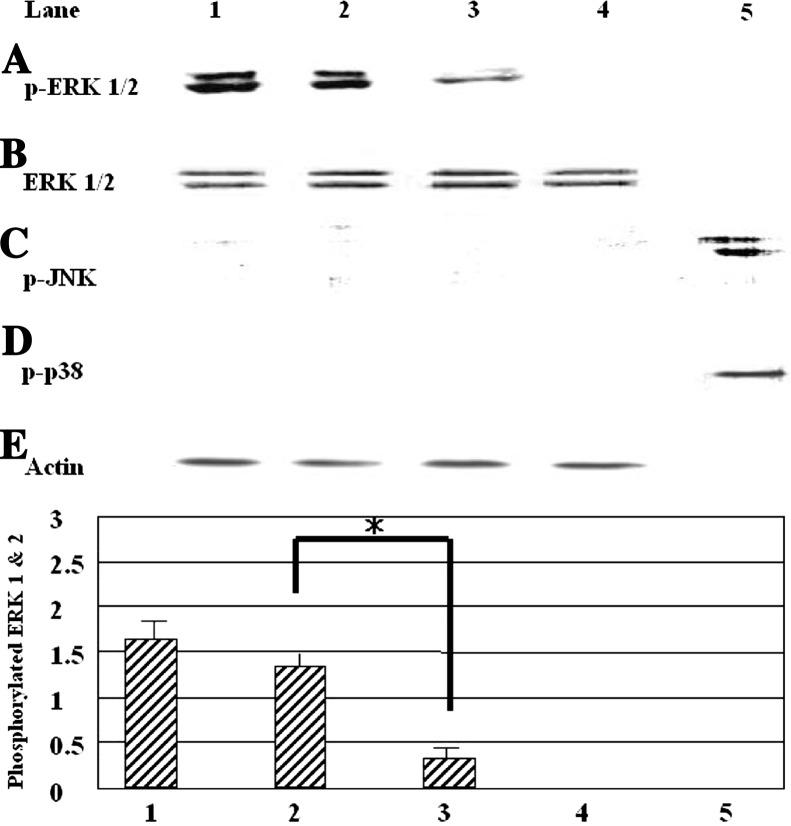
We performed western blotting for extracellular signal-regulated kinase (ERK) 1/2 and the MAPK family to detect activated ERK1/2 in PC12 cells treated with NGF or vitamin B12. K252a and U0126 inhibited the activation of ERK1/2. These results suggest that vitamin B12 stimulates the differentiation of PC12 cells via the MAPK signaling pathway. In PC12 cells treated with NGF (10 ng ml−1), vitamin B12 (100 μM) activated ERK within 5 min (Fig. 3A, lane 2). However, it did not increase total ERK (Fig. 3B). Moreover, vitamin B12 did not stimulate other members of the MAPK family, p38 and c-Jun N-terminal kinase (Fig. 3C and D).
Fig. 3.
Western blot analysis of MAPK family activation by vitamin B12. (A) Phosphorylated MAPK was detected by western blot using anti-active ERK 1/2 antibody. (B) Total ERK was detected using anti-ERK 1/2 antibody. (C) Phosphorylated JNK was detected by western blot using anti-active JNK antibody. (D) Phosphorylated p38 was detected by western blot using anti-active p38 antibody. (E) Actin was detected with anti-actin antibody. Line 1: positive control (50 ng ml−1 NGF); line 2: vitamin B12 (100 μM) and NGF (10 ng ml−1); line 3: negative control (10 ng ml−1 NGF); line 4: untreated cells; line 5: control (1 M sorbitol). PC12 cell lysates were subjected directly to 12.5% SDS-PAGE. Proteins were transferred to a nitrocellulose membrane and immunostained with monoclonal antibodies. Actin was used as an internal control for PC12 cells
Discussion
In this study, as part of a larger effort to identify new drug candidates for treating neurodegenerative disease, we investigated the ability of vitamin B12 to stimulate neurodifferentiation. Vitamin B12 stimulated the differentiation of PC12 cells in the presence of a low level of NGF. However, its neurite outgrowth-promoting activity was less than that of pheophytin a (Ina et al. in press). Vitamin B12 is known to be the antipernicious anaemia factor, required for human and animal metabolism (Krautler 2005). In a recent study, it was reported that patients with AD are particularly prone to be vitamin B12 deficiency (Kristensen et al. 1993). Although vitamin B12 has multiple biological activities, this is the first study to demonstrate its neurite outgrowth-promoting activity.
Vitamin B12 alone did not stimulate the differentiation of PC12 cells but did so in the presence of a low level of NGF. Upon further investigation using protein kinase inhibitors, we found that vitamin B12 stimulates PC12 differentiation in a manner that appears to involve the same signal transduction pathways activated by NGF. The protein kinase inhibitor K252a exerts neurotrophic effects on primary neurons and PC12 cells (Borasio 1990). K252a inhibited NGF-evoked signal transduction and blocked differentiation of PC12 cells via inhibition of tyrosine kinase activity. These results show that vitamin B12, like NGF, enhances Trk signal transduction. We then used the selective MEK inhibitor U1026 to further investigate the enhancement of MAPK activity by vitamin B12. U0126 inhibits the MEK/ERK signal pathway and the Ras oncogenic effects that are triggered by NGF, thereby blocking the production of inflammatory cytokines and matrix metalloproteinases (Favata et al. 1998; Lockman et al. 2004). U0126 has also been shown to inhibit both active and inactive MEK1 and MEK2 (Sharma et al. 2005). We found that U0126 inhibited the differentiation of PC12 cells, blocked ERK, and the phosphorylation not to stimulate the neurite outgrowth of PC12 cells induced by NGF. Thus, the differentiation effects of vitamin B12 in PC12 cells appear to be mediated by the ERK signal pathway. Furthermore, western blot analysis showed that treatment of PC12 cells with vitamin B12 increases phosphorylated ERK1/2 without affecting the level of total ERK 1/2.
In general, compounds with neurite outgrowth-promoting activity may be useful in the treatment of neurodegenerative diseases such as AD. Previous studies have demonstrated the neurite outgrowth-promoting activity of natural compounds such as picrosides 1 and 2 (Li et al. 2002), nardosinone (Li et al. 2003), Cuscuta chinensis glycoside (Jian et al. 2003), sargaquinoic acid, and sargachromenol (Tsang et al. 2001; Kamei and Tsang 2003; Tsang and Kamei 2004; Tsang et al. 2005). Picrosides 1 and 2 and nardosinone enhance NGF-induced neurite outgrowth in PC12D cells, amplifying signaling upstream of MAPK kinase in the NGF receptor-mediated intracellular signal pathway (Li et al. 1999, 2002, 2003). Cuscuta chinensis glycoside increases activation of the MAPK signal pathway (Jian et al. 2003). We reported that sargaquinoic acid and sargachromenol stimulate the TrkA-MAPK signal pathway and PKA signal pathway (Kamei and Tsang 2003; Tsang et al. 2005). In addition, the present study shows that vitamin B12 enhances the ERK signal transduction pathway and induces neurite outgrowth in PC12 cells. ERK 1 and 2 are important components of a signal transduction pathway influencing neurodifferentiation (Chang et al. 2003). When NGF binds the Trk receptor, the signal is transferred to phosphorylate each kinase in the Ras/Raf/MEK/ERK signal pathways (Szeberenyi and Erhardt 1994). Therefore, the activation of ERK by vitamin B12 is interesting to know the neurodifferentiation.
In conclusion, our results suggest that vitamin B12 can stimulate neurodifferentiation and that, like NGF, it stimulates the MAPK signaling pathway via the activation of ERK. Therefore, vitamin B12 may be a candidate compound for the development of new treatments for neurodegenerative diseases such as AD.
References
- Borasio GD. Differential effects of the protein kinase inhibitor K-252a on the in vitro survival of chick embryonic neurons. Neurosci Lett. 1990;108:207–212. doi: 10.1016/0304-3940(90)90732-O. [DOI] [PubMed] [Google Scholar]
- Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway. Int J Oncol. 2003;22:469–480. [PubMed] [Google Scholar]
- Chaudhuri TK, Paul S. Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J. 2006;273:1331–1349. doi: 10.1111/j.1742-4658.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- Chen LQ, Wei JS, Lei ZN, Zhang LM, Liu Y, Sun FY. Induction of Bcl-2 and Bax was related to hyperphosphorylation of tau and neuronal death induced by okadaic acid in rat brain. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1236–1245. doi: 10.1002/ar.a.20241. [DOI] [PubMed] [Google Scholar]
- DiProspero NA, Chen EY, Charles V, Plomann M, Kordower JH, Tagle DA. Early changes in Huntington’s disease patient brains involve alterations in cytoskeletal and synaptic elements. J Neurocytol. 2004;33:517–533. doi: 10.1007/s11068-004-0514-8. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Greene LA. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978;78:747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ina A, Hayashi KI, Nozaki H, Kamei Y (2007) Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int J Dev Neurosci in press [DOI] [PubMed]
- Iseri PK, Altinas O, Tokay T, Yuksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26:18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- Jian-Hui L, Bo J, Yong-Ming B, Li-Jia A. Effect of Cuscuta chinensis glycoside on the neuronal differentiation of rat pheochromocytoma PC12 cells. Int J Dev Neurosci. 2003;21:277–281. doi: 10.1016/S0736-5748(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, Tangalos EG, Boeve BF, Knopman DS, Braak H, Petersen RC. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Tsang CK. Sargaquinoic acid promotes neurite outgrowth via protein kinase A and MAP kinases-mediated signaling pathways in PC12D cells. Int J Dev Neurosci. 2003;21:255–262. doi: 10.1016/S0736-5748(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Gaisina IN, Petukhov PA, Sridhar J, King LT, Blond SY, Duka T, Rusnak M, Sidhu A. Highly potent and specific GSK-3beta inhibitors that block Tau phosphorylation and decrease alpha-synuclein protein expression in a cellular model of Parkinson’s disease. Chem Med Chem. 2006;1:256–266. doi: 10.1002/cmdc.200500039. [DOI] [PubMed] [Google Scholar]
- Krautler B. Vitamin B12: chemistry and biochemistry. Biochem Soc Trans. 2005;33:806–810. doi: 10.1042/BST0330806. [DOI] [PubMed] [Google Scholar]
- Kristensen MO, Gulmann NC, Christensen JE, Ostergaard K, Rasmussen K. Serum cobalamin and methylmalonic acid in Alzheimer dementia. Acta Neurol Scand. 1993;87:475–481. doi: 10.1111/j.1600-0404.1993.tb04140.x. [DOI] [PubMed] [Google Scholar]
- Law A, Gauthier S, Quirion R. Say NO to Alzheimer’s disease: the putative links between nitric oxide and dementia of the Alzheimer’s type. Brain Res Rev. 2001;35:73–96. doi: 10.1016/S0165-0173(00)00051-5. [DOI] [PubMed] [Google Scholar]
- Li P, Matsunaga K, Yamamoto K, Yoshikawa R, Kawashima K, Ohizumi Y. Nardosinone, a novel enhancer of nerve growth factor in neurite outgrowth from PC12D cells. Neurosci Lett. 1999;273:53–56. doi: 10.1016/S0304-3940(99)00629-1. [DOI] [PubMed] [Google Scholar]
- Li P, Matsunaga K, Yamakuni T, Ohizumi Y. Picrosides I and II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells. Life Sci. 2002;71:1821–1835. doi: 10.1016/S0024-3205(02)01949-5. [DOI] [PubMed] [Google Scholar]
- Li P, Matsunaga K, Yamakuni T, Ohizumi Y. Nardosinone, the first enhancer of neurite outgrowth-promoting activity of staurosporine and dibutyryl cyclic AMP in PC12D cells. Dev Brain Res. 2003;145:177–183. doi: 10.1016/S0165-3806(03)00239-6. [DOI] [PubMed] [Google Scholar]
- Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Hong HS, Wu HC, Battina SK, Rana S, Iwamoto T, Radke GA, Pettersson E, Martin GM, Hua DH, Jin LW. A novel tricyclic pyrone compound ameliorates cell death associated with intracellular amyloid-β oligomeric complexes. J Neurochem. 2006;98:57–67. doi: 10.1111/j.1471-4159.2006.03862.x. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Fountoulakis M, Dunckley T, Stephan DA, Reiman EM. Genetics, transcriptomics, and proteomics of Alzheimer’s disease. J Clin Psychiatry. 2006;67:650–670. doi: 10.4088/JCP.v67n0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis. FEBS J. 2006;273:3437–3443. doi: 10.1111/j.1742-4658.2006.05352.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- Sharma PM, Son HS, Ugi S, Ricketts W, Olefsky JM. Mechanism of SHIP-mediated inhibition of insulin- and platelet-derived growth factor-stimulated mitogen-activated protein kinase activity in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19:421–430. doi: 10.1210/me.2004-0096. [DOI] [PubMed] [Google Scholar]
- Szeberenyi J, Erhardt P. Cellular components of nerve growth factor signaling. Biochim Biophys Acta. 1994;1222:187–202. doi: 10.1016/0167-4889(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Terry RD. Alzheimer’s disease and the aging brain. J Geriatr Psychiatry Neurol. 2006;19:125–128. doi: 10.1177/0891988706291079. [DOI] [PubMed] [Google Scholar]
- Tsang CK, Sagara A, Kamei Y. Structure-activity relationship of a neurite outgrowth promoting substance purified from a brown alga, Sargassum macrocarpum and its analogues on PC12D cells. J Appl Phycol. 2001;40:349–357. doi: 10.1023/A:1017540620106. [DOI] [Google Scholar]
- Tsang CK, Kamei Y. Novel effect of vitamin K1 (phylloquinone) and vitamin K2 (menaquinone) on promoting nerve growth factor-mediated neurite outgrowth from PC12D cells. Neurosci Lett. 2002;323:9–12. doi: 10.1016/S0304-3940(01)02550-2. [DOI] [PubMed] [Google Scholar]
- Tsang CK, Kamei Y (2004) Sargaquinoic acid supports the survival of neuronal PC12D cells in a nerve growth factor-independent manner. Eur J Pharmacol 19:488, 11–18 [DOI] [PubMed]
- Tsang CK, Ina A, Goto T, Kamei Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience. 2005;132:633–643. doi: 10.1016/j.neuroscience.2005.01.028. [DOI] [PubMed] [Google Scholar]