Abstract
The gas vesicles isolated from the cells of filamentous cyanobacterium Anabaena flos-aquae were treated and sterilized with glutaraldehyde and then evaluated for their effectiveness as gas carriers in cell culture. Anchorage-dependent Vero cells were grown in a packed bed of microcarrier beads under the perfusion of Dulbecco’s Modified Eagle’s Medium with 1% serum. The culture medium supplemented with 1.8% (v/v) gas vesicles was found to support a 30% higher maximum glucose utilization rate than the same medium without gas vesicles. The gas vesicle suspension was confirmed to have no apparent effects on cell metabolism in T-flask cultures. The study results indicated that the gas vesicles, with high oxygen carrying capacity, can be used to increase the oxygen supply in cell culture systems.
Keywords: Cyanobacteria, Flocculation, Gas vesicle, Microcarrier culture, Oxygen supply, Vero cells
Introduction
The growth and maintenance of aerobic microbial and mammalian cells require ready availability of dissolved oxygen. Oxygenation, however, often becomes limiting in biological systems and processes. The root cause of this problem is the low oxygen solubility in aqueous solutions, which is approximately 8 mg l−1 at ambient temperature and pressure. Providing adequate oxygen supply to the cultures of animal and insect cells/tissues is particularly challenging because these organisms lack the cell walls to protect them from the stress caused by vigorous bubbling and agitation (Alvarez et al. 2005; Curran and Black 2005; Handa-Corrigan et al. 1989; Ma et al. 2004; Maranga et al. 2004; Oh et al. 1987).
These problems have led to attempts at enhancing oxygen supply by non-conventional methods, including the introduction of oxygen carriers such as hemoglobin, hydrocarbons and perfluorochemicals (Adlercreutz and Mattiasson 1982; Barnikol and Poetzschke 2003; Ho et al. 1990; Ju et al. 1991a; Junker et al. 1990; Nagase et al. 2005). For example, Adlercreutz and Mattiasson (1982) supplemented the growth medium with hemoglobin in their reactor for glycerol conversion to dihydroxyacetone using immobilized cells of Gluconobacter oxydans. The dihydroxyacetone production was found to increase linearly with the increasing hemoglobin concentration employed. However, the use of hemoglobin as an oxygen carrier has been limited by its high cost and its high affinity for oxygen; the latter necessitates a long residence time of hemoglobin in the reactor if the bound oxygen is to be effectively used. Researchers have similarly taken advantage of the high oxygen solubilities in perfluorochemicals (and some hydrocarbons) and used their emulsions or dispersions to increase oxygen supply to cells (Ho et al. 1990; Ju et al. 1991a; Junker et al. 1990; Nagase et al. 2005). The biologically inert perfluorochemicals have also long been pursued in formulations of artificial blood substitutes (Keipert 1995; Kim and Greenburg 2004; Remy et al. 1999). These emulsions or dispersions are, however, inherently unstable and there are potential side effects associated with the high concentrations of surfactants used for making the emulsions (Ju and Armiger 1992; King et al. 1987).
As a potential alternative to the above oxygen carriers, the naturally occurring gas-filled microcavities, the gas vesicles (GVs), were examined in this study. Having exclusively proteinaceous structures, GVs are the components of gas vacuoles which are found in cells of waterbloom-forming cyanobacteria. GVs have the form of hollow cylindrical tubes closed at each end by a hollow conical cap. Isolated from Anabaena flos-aquae, the vesicles used in this study were typically about 500 nm in length, 75 nm in width, and 2 nm in wall thickness (Walsby 1994; Walsby and Armstrong 1979; Arrington et al. 2003). The vesicle wall was made of two types of proteins: GvpA and GvpC (Hayes et al. 1992; Walsby 1994; Walsby and Hayes 1988). The smaller GvpA molecules (MW 7,397) form the inner hydrophobic shell in a tight, ribbed structure; the larger hydrophilic GvpC molecules (MW 21,985) patch on the outside of the shell (Walsby and Hayes 1988). Because GvpC molecules form the ties that cross the GvpA ribs and hold them together, the GvpC layer is primarily responsible for the mechanical strength of GVs (Walsby 1994). The vesicle wall is impermeable to liquid water but is freely permeable to gas molecules (Sundararajan and Ju 2000a; Walsby 1969; Walsby et al. 1992). As a result, the hollow space in the structure is usually filled with gas in equilibrium with the surrounding liquid. Depending on the species, the GVs formed may withstand considerable pressures, up to 37 atmospheres in a marine cyanobacterium (Walsby 1994), before collapsing irreversibly. GVs can occupy up to 10% of the cell volume. They have been assigned roles of buoyancy regulation, light shielding, and increasing cell surface area-to-volume ratio in cyanobacterial cells. It has been recognized that gas vesicles are important in providing buoyancy for planktonic cyanobacteria and helping them perform vertical migration in lakes and other aquatic systems.
As described above, GVs are essentially cylindrical “bubbles” stabilized by thin and moderately strong proteinaceous walls. The vesicle walls do not cause significant resistance to gas permeation (Sundararajan and Ju 2000a; Walsby et al. 1992). The amount of oxygen carried in the gas phase of GV can therefore be computed according to the partial pressure of oxygen in the surrounding liquid, using the ideal gas law. As shown in Table 1, GVs are expected to have superior oxygen carrying capacities than perfluorochemicals.
Table 1.
Oxygen carrying capacities of water, perfluorochemicals and gas vesicles
| ka | |
|---|---|
| Water | 9.7b |
| Perfluorodecalin | 164b |
| Perfluotripropylamine | 176b |
| Perfluorotributylamine | 156b |
| Gas Vesicles | 388c |
aValues are for 37 °C. The unit is (mol O2) m−3 MPa−1, i.e., concentration of O2 (mol m−3) per MPa of oxygen partial pressure
bData for water and perfluorochemicals taken from Reference (Reiss and Le Blanc 1982), with conversion of the unit from (mL O2) to (mol O2)
cValue calculated using the ideal gas law, i.e.,

These properties make GVs a potential candidate as gas microcarriers to provide or enhance gas exchange in low-shear environments. Compared to non-emulsified and mildly emulsified perfluorochemicals, the smaller GVs have much larger specific surface areas for gas exchange. Compared to highly emulsified perfluorochemicals that require potentially harmful, high concentrations of surfactants to maintain their emulsion stability, GVs are already stabilized by the proteinaceous walls. Compared to hemoglobin, GVs can be produced from simple cyanobacterial cultures using inexpensive media. While currently limited by the requirement of tedious downstream harvesting procedures (Kashyap et al. 1998), the production costs of GVs may be significantly reduced with further technology development.
The objective of this study was to evaluate the efficiency of GVs as oxygen carriers in animal cell culture systems. The optimization of culture conditions for high cell buoyancy, the harvesting of floated cells, and the lysis of cells to release intracellular GVs were reported earlier (Kashyap et al. 1998; Walsby and Buckland 1969). A chemical treatment procedure was also developed to cross-link the proteinaceous vesicle walls (Sundararajan and Ju 2000b). The treatment improved the resistance of GVs to protein stripping agents, which would otherwise severely weaken the vesicles. In this current work, the non-toxicity of crosslinked GVs to animal cells was examined in T-flask cultures of Vero cells. The enhanced oxygen carrying capacity of GV-supplemented medium was then evaluated in a perfusion culture of Vero cells grown in a packed bed of microcarrier beads. The experimentally observed enhancement was compared with the theoretical value computed by volume-averaging the oxygen carrying capacity of the aqueous medium and that of the suspended GVs. The potential causes of the difference in experimental and theoretical enhancements were also discussed.
Materials and methods
Production and preparation of gas vesicles
The procedures for cultivation and harvesting of the cyanobacterium Anabaena flos-aquae CCAP 1403/13f (obtained from Institute of Freshwater Ecology, Windermere, UK) as well as the procedures for the subsequent cell lysis and centrifugation to collect the released GVs have been reported elsewhere (Kashyap et al. 1998; Sundararajan and Ju 2000b; Zeleznik et al. 2002). Briefly, the culture was grown in bubble-column photobioreactors with surface illumination by Cool White light tubes. The culture was harvested at the late exponential-growth phase by flotation in the dark, because the conventional methods such as filtration and centrifugation tended to destroy the vesicles. The harvested cells were then lysed in a hypertonic (0.7 M) sucrose solution. The lysate was then gently and repeatedly centrifuged (200 × g for 8 h) to collect and concentrate GVs. The concentrated GV suspension was sterilized as follows:
The vesicles could not be autoclaved or steam sterilized because they became extremely weak after being exposed to temperatures ≥70 °C (Sundararajan and Ju 2000b). Sterilization was therefore done by treating GVs with 1% (w/v) glutaraldehyde for 3 h. The treatment also served to crosslink the GV protein structure and, thereby, to increase its resistance against protein stripping agents (Sundararajan and Ju 2000b). Due to its highly toxic nature, all excess unused glutaraldehyde had to be removed. Dialysis, using Spectra/Por® regenerated cellulose membranes with a molecular weight cut-off of 50,000, was performed for this purpose. The dialysis tubes containing GV suspensions were first transferred to glass jars filled with 1% (w/v) glutaraldehyde. This was done to chemically sterilize the entire dialysis assembly. After 3 hours, the glutaraldehyde solution was replaced with sterile distilled water. The dialysis was continued for 5 more days with daily replacement of sterile water. The dialyzed GV suspension was then transferred to a storage vial and handled aseptically thereafter.
Animal cell culture
Cell line and media
Vero cells, a continuous anchorage-dependent cell line isolated from African green monkey kidney cells, were obtained from American Type Culture Collection (ATCC). Stock Vero cells were grown and maintained in 75-cm2 T-flasks (Sigma Chemicals Co.) with 20 mL of Minimum Essential Medium (MEM) (Sigma) supplemented with 10% Newborn Calf Serum (NCS) (Sigma). The T-flasks were kept in a Stericult 200 Incubator (Forma Scientific, Inc.) maintained at 37 °C, 5% CO2 and 95% relative humidity. A one-half medium change was done every other day inside a vertical laminar flow hood (Nuaire Class II Type A/B3), with subculturing done upon confluence. Stock cultures were maintained in liquid nitrogen.
The Dulbecco’s Modified Eagle’s Medium (DMEM) with higher concentrations of glucose and glutamine was used for the perfused microcarrier cultures. As described later, the serum concentration in media needed to be lowered to minimize the GV flocculation caused by certain serum components. Vero cells were therefore gradually acclimated to medium of 1% serum over a period of weeks. The final medium composition used in the perfusion study is given in Table 2.
Table 2.
Medium composition used in perfusion microcarrier culture of Vero cells
| Component | Volume (mL per L) |
|---|---|
| 10X DMEM* | 100 |
| NaHCO3 (7.5%) | 49.3 |
| Glutamine (200 mM) | 20 |
| Nystatin (10,000 units per mL) | 24 |
| Penicillin-Streptomycin Solution | 10 |
| Newborn Calf Serum | 10 |
| Folic Acid Solution (0.4% w/v) | 1 |
| Gas Vesicle Suspension (2.3% v/v) or Distilled Water | 785.7 |
* Dulbecco’s Modified Eagle’s Medium (DMEM), with 4 g L−1 of glucose
Attachment of cells to microcarriers
A 200-mL spinner flask was used for initial cell attachment to microcarrier beads. All glassware was siliconized prior to use. The glass microcarrier beads (Sigma) used had diameters of 150–210 μm and a density of 1.03 g cm−3. Accordingly, 1 g of the beads contained approximately (2.0–5.5) × 105 beads and provided a surface area of 140–390 cm2 for cell attachment. The beads, ∼4 g or (0.8–2.2) × 106 beads, were suspended in tissue culture grade water and sterilized by autoclaving at 121 °C, 124 kPa for 15 min. The water was discarded and the beads soaked in a small amount of culture medium in a 37 °C incubator for 30 min. The medium was next discarded and the beads transferred to the spinner flask with 75 mL of freshly inoculated medium. From experience the appropriate inoculum concentration was found to be about 2 × 104 cells per mL, corresponding to 1.5 × 106 cells or about one cell per bead. The inoculated spinner flask was kept in the CO2 incubator on top of a magnetic stirrer for 18 h. The stirring was maintained at the lowest speeds (60–80 rpm) required for bead suspension. After 18 h, the beads were visually checked under a microscope (Nikon TMS) for cell attachment.
Perfusion apparatus
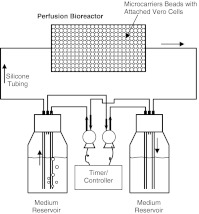
As shown in Fig. 1, the perfusion apparatus consisted of (1) a packed-bed column reactor; (2) two peristaltic pumps (Fisher Scientific); (3) two medium reservoirs; (4) two programmable controllers (VWR Scientific), used to turn the pumps on and off at chosen intervals; and (5) silicone tubing. The reactor was a thin glass column, 1 cm in diameter and 5 mL in volume. The glass column was fitted with holed Teflon stoppers. The inserted end of each stopper had a glued stainless steel screen (with 104-μm grids); the hole at the other end was connected to silicone tubing. The reservoirs were 40-mL glass vials; each had a screw cap with three snugly fitted glass tubings. One of the glass tubings, extended all the way to the bottom of the vial, was connected to the column reactor via silicone tubing, for circulating the medium to and from the reservoir. The other two glass tubings were used for air inlet and outlet in a specific manner as described in the following.
Fig. 1.
Experimental set-up for perfusion culture. Vero cells were grown on glass microcarrier beads packed in the bioreactor. The perfusion medium was passed through the bioreactor gently, driven by the air pump-created headspace pressure difference between the two medium reservoirs. The two pumps were synchronously turned on and off, using the programmable controllers, to reverse the perfusion flow direction when the medium volume in the source reservoir dropped to about 3 mL (and that in the sink reservoir accumulated to about 12 mL)
It was found earlier that pumping a GV suspension using peristaltic pumps would destroy the vesicles, presumably by the grinding effect. This perfusion system was therefore designed to circulate medium by headspace pressurization. One of the two remaining glass tubings was pushed to the bottom of the vial, while the other was short and inserted just below the top of the vial. The longer glass tubing was connected by silicone tubing to the outlet of one of the peristaltic pumps; the shorter one was connected to the inlet of the other pump. The loop was complete once the tubings from the other reservoir were connected similarly. The on/off times of the two pumps were synchronized using the programmable controllers such that one pump was switched on the moment the other was switched off. At any instant, air from the head space of one vial was sucked out and sparged into the medium in the other vial. This raised the pressure in the latter vial and pushed the medium to flow from that vial through the microcarrier reactor and finally into the other vial. This flow was reversed once the controllers reversed the on/off switch of the pumps.
Operation of perfused culture
After all parts were autoclaved separately, the microcarrier beads with attached cells were pipetted into the glass column stoppered at one end. The beads formed a packed bed once the medium drained off. The mesh glued to the end of the Teflon stopper prevented the beads from draining off. After being fully packed with beads, the column was fitted with the other Teflon stopper. The reservoirs were next added with medium: 10 mL in one and 5 mL in the other. The column was held vertically at all times by a clamp stand. After connecting the air-circulating silicone tubing to the peristaltic pumps, the entire assembly (except the programmable controllers) was transferred into a CO2 incubator. The programmed on/off switches were next begun to perfuse the medium through the packed bed of beads. Medium samples were taken daily for metabolite assays. The spent medium was replaced with fresh medium every 48 h.
Analytical methods
The volume of GVs in a sample was determined using the capillary compression apparatus as described elsewhere (Kashyap et al. 1998; Walsby 1982). Briefly, the sample was filled into a reservoir (1.03 mL) attached to one end of the compression tube that had a capillary of 200 μm in diameter and a capacity of 31.4 nL per mm in length. The filled tube was then placed in a glass pressure tube pre-filled with cold water (at 19 °C). With a special procedure, water was pulled into the capillary by the capillary force while forming a trapped bubble between the sample and the water. One end of the pressure tube was connected to a compressed air cylinder. The assembly was then mounted on a movable steel base that was connected to the sliding arm of a vernier caliper. A fixed microscope was used to observe the meniscus of the trapped air bubble in the capillary. The meniscus was allowed to stabilize and the reading (a) on the vernier noted. The chamber was then pressurized to collapse the GVs in the sample. After the pressure was released and the system re-equilibrated, the bubble had moved toward the sample reservoir, reflecting the reduction in sample volume due to the collapse of GVs. The new meniscus reading (b) was again noted, and the GV volume could be calculated from the difference, (a)–(b). The standard deviation of the procedure was 15–20% with triplicate measurements.
Glucose concentration was measured by the glucose oxidase method using the diagnostic kit (Sigma 510; Sigma Chemicals Co., St. Louis, MO). Glutamine concentration was measured using the Glutamine Assay Kit from Sigma Biosciences (GLN-2). To follow cell growth in T-flasks, viable cell counts were determined by the Trypan Blue dye-exclusion method. Without a simple, accurate procedure for direct cell enumeration in the perfusion culture, glucose utilization rate (GUR) was determined along the experiment and used as an indicator of the cell activity.
Results
T-flask cultures in media with and without chemically treated GVs
Batch cultures of Vero cells in T-flasks were carried out in MEM without and with 0.2% (v/v) of the glutaraldehyde-treated and then dialyzed GVs. The purpose was twofold: (1) to ensure total sterility of the GV preparation, and (2) to look for any negative effects on cell growth or metabolism due to the presence of any residual glutaraldehyde or of the crosslinked GVs themselves. Note that this experiment in T-flasks was not designed to show the enhanced oxygen carrying capacity of the GV-supplemented medium. In the unmixed T-flask cultures with a large gas-liquid interface, the rate of oxygen supply to cells attached on the bottom surface of the flasks was governed by the rate of oxygen permeation through the medium, not by the equilibrium amount of oxygen carried in the medium. While GVs (and other oxygen carriers such as perfluorochemicals) could increase the permeation rate and the increase could be estimated using the Fricke’s equation (Sundararajan and Ju 2000a; Ju et al. 1991b), in dilute GV suspensions the increase in oxygen permeability would not be nearly as apparent as the increase in oxygen carrying capacity. For example, at the 0.2% (v/v) concentration used in this experiment, GVs would not be expected to increase the oxygen permeability by more than 2% (Sundararajan and Ju 2000a). The latter value was calculated using the Fricke’s equation with the assumptions of (1) uniform distribution of GVs in the medium and (2) no mass transfer resistance across the proteinaceous walls of GVs. Accordingly, no appreciable oxygen supply-enhancing effects of GVs were anticipated in this experiment.
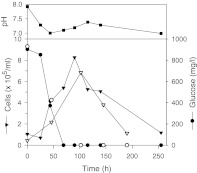
For direct comparison, the results obtained in both GV-free and GV-supplemented systems are shown in Fig. 2. The culture growth was quantified by cell number concentrations from trypsinized sacrificial flasks. The pH fluctuated but remained in the physiologically suitable range of 7.0–7.6. (The apparently higher than normal pH of the starting medium was the value measured before the medium was equilibrated with the 5% CO2 atmosphere in the incubator.) The cell number increased up to about 80–100 h in both systems. The profiles of glucose consumption were practically the same and they roughly mirrored the profiles of cell growth, considering the inevitable scattering of experimental data, particularly the viable cell numbers counted from the trypsinized sacrificial flasks. Glucose was shown to be the limiting nutrient in both systems. No significantly negative effects were evident by either the residual glutaraldehyde or the crosslinked GVs themselves.
Fig. 2.
Profiles of cell growth, glucose consumption and pH change observed in T-flasks (filled symbols for GV-free medium, open symbols for GV-supplemented medium)
Effects of serum concentration on gas vesicle flocculation and cell growth
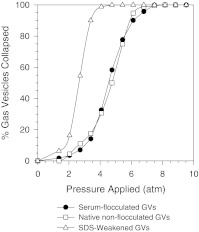
One important observation from the above T-flask study was the occurrence of flocculation among GVs, whereupon a homogeneously turbid suspension turned into one with white flakes floating around. By suspending GVs in culture media with and without serum it was found that serum was responsible for the flocculation. Two possibilities existed for the occurrence of this phenomenon: (1) the outer hydrophilic GV protein (GvpC) was stripped by serum components, resulting in the flocculation to minimize the exposure of the inner, less hydrophilic GvpA shells; and (2) certain serum components functioned as flocculants that bridged among the GVs. The first case had been reported before when GV suspensions were treated with urea and detergents like SDS (Hayes et al. 1992; Sundararajan and Ju 2000b; Walsby and Hayes 1988). The detachment of GvpC from the surface would be accompanied by a significant weakening of the GVs, and this weakening could be detected from the pressure collapse curves of GV suspensions generated using 90°-nephelometry (Sundararajan and Ju 2000b; Walsby 1994; Walsby and Hayes 1988). Briefly, intact GVs scatter light strongly because of the enclosed gas space. This property is lost with the collapse of the vesicles. The change in intensity of scattered light of a GV suspension can therefore be easily measured with a pressure nephelometer to follow the collapse of GVs under different pressures and assess the mechanical strength of the vesicles. To determine if the stripping of GvpC was responsible for flocculation, the pressure collapse curves were generated for the serum-flocculated GVs and compared with those from the GVs exposed to 2.5% (w/v) SDS (Fig. 3). The flocculated GVs clearly retained their strength, suggesting that the second mechanism was responsible for the flocculation.
Fig. 3.
Pressure collapse curves of gas vesicles. The scattered light intensity at 90° (Nephelometric Turbidity Unit, NTU) fell from the initial reading, NTUi, as the vesicles collapsed under increasing pressure. The final reading, NTUf, was taken when no further decrease in NTU was observed with increasing pressure. The fraction of GVs collapsed at any pressure was estimated as (NTUi - NTU)/(NTUi–NTUf)
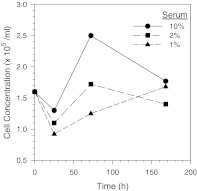
To solve the GV flocculation problem, it was decided to lower the serum concentration in the medium. In a study done in test tubes, 1% was about the highest serum concentration at which no flocculation was observed (detailed data not shown). It is however well known that the presence of certain serum components like glycoproteins, growth factors and hormones in culture media are essential for unlimited cell growth. In a subsequent study, the growth profiles of Vero cells in media with different serum concentrations (10%, 2%, and 1%) were observed in T-flasks. As shown in Fig. 4, the medium with 1% serum was found to support growth of Vero cells but at a significantly slower rate. A gradual acclimation of Vero cells to the medium containing 1% serum was therefore undertaken in this study during the early stage of the perfusion culture study.
Fig. 4.
Growth of non-acclimated Vero cells in MEM with different serum concentrations
Perfusion culture studies
For proper demonstration of the beneficial effects associated with the improved oxygen carrying capacity in GV-supplemented medium, it was important that cell growth was not limited by any factor other than the availability of oxygen to the cells. Two major potential factors are (1) depletion of main energy sources, i.e., glucose and/or glutamine (Ju and Armiger 1990; Kaiser et al. 2005; Pithon-Curi et al. 2004); and (2) accumulation of inhibitory metabolites such as lactate (Nahapetian et al. 1986; Pithon-Curi et al. 2004) and ammonia (Imamura et al. 1982; Thilly et al. 1982; Wong et al. 2004). These were carefully eliminated in the perfusion culture study, as described in the following:
The circulating medium was changed every 2 days. A MEM-based medium was originally used. The pH changed but stayed within the physiologically suitable range (7.2–7.4), suggesting that there was no inordinate accumulation of either (pH-lowering) lactate or (pH-raising) ammonia to growth-inhibitory levels. However, both glucose and glutamine were consumed rapidly: glucose was completely depleted and glutamine was in very low concentrations before the end of the perfusion cycle (data not shown). To continue operating the perfusion system through 2-day cycles, this necessitated a switch from MEM to DMEM. Test perfusion cultures with DMEM further confirmed that glutamine was never depleted as long as glucose was still available. Therefore, only glucose concentration needed to be routinely measured to ensure no nutrient limitation.
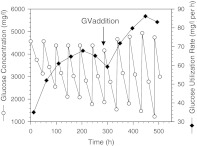
With the experience gathered from the test perfusion runs, the experiment to demonstrate the oxygen carrying capacity of GV-supplemented media was conducted. Cells had been acclimatized for one month in DMEM containing 1% serum before they were attached to the microcarrier beads. The 2-day perfusion cycle was strictly adhered to. The perfusion was run with GV-free medium for 288 h (12 days), after which the medium was switched to the medium supplemented with 1.8% (v/v) GVs. Profiles of glucose concentration and GUR during this perfusion experiment are shown in Fig. 5. GUR steadily increased during the first week of perfusion indicating a corresponding cell growth. A slight drop in GUR occurred in the early part of the second week, presumably because the increasing oxygen demand of the cells could no longer be properly met by the oxygen carried into the bioreactor with the circulating medium. After the switch to the GV-containing medium at 288 h, the increase in GUR resumed. A new maximum of about 30% higher GUR was reached 10 days later. The significant benefit of using GVs as gas microcarriers to enhance the oxygen supply in cell cultures was clearly demonstrated.
Fig. 5.
Profiles of glucose concentration and glucose utilization rate from a perfusion culture in DMEM with 1% serum. At 288 h, after the glucose utilization rate peaked and started to decline, GVs (1.8%) were added to the medium to enhance the oxygen supply to cells
Discussion
Although significant, the 30% increase in cell activity (indicated by GUR) achieved with 1.8% GVs was lower than the potential increase predicted theoretically, as discussed in the following:
In the perfusion system used in this study, the oxygen supply to the bioreactor was achieved by external oxygenation of the circulating culture medium. An oxygen balance can be done around the bioreactor. At (pseudo)steady state, the difference between input and output rates of oxygen carried by the circulating medium is equal to the oxygen consumption rate by cells, i.e.,
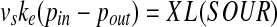 |
1 |
where vs is the medium circulation velocity (cm s−1), ke is the oxygen carrying capacity (or effective solubility) of the circulating medium (per unit partial pressure of O2, i.e., μmol cm−3 MPa−1 = mol m−3 MPa−1), p is the oxygen partial pressure in the circulating medium (MPa), X is the viable cell concentration (number of cells per cm−3), L is the length of the bioreactor (cm), and SOUR is the specific oxygen uptake rate per cell (μmol-O2 s−1), respectively. Subscripts in and out denote inlet and outlet of the bioreactor.
In the perfusion experiment, GUR was used to indicate the (volumetric) cell activity. In Eq. (1),
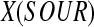 is the corresponding cell activity assuming that the rates of glucose and oxygen consumption were stoichiometrically correlated and the correlation did not change with the GV supplementation. vs and L were kept constant in the perfusion experiment before and after the switch to GV-containing medium. With adequate external medium oxygenation, pin would also be constant at ∼0.021 MPa. Equation (1) can therefore be transformed to the following proportional relationship:
is the corresponding cell activity assuming that the rates of glucose and oxygen consumption were stoichiometrically correlated and the correlation did not change with the GV supplementation. vs and L were kept constant in the perfusion experiment before and after the switch to GV-containing medium. With adequate external medium oxygenation, pin would also be constant at ∼0.021 MPa. Equation (1) can therefore be transformed to the following proportional relationship:
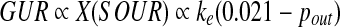 |
2 |
As shown in Fig. 5, the perfusion culture’s GUR reached two different maxima in the GV-free and GV-containing media, presumably due to oxygen limitation because other potential limiting factors had been largely eliminated as described earlier. It is reasonable to assume that the two oxygen-limiting conditions occurred when the outlet oxygen partial pressures (pout) were similarly low (if not zero). Accordingly, Eq. (2) can be further simplified to
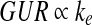 |
3 |
The oxygen carrying capacity (ke) of the GV suspension can be estimated using the volume-average rule, i.e.,
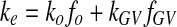 |
4 |
ko and kGV are the oxygen solubilities in the GV-free medium and the gas phase inside GV, respectively. fo and fGV are their volume fractions. The values for ko and kGV at 37 °C are given in Table 1, assuming that ko in water approximates that in the GV-free medium. By using Eq. (4) and the values of ko and kGV, the values of ke can be calculated for the media supplemented with different volume fractions of GV. The computed % increases in oxygen carry capacity are listed in Table 3. At 1.8% GV supplementation, as in the perfusion experiment, an about 70% increase in ke was expected.
Table 3.
Computed oxygen carrying capacity in media with different volume fractions of gas vesicles at 37 °C
| Gas vesicle volume fraction (%) | % Increase in oxygen carrying capacity (%) |
|---|---|
| 0 | 0 |
| 0.5 | 19.5 |
| 0.77 | 30.0 |
| 1.0 | 39.0 |
| 1.5 | 58.5 |
| 1.8 | 70.1 |
| 2.0 | 77.9 |
| 5.0 | 194.8 |
| 10.0 | 389.7 |
| 20.0 | 779.4 |
The 30% increase in GUR observed experimentally with the supplementation of 1.8% GVs is clearly lower than the 70% increase predicted theoretically from the enhanced oxygen carrying capacity. GV flocculation was likely the most important cause for the above discrepancy. In the earlier study for evaluating the effect of serum concentration on the GV flocculation in small vials, there was no visible flocculation in the medium containing 1% serum. This observation was used as a guideline for the perfusion experiment. Nonetheless, GV flocculation still occurred in the perfusion experiment, presumably due to the much prolonged exposure to serum. The flocculated GVs appeared as tiny white flakes that tended to float to the surface of the medium reservoirs and/or stick to the glass surface. Some might also become trapped inside the packed bed. As a result of the flocculation, there were fewer GVs being perfused with the medium. As shown in Table 3, if GV flocculation was solely responsible for the discrepancy between the experimental and theoretical enhancements, the GV content in the medium would have decreased from 1.8% to about 0.8% during the experiment.
Other non-idealities in the bioreactor might have also contributed to the lower enhancement obtained. The environments inside the packed bed were not homogeneous: various gradients of pH, dissolved oxygen level, and nutrient and metabolite concentrations were expected. Possible flow channeling would exacerbate the non-uniform distributions of these properties. Formation of multiple layers of anchorage-dependent cells on microcarrier beads has also been reported (Brenner and Huelser 1996; Shima et al. 1988).
Further studies to minimize GV flocculation and to evaluate the perfusion culture at different levels of GV supplementation are required to clearly identify the cause(s) for the discrepancy. Unfortunately, the current procedures for producing, harvesting and chemically treating the cyanobacterial GVs are still too laborious to allow the complete and thorough investigation. Nevertheless, the results of this study demonstrated the significant benefits achievable with the use of GVs as oxygen carriers in low-shear animal cell cultures.
Conclusions
The oxygen carrying capacity of GV-supplemented culture medium was estimated using the volume-average rule. It was shown that the maximum cell activity supportable in perfusion cell culture could be substantially increased even with a low volume fraction of GVs. With Vero cell culture in T-flasks, the glutaraldehyde-treated GVs were shown not to have significant effects on cell metabolism such as cell growth and glucose consumption. GVs, however, would flocculate in the presence of serum. In the perfusion study of acclimated Vero cells in DMEM with 1% serum, the addition of 1.8% GVs in the medium enhanced the maximum glucose utilization rate by about 30%. The feasibility of using cyanobacterial GVs as oxygen carriers in cell culture was demonstrated.
Acknowledgements
The work was supported by a grant to Ohio Bioprocessing Research Consortium from Ohio Board of Regents and a Faculty Research Grant from The University of Akron.
References
- Adlercreutz P, Mattiasson B. Oxygen supply to immobilized cells: Oxygen supply by hemoglobin or emulsions of perfluorochemicals. Eur J Appl Microbiol Biotechnol. 1982;16:165–170. doi: 10.1007/BF00505826. [DOI] [Google Scholar]
- Alvarez MM, Guzman A, Elias M. Experimental visualization of mixing pathologies in laminar stirred tank bioreactors. Chem Eng Sci. 2005;60:2449–2457. doi: 10.1016/j.ces.2004.11.049. [DOI] [Google Scholar]
- Arrington SA, Zeleznik MJ, Ott DW, Ju L-K. Effects of polyethyleneimine on cyanobacterium Anabaena flos-aquae during cell flocculation and flotation. Enz Microb Technol. 2003;32:290–293. doi: 10.1016/S0141-0229(02)00294-6. [DOI] [Google Scholar]
- Barnikol W, Poetzschke H (2003) Use of hemoglobin, myoglobin or their derivatives as oxygen carriers for the controlled oxygen supply of microorganisms, cell and tissue cultures and organs. Ger Offen 10220990
- Brenner J, Huelser DF. Production of tissue plasminogen activator (tPA) in two and three dimensionally growing cultures of Bowes melanoma cells. Biotechnol Bioeng. 1996;51:422–433. doi: 10.1002/(SICI)1097-0290(19960820)51:4<422::AID-BIT5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Curran SJ, Black RA. Oxygen transport and cell viability in an annular flow bioreactor: Comparison of laminar couette and Taylor-vortex flow regimes. Biotechnol Bioeng. 2005;89:766–774. doi: 10.1002/bit.20361. [DOI] [PubMed] [Google Scholar]
- Handa-Corrigan A, Emery AN, Spier RE. Effect of gas-liquid interfaces on the growth of suspended mammalian cells: mechanisms of cell damage by bubbles. Enz Microb Technol. 1989;11:230–235. doi: 10.1016/0141-0229(89)90097-5. [DOI] [Google Scholar]
- Hayes PK, Buchholz B, Walsby AE. Gas vesicles are strengthened by the outer-surface protein, GvpC. Arch Microbiol. 1992;157:229–234. doi: 10.1007/BF00245155. [DOI] [PubMed] [Google Scholar]
- Ho CS, Ju L-K, Baddour RF. Enhancing penicillin fermentations by increased oxygen solubility through the addition of n-hexadecane. Biotechnol Bioeng. 1990;36:1110–1118. doi: 10.1002/bit.260361106. [DOI] [PubMed] [Google Scholar]
- Imamura T, Crespi CL, Thilly WG, Brunengraber H. Fructose as a carbohydrate source yields stable pH and redox parameters in microcarrier cell culture. Anal Biochem. 1982;124:353–358. doi: 10.1016/0003-2697(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Ju L-K, Armiger WB. Enhancing oxygen transfer in surface-aerated bioreactors by stable foams. Biotechnol Prog. 1990;6:262–265. doi: 10.1021/bp00004a005. [DOI] [PubMed] [Google Scholar]
- Ju L-K, Armiger WB. Use of perfluorocarbon emulsions in cell culture. BioTechniques. 1992;12:258–263. [PubMed] [Google Scholar]
- Ju L-K, Lee JF, Armiger WB. Enhancing oxygen transfer in bioreactors by perfluorocarbon emulsions. Biotechnol Prog. 1991a;7:323–329. doi: 10.1021/bp00010a006. [DOI] [Google Scholar]
- Ju L-K, Lee JF, Armiger WB. Effect of the interfacial surfactant layer on oxygen transfer through the oil/water phase boundary in perflurocarbon emulsions. Biotechnol Bioeng. 1991b;37:505–511. doi: 10.1002/bit.260370603. [DOI] [PubMed] [Google Scholar]
- Junker BH, Hatton TA, Wang DIC. Oxygen transfer enhancement in aqueous/perfluorocarbon fermentation systems: I. Experimental observations. Biotechnol Bioeng. 1990;35:586–597. doi: 10.1002/bit.260350606. [DOI] [PubMed] [Google Scholar]
- Kaiser GRRF, Monteiro SC, Gelain DP, Souza LF, Perry MLS, Bernard EA. Metabolism of amino acids by cultured rat Sertoli cells. Metabol Clin Exp. 2005;54:515–521. doi: 10.1016/j.metabol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kashyap S, Sundararajan A, Ju L-K. Flotation characteristics of cyanobacterium Anabaena flos-aquae for gas vesicle production. Biotechnol Bioeng. 1998;60:636–641. doi: 10.1002/(SICI)1097-0290(19981205)60:5<636::AID-BIT14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Keipert PE. Use of oxygent, a perfluorochemical-based oxygen carrier, as an alternative to intraoperative blood transfusion. Artif Cells Blood Subst Immobil Biotechnol. 1995;23:381–394. doi: 10.3109/10731199509117954. [DOI] [PubMed] [Google Scholar]
- Kim HW, Greenburg AG. Artificial oxygen carriers as red blood cell substitutes: a selected review and current status. Artif Organs. 2004;28:813–828. doi: 10.1111/j.1525-1594.2004.07345.x. [DOI] [PubMed] [Google Scholar]
- King AT, Mulligan BJ, Lowe KC. Perfluorochemicals and cell culture. Bio/Technol. 1987;7:1037–1042. [Google Scholar]
- Ma N, Chalmers JJ, Aunins JG, Zhou W, Xie L. Quantitative studies of cell-bubble interactions and cell damage at different pluronic F-68 and cell concentrations. Biotechnol Prog. 2004;20:1183–1191. doi: 10.1021/bp0342405. [DOI] [PubMed] [Google Scholar]
- Maranga L, Cunha A, Clemente J, Cruz P, Carrondo MJT. Scale-up of virus-like particles production: effects of sparging, agitation and bioreactor scale on cell growth, infection kinetics and productivity. J Biotechnol. 2004;107:55–64. doi: 10.1016/j.jbiotec.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Nagase K, Kohori F, Sakai K, Nishide H. Rearrangement of hollow fibers for enhancing oxygen transfer in an artificial gill using oxygen carrier solution. J Memb Sci. 2005;254:207–217. doi: 10.1016/j.memsci.2005.01.008. [DOI] [Google Scholar]
- Nahapetian AT, Thomas JN, Thilly WG. Optimization of environment for high density vero cell culture: effect of dissolved oxygen and nutrient supply on cell growth and changes in metabolites. J Cell Sci. 1986;81:65–103. doi: 10.1242/jcs.81.1.65. [DOI] [PubMed] [Google Scholar]
- Oh SKW, Nienow AW, Al-Rubeai M, Emery AN. On the evaluation of gas-liquid interfacial effects on hybridoma viability in bubble column bioreactors. J Biotechnol. 1987;12:45–62. doi: 10.1016/0168-1656(89)90128-4. [DOI] [PubMed] [Google Scholar]
- Pithon-Curi TC, Pires Melo M, Curi R. Glucose and glutamine utilization by rat lymphocytes, monocytes and neutrophils in culture: A comparative study. Cell Biochem Funct. 2004;22:321–326. doi: 10.1002/cbf.1109. [DOI] [PubMed] [Google Scholar]
- Reiss JG, Le Blanc M. Solubility and transport phenomena in perfluorochemicals relevant to blood substitution and other biomedical applications. Pure Appl Chem. 1982;54:2383–2406. [Google Scholar]
- Remy B, Deby-Dupont G, Lamy M. Red blood cell substitutes: fluorocarbon emulsions and haemoglobin solutions. Brit Med Bull. 1999;55:277–298. doi: 10.1258/0007142991902259. [DOI] [PubMed] [Google Scholar]
- Shima M, Seino Y, Tanaka H, Kurose H, Ishida M, Yabuuchi H, Kodama H. Microcarriers facilitate mineralization in MC3T3-E1 cells. Calcif Tissue Int. 1988;43:19–25. doi: 10.1007/BF02555163. [DOI] [PubMed] [Google Scholar]
- Sundararajan A, Ju L-K. Evaluation of oxygen permeability of gas vesicles from cyanobacterium Anabaena flos-aquae. J Biotechnol. 2000a;77:151–156. doi: 10.1016/S0168-1656(99)00223-0. [DOI] [PubMed] [Google Scholar]
- Sundararajan A, Ju L-K. Glutaraldehyde treatment of proteinaceous gas vesicles from cyanobacterium anabaena flos-aquae. Biotechnol Prog. 2000b;16:1124–1128. doi: 10.1021/bp000123n. [DOI] [PubMed] [Google Scholar]
- Thilly WG, Barngrover D, Thomas JN (1982) Microcarriers and the problem of high density cell culture. In: From gene to protein: translation into biotechnology. Academic Press, New York, pp 75–103
- Walsby AE. The permeability of blue-green algal gas vacuole membranes to gas. Proc R Soc Lond Ser B. 1969;173:235–255. doi: 10.1098/rspb.1969.0049. [DOI] [Google Scholar]
- Walsby AE. The elastic compressibility of gas vesicles. Proc R Soc Lond B. 1982;216:355–368. [Google Scholar]
- Walsby AE. Gas vesicles. Microbiol Rev. 1994;58:94–144. doi: 10.1128/mr.58.1.94-144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby AE, Armstrong RE. Average thickness of the gas vesicle wall in Anabaena flos-aquae. J Mol Biol. 1979;129:279–285. doi: 10.1016/0022-2836(79)90281-X. [DOI] [PubMed] [Google Scholar]
- Walsby AE, Buckland B. Isolation and purification of intact gas vesicles from a blue-green alga. Nature (London) 1969;224:716–717. doi: 10.1038/224716a0. [DOI] [Google Scholar]
- Walsby AE, Hayes PK. The minor cyanobacterial gas vesicle protein, GVPc, is attached to the outer surface of the gas vesicle. J Gen Microbiol. 1988;134:2647–2657. [Google Scholar]
- Walsby AE, Revsbech NP, Griffel DH. The gas permeability coefficient of the cyanobacterial gas vesicle wall. J Gen Microbiol. 1992;138:837–845. [Google Scholar]
- Wong DCF, Wong KTK, Goh LT, Heng CK, Yap MGS. Impact of dynamic online fed-batch strategies on metabolism, productivity and N-glycosylation quality in CHO cell cultures. Biotechnol Bioeng. 2004;89:164–177. doi: 10.1002/bit.20317. [DOI] [PubMed] [Google Scholar]
- Zeleznik MJ, Segatta JM, Ju L-K. Polyethyleneimine-induced flocculation and flotation of cyanobacterium Anabaena flos-aquae for gas vesicle production. Enz Microb Technol. 2002;31:949–953. doi: 10.1016/S0141-0229(02)00158-8. [DOI] [Google Scholar]