Abstract
The aim of this study was to develop optimal conditions for selective adhesion and isolation of mesenchymal progenitor cells (PCs) from cord blood and to determine their potential for osteogenic differentiation. Mononuclear cells (MNCs) were isolated by Ficoll-Paque gradient and plated onto 48-well culture plates precoated with: human or bovine collagen type I, human collagen type IV, fibronectin or matrigel. Cultures were incubated in αMEM containing fetal calf serum. Viability of the adherent cells was determined by alamarBlue® assay after 2, 3, and 4 weeks. After 4 weeks in culture, cells were typsinized and replated. Primary cultures were analyzed by histochemistry and third passage cells by FACS. Isolated fibroblast-like cells were cultured in the presence of osteogenic factors and differentiation determined by Alizarin Red S staining, RT-PCR and electron dispersive spectroscopy (EDS). MNCs adhered to all types of matrices with the greatest adhesion rates on fibronectin. These cells were CD45+, CD105+, CD14+, CD49a+, CD49f+, CD44+ and CD34−. The highest incidence of PCs was observed on fibronectin and polystyrene. Passages were CD45−, CD14−, CD34− and weakly CD105+. Primary cultures expressed endothelial/macrophage RNA markers whether cultured on fibronectin or polystyrene and these markers decreased upon passage. The best osteogenic differentiation was observed in MPCs cultured in osteogenic medium containing Vit D3 and FGF9. These cells expressed the bone-related mRNA, collagen type I, core binding factor I (Cbfa I), osteocalcin and osteopontin. EDS of deposits produced by these cells demonstrated a calcium/phosphate ratio parallel to hydroxyapatite. It was concluded that fibronectin increased adhesion rates and isolation potential of cord blood mesenchymal progenitor cells.
Keywords: Adhesion molecules, Cord blood, Progenitor cells, Osteogenesis
Introduction
Mesenchymal progenitor cells are multipotential cells which are thought to act as long lasting precursors for connective tissues (Arinzeh 2005; Bruder et al. 1994; Bhagavati and Xu 2004; Dazzi et al. 2005; Dennis and Charbord 2002; Ge et al. 2005). To date, the most common source for harvesting of MSCs is bone marrow (Grove et al. 2004; Herzog et al. 2003) and fat tissue (Dicker et al. 2005; Zuk et al. 2001), but due to the invasive procedures necessary for the aspiration of sufficient numbers of target cells, alternative pools are being studied. It has been accepted that umbilical cord blood (UCB) and peripheral blood (PB) are resources for hematopoetic stem cells which can be utilized in place of bone marrow transplants (Cohen and Nagler 2004; Fibbe et al. 2001; Kogler et al. 2005; Rogers and Casper 2004; Muguruma et al. 2006) following myeloablation. In addition there have been several reports of the presence of circulating endothelial progenitors in CB and PB (Silva et al. 2005; Murohara et al. 2000; Hildbrand et al. 2004). On the contrary to bone marrow and PB-derived progenitors, cord blood progenitors are thought to possess a higher cell cycle rate. This property should favor the growth of cord blood progenitors compared with adult PB. In spite of reports of in vitro skeletal myogenic (Gang et al. 2004), hepatic (Hong et al. 2005), neural (Jeong et al. 2004), osteogenic, chondrogenic and adipogenic differentiation (Chang et al. 2006) of UCB-derived MNCs, controversy still exists as to the identity of these cells and the reproducibility of the various isolation and culture protocols.
Efforts to isolate pure cultures of these multipotential cells from samples of fresh cord blood while employing the plastic adhesion method with variations of supplemented cell media have demonstrated levels of success ranging from 7.4% (Kang et al. 2004), 24% (Erices et al. 2000) to 40.3% (Kogler et al. 2004). Bieback et al. (2004) developed optimized isolation and culture conditions and were able to reach a 63% rate of isolation. Their study determined 4 criteria which significantly affected the success rate of MPC isolation; (1) time of culture (not >15 h from collection), (2) volume of sample (not <33 ml), (3) number of MNCs/ml (not <108) and (4) minimal state of hemolysis of sample. The goals of the present study were to determine if the addition of protein adhesion molecules to the surface of plastic culture plates would assist in increasing the incidence of MPC isolation from fresh samples of cord blood, to identify the cells which bind to the plate surface and to evaluate the osteogenic potential of the resultant mesenchymal isolates.
Materials and methods
Coating of plates
Forty-eight-well (1.1 cm2) polystyrene culture plates (Nunc) were coated prior to initiation of cell culture with either human collagen types I and III, bovine collagen types I and III (both Gibco-Invitrogen, USA), collagen type IV (Sigma, USA), fibronectin (Biological Industries, Israel) or Cultrex Basement Membrane Extract (Trevigen, USA). Coating was accomplished in the following manner: (1) Working solutions of human collagen type IV (Col IV), bovine collagen (b-Col) and human collagen (h-Col) (97% type I and 3% type II) were prepared by diluting to a concentration of 100 μg ml-1 with 0.25% acetic acid. 0.5 ml of collagen were spread evenly over the surface, followed by incubation overnight (o/n) at 4°C. Excess matrix was removed, the plates dried under UV for 20 min followed by two washes with phosphate buffered saline (PBS). (2) Fibronection was diluted (5 μg ml−1) in Minimal Essential Medium-Alpha with 1,000 mg/ml d-glucose (μMEM—Biological Industries, Israel), added to culture dishes and incubated (60 min, 37°C, 5% CO2) prior to seeding of the cord blood cells. (3) Cultrex was diluted with cold serum-free medium (0.1 mg ml−1) and a sufficient volume necessary to cover the surface area of each well was applied prior to incubation for 60 min at 37°C. Each trial consisted of six replicates/plate of each coating and three plates/sample i.e. a total of 18 replicate wells of each coating/experiment. Cells were also seeded onto non-coated polystyrene culture plates as well as 4-well polystyrene chamber slides (Lab-Tek, USA). The chamber slides were employed for histochemical staining. A total of six cord blood samples from different donors were studied in this manner.
Isolation and culture of mononuclear cells
Fresh cord blood was collected after full-term deliveries in accordance with local ethical standards and after informed consent from the donors. MNCs were isolated in Uni-SepMAXI tubes (NovaMed, Israel) using Lymphoprep™ (density 1.007 g ml−1—osmolality 290 mOSm) (Axis Sheild, Norway) according to the manufacturer’s protocol. Briefly, 10 ml of Lymphoprep solution were overlaid onto the separation membrane followed by centrifugation (400 × g, 1 min, RT), after which undiluted cord blood (15 ml) was added above the membrane and centrifuged (1,000 × g, 30 min, RT, no brake). The resultant mononuclear layer was then removed with the aid of a Pasteur pipette, washed twice with PBS and resuspended at a concentration of 25 × 106 cells ml−1 in αMEM supplemented with 20% FCS, glutamine (4 mM), penicillin (100 U/ml), streptomycin (100 μg ml−1), amphotericin (0.25 μg ml−1) and 1% non-essential amino acids (all from Biological Industries, Israel). The cells were then seeded onto tissue culture plates in the following volumes: 1 ml for 48-well (1.1 cm2 area) polystyrene dishes and 0.6 ml for 4-well chamber slides. Following two days incubation, the cultures were washed twice with PBS and fresh medium added. Subsequent to 2 weeks in culture the concentration of FCS was reduced to 10% (basic medium). Unless otherwise noted all cultures were maintained at 37°C, 5%CO2 in a moist atmosphere and medium changed twice weekly. Adherent cells were detached from the primary (P0) culture plates by trypsinization (0.05% trypsin/0.25% EDTA- Biological Industries, Israel) after 4 weeks and replated onto freshly coated plates. These cells were termed P1. The number of wells in which putative PCs developed following the first passage was determined from each experiment and the average number from all experiments calculated. P1 cells were collected and recultured (P2) for use in the differentiation experiments and for FACS.
Cell proliferation assay
Cell attachment, survival and proliferation were monitored employing the alamarBlue® assay (Serotec, Oxford UK ) 2, 3 and 4 weeks following initial culture. The medium was removed from the culture wells, replaced with 10% (v/v) alamarBlue® in αMEM without FCS and incubated for 2 h (37°C, 5% CO2). Redox levels of the alamarBlue® were determined fluorometrically employing a spectrofluormeter microtiter well-plate reader (Fluostar Galaxy, USA) at excitation 530 nm/emission 590 nm with an internal gain of 10. Results were noted as fluorescence emission intensity units (FEIUs) and normalized relative to the level of adhesion to uncoated polystyrene culture wells.
Osteogenic differentiation
P1 PCs were replated onto 6-well polystyrene plates at a concentration of 4 × 104/well in αMEM (10% FCS). Cultures were allowed to reach ∼60% confluence before differentiation was initiated. Three different types of osteogenic differentiation medium, based on αMEM supplemented with 0.1 uM dexamethasone, 10 mM β-glycerolphosphate, 50μM ascorbic acid (all from Sigma-Aldrich USA) (basic osteogenic medium), were employed in order to induce differentiation. PCs were incubated with either basic osteogenic medium alone or enriched with 10 ng/ml recombinant FGF-9 , 10−7M 1,25-(OH)2 vitamin D3 (Sigma-Aldrich, USA) or FGF-9 together with Vit D3 at the same concentrations as mentioned previously. Cultures were maintained for 18 days for RT-PCR and 21 days for calcium deposit analysis.
Calcium analysis
The presence of calcium deposits was determined in 3-week cultures by Alizarin Red S and electron dispersive spectroscopy (EDS). For Alizarin Red S staining, cultures were prefixed with 95% ethanol (4°C, 10 min) and stained (30 min) with a 2% solution of Alizarin Red S (pH 4.3) followed by intense washing with double distilled water (ddw).
Prior to EDS analysis the same cultures were fixed with 4% glutaraldehyde, washed intensively with ddw, dehydrated in graded alcohols (70, 95 and 100%, three changes, 10 min each) and vacuum dried. Charcoal adhesive tape was used to lift small samples of each culture which were subsequently examined utilizing a Jeol JSM-35 C scanning electron microscope at 15 kV with attached Kevex energy dispersive spectrophotometer.
Histochemistry
Histochemical staining was carried out on 2-week P0 cord blood cultured in 4-well chamber slides. General cell morphology was observed by light microscopy (100×) following staining with Modified Giemsa Stain (Sigma, USA) employing the manufacturers instructions.
Naphthol AS-D chloroacetate (NCAE) and α-naphthyl acetate esterase (NAE) activity was determined employing a Sigma Diagnostics kit using the manufacturer’s instructions (procedure 91). Cells were scaled as positive, weakly positive or negative according to the intensity of red (NCAE) or black (NAE) granular staining apparent in the cytoplasm.
Tartrate-resistant acid phosphatase (TRAP) staining was carried out employing the protocol described in the Beckton Dickinson technical bulletin 445. The presence of a deep red sediment within the cells was considered a positive reaction.
RT-PCR
RNA was prepared from cells (P0, P1 or P3 cells in differentiation) collected from cultures using a Qiagene mini-kit and according to the manufacturer’s instructions. Reverse transcription was carried out employing a final concentration of 0.5 μg random primers (Promega, USA)/1μg RNA, 500 μM dNTP mix, 10U RNase inhibitor, 40 U M-MuLV reverse transcriptase (RT) and its accompanying buffer (25 μl final volume). The RT program consisted of heating the mRNA and random primers (final volume 15 μl) for 5 min at 70°C followed by 5 min 0°C. A mixture of buffer, enzymes and dNTPs were added and heated to 39°C (1 h) followed by 90°C (10 min). Each PCR reaction was accomplished using ∼100 ng cDNA, 400 nM each of sense and antisense primers (Table 1), 200 μM dNTP mix, 0 4U/reaction of Taq polymerase and its accompanying buffer (final volume 25 μl). The PCR program consisted of 5 min 94°C denaturization followed by 29 cycles of 94°C (3 min), annealing (45 s), elongation (1 min) and terminating with 5 min elongation. Both RT and PCR were carried out in PTC-100™ Thermal Control (MJ Research, US). PCR products were resolved on 1.5% agarose gels containing ethidium bromide.
Table 1.
List of primer names, sequence and predicted size
| Primer name | Sequence (5'–3') | Predicted size (bp) |
|---|---|---|
| Glyceraldehyde-3-p dehydrogenase | ||
| Sense | tgaacgggaagctcactgg | 307 |
| Antisense | tccaccaccctgttgctgta | |
| Collagen type I | ||
| Sense | tgacgagaccaagaactg | 600 |
| Antisense | ccatccaaaccatgaaacc | |
| Bone sialoprotein | ||
| Sense | atttccagttcagggcagtag | 447 |
| Antisense | acactttcttcttccccttct | |
| Core binding factorα1 | ||
| Sense | taccagaccgagaccaacagag | 310 |
| Antisense | caccaccgggtcacgtcgc | |
| Osteocalcin | ||
| Sense | tcacactcctcgccctattgg | 371 |
| Antisense | tcacactcctcgccctattgg | |
| Osteopontin | ||
| Sense | agaccccaaaagtaaggaagaag | 541 |
| Antisense | gacaaccgtggaaacaaataag | |
| VEGFR 1 | ||
| Sense | gtcacagaagaggatgaaggtgtcta | 414 |
| Antisense | cacagtccggcacgtaggtgatt | |
| VEGFR 2 | ||
| Sense | ctggcatggtcttctgtgaagca | 795 |
| Antisense | aataccagtggatgtgatgccgg | |
| TIE-2 | ||
| Sense | tctgtgctgttccttcttgc | 376 |
| Antisense | cttgagtaacttccagcgga | |
| eNOS | ||
| Sense | caccgtttgcccacccttcg | 434 |
| Antisense | gcccactgggagccgacact | |
Flow cytometry
For flow cytometry analysis, P0 and P1 cultures were detached from their culture plates with trypsin/EDTA (on ice) and labeled with the following monoclonal antibodies: CD49a-FITC (P1H5-SantaCruz), CD49f-FITC (MCA1457F, Serotec), CD105 (266; BD PharMingen), CD44Std. (SFF-304; Bender MedSystems), CD34-FITC (Class III 581; Serotec), CD45-FITC (2B11; Serotec) CD14 (MEM-18, Abcam, USA). Acquisition and analysis was performed on a FACSCaliber flow cytometer (Becton Dickenso, USA). All antibodies were used at a concentration of 0.5 μg/106 cells in a volume of 100 μl unless otherwise recommended by the manufacturer.
Statistical analysis
Intra and inter plate assay differences were determined prior to interpretation of the data. The affect of adhesion matrices on adhesion and isolation of PCs was examined using a one-way analysis of variance (ANOVA) with the aid of the program, GraphPad Prism, Version 4 (GraphPad Software, USA). The Dunnet post-test was employed for comparison of the experimental groups. In all cases, P < 0.05 with a Cl of 95% was considered significant.
Results
Isolation and proliferation of PCs on different matrices
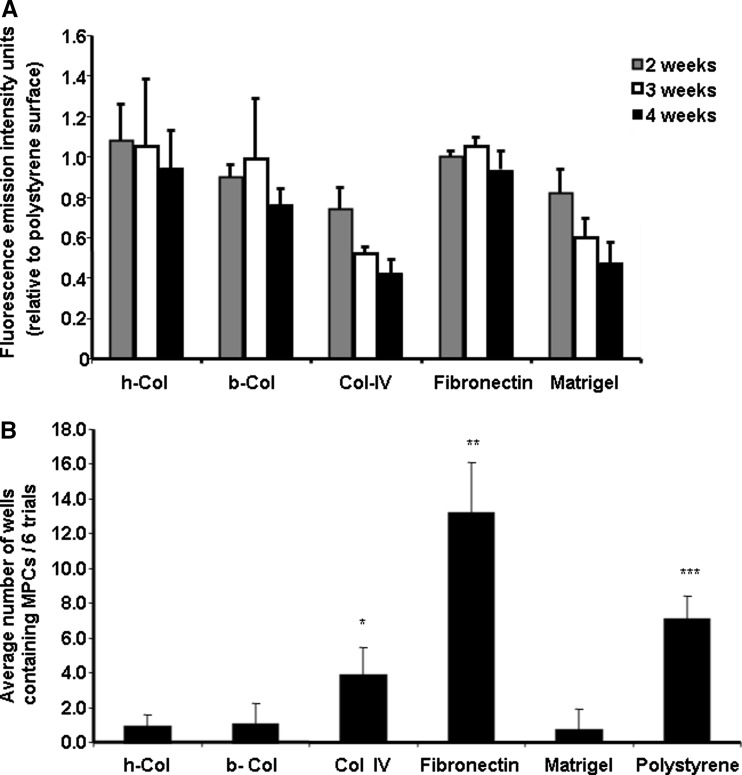
The relative numbers of cells which adhered to the various matrices was determined by the alamarBlue Assay 2, 3, and 4 weeks after initial culture (Fig. 1a). The average intra plate disparity was 5.2% and the inter plate 8.3%. The lowest number of residual cells recorded following 2 weeks in culture was observed on the collagen type IV surface. The numbers decreased significantly after 3 and 4 weeks incubation (29.8 and 18.0% respectively, P < 0.05). A similar profile was observed with the matrigel coating. Human collagen and fibronecton films initiated adhesion levels which were equivalent to those observed in uncoated polystyrene plates (1.08 ± 1.78 and 0.99 ± 0.315 FEIUs relative to the polystyrene plates). A slight but insignificant decrease in the cell numbers occurred during the next two weeks with these two matrices while the number of cells in the fibronectin coated plates remained constant.
Fig. 1.
(a) Relative percentage of cord blood cells which adhered to coated plates. Mononuclear cord blood cells were isolated by Ficoll-Paque gradient and seeded onto 48-well polystyrene culture plates or the same plates pre coated with either h-Col I, b-Col I, human Col IV, fibronectin or matrigel. The presence of live adherent cells was determined 2, 3 and 4 weeks post seeding employing the alamarBlue™ assay and results normalized relative to the level of cell adhesion on uncoated polystyrene culture wells. Redox levels were determined fluorometrically at excitation 530 nm and emission 590 nm with an internal gain of 10. Results are noted as fluorescence emission intensity units and represent the average numbers from six separate experiments with 18 replicates/experiment ± standard deviation. (b) Percentage of PC isolations on different adhesion proteins. Adherent cells were trypsinized from P0 cultures and replated onto matrix coated plates. The number of wells in which PCs developed were counted and displayed on a bar graph. Each bar represents the average number of wells in which PCs developed in all six trials ± standard deviation. Significant differences in PC isolation were observed between protein coatings with fibronectin > polystyrene > collagen type IV > human collagen type I (P < 0.05, CI 95)
Long-term adherent cells were recultured onto freshly coated culture wells. Most of the P0 cells did not adhere and were lost upon passage. The average number of P0 wells (18 replications per experiment) in which PCs were observed microscopically in all six experiments were; fibronectin 13.17 ± 2.93, polystyrene 7.0 ± 1.29, collagen type IV 3.83 ± 1.6, human collagen 0.83 ± 0.75, bovine collagen 1.0 ± 1.26 and matrigel 0.67 ± 1.21 coated wells (Fig. 1b).
Classification of adherent cells
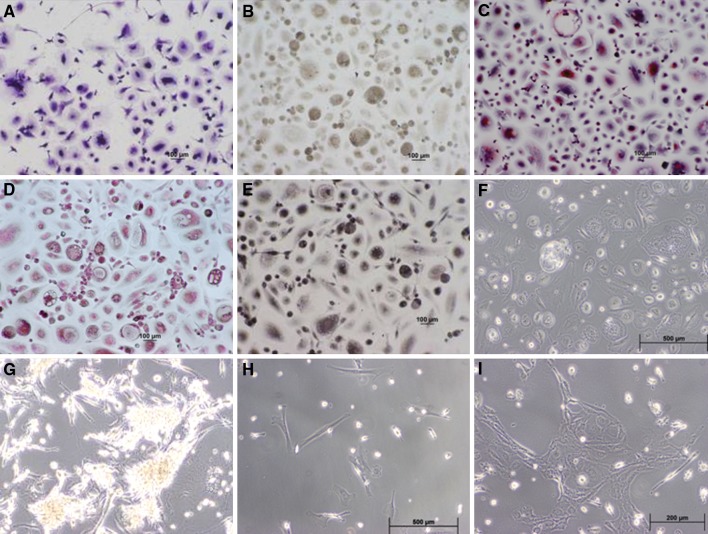
Within 24 h MNCs adhered in large numbers to all surfaces regardless of the coating. Morphology and staining properties were similar with all of the adhesion proteins with the exception of the matrigel. A representation of the cells found on the polystyrene surface is shown in Fig. 2. The h-Col and b-Col appeared unstable and small areas of the matrix became partially detached with time. All cultures, excluding the matrigel, developed into a field of flattened single or multinucleated cells containing distinct cytoplasm (Fig. 2f). Cells cultured on the matrigel tended to clump together forming small mounds (Fig. 2g).
Fig. 2.
Morphology and histochemistry of P0 adherent cells. Cord blood cells which adhered to polystyrene surfaces for 2 weeks were stained with (a) Giemsa; (b) Alkaline phosphatase; (c) Tartrate-resistant acid phosphatase; (d) Napthol AS-D chloroacetate; (e) α-napthyl acetate esterase; (f) P0 culture on polystyrene (unstained); (g) Morphology of clusters which developed on matrigel coated plates; (h) P1 culture; (i) P2 culture. (Magnification 100×) Two major types; elongated thin cells carrying a funnel-like cytoplasmic extension containing a small but distinct nucleus and larger cells containing cytoplasm surrounding a central nucleus were observed. The rounded population was weakly alkaline phosphates positive, TRAP reactive as well as NCAE and NAE positive. The fibroblast-like elongated cells were mostly alkaline and TRAP negative and weakly NAE and NCAE positive
Cells which adhered to the various coated and noncoated surfaces for a period of two weeks were identified by histochemical and enzymatic stains. Giemsa staining revealed a mixture of cell morphologies with two major types; elongated thin cells carrying a funnel-like cytoplasmic extension containing a small but distinct nucleus and larger cells containing cytoplasm surrounding a central nucleus (Fig. 2a). The rounded population was composed of a substantial number of larger multinucleated cells. These cells were weakly alkaline phosphates positive (Fig. 2b), TRAP (Fig. 2c) reactive as well as NCAE (Fig. 2d) and NAE (Fig. 2e) positive. The fibroblast-like elongated cells were mostly alkaline and TRAP negative and weakly NAE and NCAE positive. Cells cultured on matrigel produced clusters with elongated cells extending from the periphery (Fig. 2g). Staining was poor and not definitive (Results not shown).
P1 positive cultures on all of the plate surfaces revealed elongated fibroblast cells which developed randomly and which replicated very slowly (Fig. 2h). P2 cultures replicated more rapidly and formed of loosely woven clusters (Fig. 2i).
FACS and RT-PCR analysis of P0 and P1 cultures
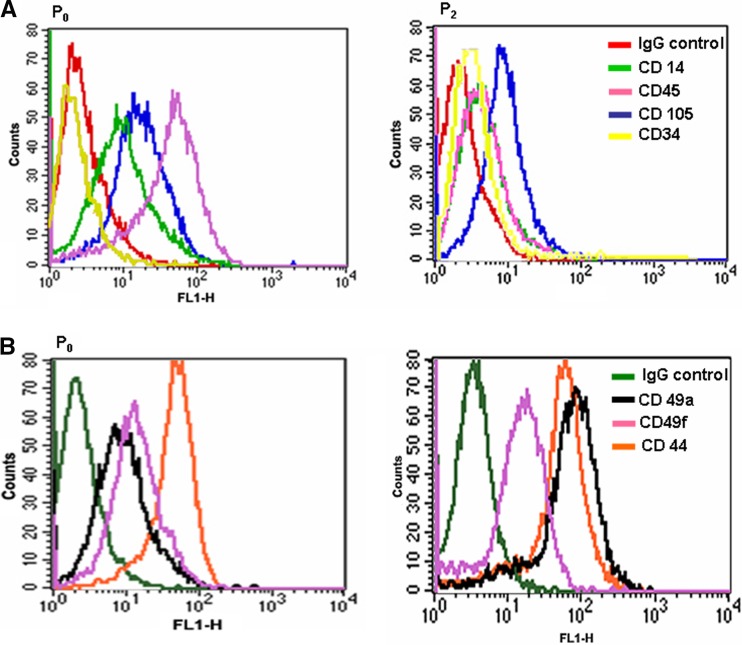
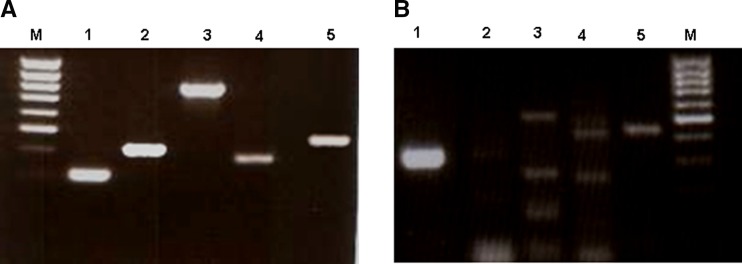
P0 (Fig. 2f) and P3 cultures (Fig. 2i) were typsinized from polystyrene plates and analyzed by FACS and RT-PCR. A large portion of the primary population strongly expressed CD45 and CD44-standard, weakly CD14, CD105, CD49a, CD49f and negative for CD34 (Fig. 3a). PCs which developed after passage did not display the markers for CD45, CD14 or CD34 while both CD105 and CD49f remained stable and expression of CD49a and CD44 increased (Fig. 3b). RT-PCR of the primary cord blood cultures expressed RNA sequences for VEGFR1, VEGFR2, TIE2 and E-nos (Fig. 4a) while only the endothelial marker eNOS was weakly observed in the P1 passage of the same cells (Fig. 4b).
Fig. 3.
FACS analysis of P0 and P2 cultures employing markers (a) CDs 14, 105, 45, 34; (b) 49a, 49f, and 44. FACS analysis determined that the majority of P0 cells were of the macrophage/monocyte lineage while P3 cultures demonstrated typical mesenchymal markers
Fig. 4.
RT-PCR of cord blood cells cultured on polystyrene plates. 5a P0 cord blood culture after 2 weeks adhesion: left to right: M 100 bp marker (top to bottom 1,031, 900, 800, 700, 600, 500, 400, 300, 200 bp); 1 GAPDH; 2 VEGFR1; 3 VEGFR2; 4 TIE2; 5 eNOS. 5b P1 cord blood culture (PCs) after 2 weeks in culture: 1 GAPDH; 2 VEGFR1; 3 VEGRF2; 4 TIE2; 5 eNOS; M marker (top to bottom 1,031, 900, 800, 700, 600, 500, 400, 300,200 bp). Primary cord blood cultures expressed RNA sequences for VEGFR1, VEGFR2, TIE2 and E-nos (a) while only eNOS was weakly observed in the P1 passage of the same cells (b)
Osteogenic differentiation of isolated MPCs
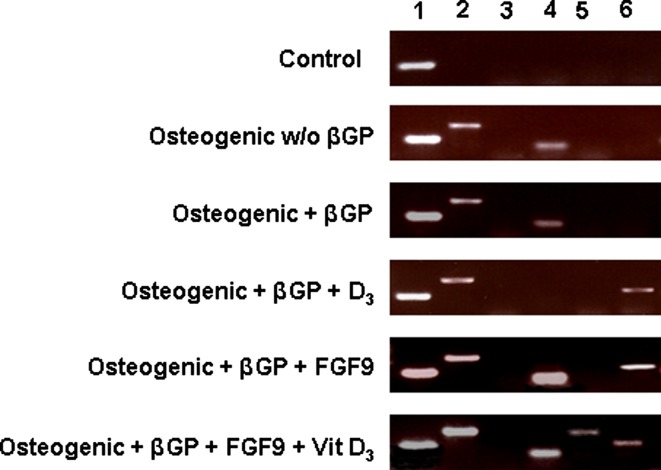
Isolated fibroblast-like cord blood cells (P2) were cultured onto 6-well polystyrene plates and exposed to “osteogenic medium” with or without β glycerophosphate, FGF9 and/or Vit D3. Subsequent to 18 days in culture, control cultures, incubated in basic medium only, express RNA for the housekeeping gene GAPDH. The addition of ascorbic acid and dexamethasone to the growth medium resulted in the appearance of RNA associated with collagen type 1 and CBFAI. Adding β-glycerophosphate to the osteogenic media did not alter this profile. The enrichment with Vit D3 induced the production of mRNA for osteopontin but eliminated the presence of CBFA1, while Addition of FGF9 in place of D3 resulted in strong CBFAI expression together with osteopontin and collagen type I. Only when both Vit D3 and FGF9 were added to the culture media was osteocalcin observed (Fig. 5). BSP expression was not observed in any of the above mentioned culture conditions although it did occur in human bone marrow osteoprogenitors cultured in our lab in osteogenic basic medium (results not shown).
Fig. 5.
RT-PCR of PCs (P2) cultured for 18 days in standard medium and in medium designed for osteogenic differentiation. 1 GAPDH; 2 collagen type I; 3 BSP; 4 CBFAα1; 5 osteocalcin; 6 osteopontin. (w/o without; βGP β-glycerophosphate)
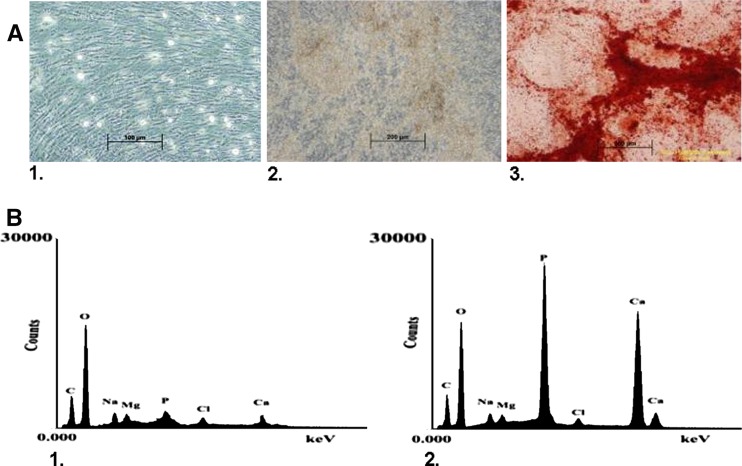
Compared to PCs cultured in basic medium (Fig. 6a-1), only cells exposed to osteogenic medium containing both Vit D3 and FGF9 produced large deposits of calcium (Fig. 6a-2) as seen by Alizarin staining (Fig. 6a-3). Cells which had been induced with each vehicle alone produced small and scattered mineralized matrix deposits. EDS analysis of the control culture (Fig. 6b-1) and the osteogenic cultures containing sediment (Fig. 6b-2) revealed a calcium to phosphate ratio of 1.68 which is parallel to that of hydroxyapatite.
Fig. 6.
PCs were cultured in standard medium or osteogenic medium containing both FGF9 and Vit D3 for a period of 21 days. The presence of calcium deposits was determined by Alizarin Red S and Electron Dispersive Spectroscopy. (a1) PCs cultured in standard medium; (a2) PCs cultured in osteogenic medium containing FGF9 and Vit D3 demonstrating calcium deposits; (a3) Alizarin Red staining culture observed in (a2). Red staining indicates presence of calcium deposits. (b) EDS analysis of culture (a1) and (a2). (b1) EDS of control culture (a1) with very minimal presence of calcium and phosphate; (b2) EDS of osteogenic culture (a2) with strong a signal for phosphate and calcium demonstrating a ratio similar to hydroxyapatite
Discussion
The interaction of cell receptors with extra cellular matrix (ECM) adhesion proteins provides signals which can affect the survival of adherent cells. Salasznyk et al. (2004) assessed the contribution of extra cellular matrix proteins on the ability of MSCs to adhere to ECM proteins found in bone marrow. While employing 30-min adhesion assays they determined that fibronectin, Col I, Col IV and vitronectin supported approximately 6-to 8-fold greater static adhesion compared to the negative control (culture well blocked with dairy creamer) or laminin. We examined the long-term adhesion and possible replication potential of mononuclear cord blood cells 2, 3 and 4 weeks following seeding onto five different pro-adhesive proteins. Over time, the numbers of adherent cells changed only slightly on the standard polystyrene surface, as well as on the h-Col, b-Col and fibronectin coated plates; indicating absence of cell proliferation and perhaps characteristic of the state of differentiation of the adherent cells. In comparison, cells attached poorly and decreasing metabolic activity and/or numbers was determined in plates coated with matrigel and Col IV (Fig. 1a). No obvious changes in cell morphology were perceptible in any of the plates and PC colonies did not develop during the 4 week incubation period. Only following trypsinization and reculture did the MPCs become visible (Fig. 1b). A significant difference (P < 0.05, CI 95%) was observed in the percentage of positive culture wells between the various matrices. The largest number of clones was isolated on fibronectin (73.2%) while 1.9-fold fewer isolations were observed with uncoated plates (38.89%). Although collagen type IV appeared to be a poor candidate for adhesion, it proved to be a more efficient matrix (21.3%) for the isolation of PCs than type I human (4.63%) and bovine (4.63%) collagens. Matrigel coating, which produced a very different initial adhesion pattern, also demonstrated very limited potential for isolation of the target cells. Fibronectin is a large multidomain glycoprotein found in connective tissue, on cell surfaces, and in plasma. It is a potential ligand for most cells involved in cellular processes, including tissue repair, cell migration and adhesion. It is known to interact with cell-surface receptors on a variety of cells including fibroblasts, neurons and phagocytes. The RGD motif found in fibronectin is the most important recognition site for about half of all known integrin cellular receptors and these receptors have a dominating role in the anchoring of cells to the ECM. A striking feature of many integrins is their ability to bind multiple ligands such as fibronectin, vitronectin, collagens and laminin (Jiang et al. 2006; Keselowsky et al. (2005). When examined by FACS, P0 and P3 cultures consistently expressed high levels of integrins CD49a and CD49f (Fig. 3b). This would explain the superior adhesion observed with the fibronectin, h-Col and b-Col. Although α2 integrin is a known receptor for Col IV (Keselowsky et al. 2005), isolated MNCs did not bind well or remain attached to this matrix.
Positive NAE, NCAE and general morphology indicated that the majority of the adherent primary cells are related to the monocyte/macrophage lineage (Fig. 2a, d, e). Within this complex, cells presenting staining properties (TRAP, Fig. 2c) and morphology (multiple nuclei) of osteoclasts were also evident in fairly large numbers. TRAP positive osteoclasts, in their precursor stage are found in the PB among the monocytic population (Shalhoub et al. 2000) but, although the expression of TRAP is regarded as an osteoclast marker, under certain conditions, cells of the macrophage/dendritic cell family also express TRAP (Tsuboi et al. 2003). FACS analysis further fortified the monocyte/macrophage identity of the primary adherent cells which demonstrated 90% positive for the leukocyte-common antigen CD45 and negative for CD34 (Fig. 3a). During the 4 weeks of culture, the population of adherent cells observed on all coatings, excluding the matrigel, consisted of a heterogenic mixture of hematopoietic cells, monocytes/macrophages and pre osteoclasts.
Paradoxically, a greater isolation rate of PCs was observed on Col IV plates than with collagen type I. PC colonies were not observed in P0 cultures, but became apparent only after transfer to fresh plates (Fig. 2h). The enhanced adhesion to fibronectin in the primary cultures resulted in an increased incidence of PC isolation (Fig. 1b) when compared to the other coatings and the standard polystyrene culture plates. Protein adhesion molecules in the ECM trigger changes in the cytoplasmic domains which eventually alter their interaction with cytoskeletal and/or other proteins that regulate cell adhesion, growth and migration (Damsky and Ilic 2002). A 1.9-fold increase in PC colony numbers compared to the polystryrene plates would represent a significant effect of the fibronectin coating on these cells. The large and varied cell population which adhered to the culture plates in P0 could be analogous to the micro environment present in the native cord blood state. Similar to the niche which has been observed in bone marrow (Diaz-Flores et al. 2006; Yin and Li 2006), replication of the non differentiated cells did not occur until appropriate signals have been initiated. It would seem that separation of the PCs from the hematopoetic population by the process of trypsinization induced a modification in these cells which initiated division. After passage, adherent cells were constituted by a homogeneous population of PCs which exhibited a fibroblast-like morphology (Fig. 2h) and typical mesenchymal-like immunophenotypes.
RT-PCR analysis of P0 produced products for the Receptor Tyrosine kinases VEGFR 1, VEGFR 2 and TIE 2 as well as the endothelial eNOS (Fig. 4a). Buhring et al. (1999) described these genes in normal hematopoietic tissue and define them as bipotent hematopoietic/endothelial precursors. Following trysinization and reculture, with the exception of the declining eNOS signal, expression of these RNA sequences was progressively lost after cell proliferation, demonstrating the dominance of a unique type of cells.
To examine whether the P2 generation of PCs could be induced to differentiate into mature osteoblasts and produce a mineralized matrix in culture we exposed these cells to various soluble osteogenic supplements and assayed for the presence of RNA differentiation markers (Fig. 5). During 18 days in culture, no morphological transition from elongated to cuboidal shaped cells was noted. Cells that were cultured in standard medium expressed only GAPDH while the addition of ascorbic acid and dexamethasone stimulated the upregulation of the early osteogenic gene, collagen type I and Cbfa I. These markers remained stable regardless of the additives employed. Glucocorticoids such as dexamethasone promote osteoblastic differentiation in in vitro models employing bone marrow derived mesenchymal cells. This is thought to take place through the coordinated action of dexamethasone and the transcription factor Runx2/Cbfa 1 which in turn induces osteocalcin expression and terminal mineral deposition (Ducy et al. 1997; Yang et al. 2003; Kundu et al. 2002). β-glycerophosphate is added to bone marrow-derived osteogenic cultures in order to provide a source of phosphate necessary for construction of the hydroxyapatite configuration (Bellows et al. 1992). Calcium deposits were not apparent in cord blood PCs cultured in the presence of β-glycerophosphate, nor was the genetic expression pattern altered. Osteopontin, an intermediate marker expressed during osteoblast maturation, appeared subsequent to the addition of Vit D3, but at the same time Cbfa 1 receded. When cells were cultured with 10 ng/ml FGF9, Cbfa I and osteopontin were present. Only when osteogenic medium was enriched with both Vit D3 and FGF9 was the late marker, osteocalcin, expressed and considerable amounts of hydroxyapatite crystals (Fig. 6b-1 and 2) deposited (Fig. 6a-2 and 3).
Bsp 1 was not detected in any of the osteogenic cultures. Temporal sampling of these cultures would have been advantageous for better defining the window of expression of each marker and may have increased the possibility of observing Bsp 1.
The genetic markers of differentiation expressed by the PCs, and the phenotypic display of matrix production, is comparable to intramembranous ossification. Variation in the projected appearance of each marker, as affected by the osteogenic supplements when compared to osteoblasts, could be due to a large degree on differenced in osteogenic cell type, culture conditions and the ECM environment.
Conclusions
Various preparative protocols have been proposed using surface antigen markers for the acquisition of mesenchymal progenitor cells from cord blood. Since MPCs have been defined as adherent cells we proposed a simple method of adherence to a specific adhesion matrix for the isolation of these cells. PCs derived from different donors using the same culture procedure yielded consistent and reproducible surface marker and gene expression profiles when cultured under standard or osteogenic conditions. Isolated cells could be induced to differentiate into osteoblasts and to produce a hydroxyapatite mineralized matrix. Strong alkaline phosphatase activity is a well documented marker of early osteoblast differentiation. Within the adherent cell population (P0), positive alkaline phosphatase cells were not observed indicating that the cells which later developed into osteoprogenitors were not present in the primary cell population as osteoblasts.
Whether the model of differentiation observed with cord blood PCs is truly osteogenisis or just an artifact of in vitro culture can only be determined with in vivo models involving bone defects which are greater than the critical size necessary for natural healing.
Acknowledgments
This research was supported by the Israeli Ministry of Commerce Magnet Bereshit Grant No. 2004473.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s10616-007-9077-0
References
- Arinzeh TL. Mesenchymal stem cells for bone repair: preclinical studies and potential orthopedic applications. Foot Ankle Clin. 2005;10(4):651–665. doi: 10.1016/j.fcl.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Bellows CG, Heersche JN, Aubin JE. Inorganic phosphate added exogenously or released from beta-glycerophosphate initiates mineralization of osteoid nodules in vitro. Bone Miner. 1992;17(1):15–29. doi: 10.1016/0169-6009(92)90707-K. [DOI] [PubMed] [Google Scholar]
- Bhagavati S, Xu W. Isolation and enrichment of skeletal muscle progenitor cells from mouse bone marrow. Biochem Biophys Res Commun. 2004;318(1):119–124. doi: 10.1016/j.bbrc.2004.03.192. [DOI] [PubMed] [Google Scholar]
- Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22(4):625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56(3):283–294. doi: 10.1002/jcb.240560303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhring HJ, Seiffert M, Bock TA et al (1999) Expression of novel surface antigens on early hematopoietic cells. Ann N Y Acad Sci 872:25–38; discussion 38–29 [DOI] [PubMed]
- Chang YJ, Shih DT, Tseng CP, et al. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006;24(3):679–685. doi: 10.1634/stemcells.2004-0308. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Nagler A. Umbilical cord blood transplantation–how, when and for whom? Blood Rev. 2004;18(3):167–179. doi: 10.1016/S0268-960X(03)00064-X. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Ilic D. Integrin signaling: it’s where the action is. Curr Opin Cell Biol. 2002;14(5):594–602. doi: 10.1016/S0955-0674(02)00368-X. [DOI] [PubMed] [Google Scholar]
- Dazzi F, Ramasamy R, Glennie S, et al. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2005;20(3):161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20(3):205–214. doi: 10.1634/stemcells.20-3-205. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Jr, Madrid JF, Gutierrez R, et al. Adult stem and transit-amplifying cell location. Histol Histopathol. 2006;21(9):995–1027. doi: 10.14670/HH-21.995. [DOI] [PubMed] [Google Scholar]
- Dicker A, Le Blanc K, Astrom G, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308(2):283–290. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- Fibbe WE, Noort WA, Schipper F, et al. Ex vivo expansion and engraftment potential of cord blood-derived CD34+ cells in NOD/SCID mice. Ann N Y Acad Sci. 2001;938:9–17. doi: 10.1111/j.1749-6632.2001.tb03569.x. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Jeong JA, Hong SH, et al. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22(4):617–624. doi: 10.1634/stemcells.22-4-617. [DOI] [PubMed] [Google Scholar]
- Ge Z, Goh JC, Lee EH. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14(8):573–583. doi: 10.3727/000000005783982819. [DOI] [PubMed] [Google Scholar]
- Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22(4):487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102(10):3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- Hildbrand P, Cirulli V, Prinsen RC, et al. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;104(7):2010–2019. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- Hong SH, Gang EJ, Jeong JA, et al. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330(4):1153–1161. doi: 10.1016/j.bbrc.2005.03.086. [DOI] [PubMed] [Google Scholar]
- Jeong JA, Gang EJ, Hong SH, et al. Rapid neural differentiation of human cord blood-derived mesenchymal stem cells. Neuroreport. 2004;15(11):1731–1734. doi: 10.1097/01.wnr.0000134846.79002.5c. [DOI] [PubMed] [Google Scholar]
- Jiang XS, Chai C, Zhang Y, et al. Surface-immobilization of adhesion peptides on substrate for ex vivo expansion of cryopreserved umbilical cord blood CD34+ cells. Biomaterials. 2006;27(13):2723–2732. doi: 10.1016/j.biomaterials.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TJ, Yeom JE, Lee HJ, et al. Growth kinetics of human mesenchymal stem cells from bone marrow and umbilical cord blood. Acta Haematol. 2004;112(4):230–233. doi: 10.1159/000081281. [DOI] [PubMed] [Google Scholar]
- Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci USA. 2005;102(17):5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200(2):123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler G, Radke TF, Lefort A, et al. Cytokine production and hematopoiesis supporting activity of cord blood-derived unrestricted somatic stem cells. Exp Hematol. 2005;33(5):573–583. doi: 10.1016/j.exphem.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Kundu M, Javed A, Jeon JP, et al. Cbfbeta interacts with Runx2 and has a critical role in bone development. Nat Genet. 2002;32(4):639–644. doi: 10.1038/ng1050. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Yahata T, Miyatake H, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107(5):1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- Murohara T, Ikeda H, Duan J, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105(11):1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18(6):893–908. doi: 10.1016/j.bpobgyn.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Salasznyk RM, Williams WA, Boskey A, et al. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004(1):24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalhoub V, Elliott G, Chiu L, et al. Characterization of osteoclast precursors in human blood. Br J Haematol. 2000;111(2):501–512. doi: 10.1046/j.1365-2141.2000.02379.x. [DOI] [PubMed] [Google Scholar]
- Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- Tsuboi H, Matsui Y, Hayashida K, et al. Tartrate resistant acid phosphatase (TRAP) positive cells in rheumatoid synovium may induce the destruction of articular cartilage. Ann Rheum Dis. 2003;62(3):196–203. doi: 10.1136/ard.62.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wei D, Wang D, et al. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 2003;18(4):705–715. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116(5):1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]