Abstract
The necessity to perform serum-free cultures to produce recombinant glycoproteins generally requires an adaptation procedure of the cell line to new environmental conditions, which may therefore induce quantitative and qualitative effects on the product, particularly on its glycosylation. In previous studies, desialylation of EPO produced by CHO cells was shown to be dependent on the presence of serum in the medium. In this paper, to discriminate between the effects of the adaptation procedure to serum-free medium and the effects of the absence of serum on EPO production and glycosylation, adapted and non-adapted CHO cells were grown in serum-free and serum-containing media. The main kinetics of CHO cells were determined over batch processes as well as the glycosylation patterns of produced EPO by HPCE-LIF. A reversible decrease in EPO production was observed when cells were adapted to SFX-CHOTM medium, as the same cells partially recovered their production capacity when cultivated in serum-containing medium or in the enriched SFMTM serum-free medium. More interestingly, EPO desialylation that was not observed in both serum-free media was restored if the serum-independent cells were recultured in presence of serum. In the same way, while the serum-independent cells did not release a sialidase activity in both serum-free media, a significant activity was recovered when serum was added. In fact, the cell adaptation process to serum-free conditions did not specifically affect the sialidase release and the cellular mechanism of protein desialylation, which appeared to be mainly related to the presence of serum for both adapted and non-adapted cells.
Keywords: Capillary electrophoresis, Cell adaptation, CHO cells, Murine recombinant EPO, N-glycosylation, Serum-free medium, Sialylation
Introduction
Mammalian cells are being used for the industrial production of a number of recombinant therapeutic glycoproteins because of their ability to perform post-translational modifications that closely mimic those of the human proteins. N-glycosylation has widely been reported to exert significant effects on several properties of glycoproteins, including antigenicity, solubility, protein folding, protease resistance, pharmacokinetics or biological activity (Schauer 1988; Kobata 1992; Yusa et al. 2005). Moreover, sialylation is known to be able to influence the in vivo circulatory lifetime of these proteins, which is crucial to their therapeutic value (Dordal et al. 1985; Takeuchi et al. 1989; Imai et al. 1990; Misaizu et al. 1995; Koury 2003; Yuen et al. 2003; Elliott et al. 2004).
During industrial process development, adaptation of the recombinant cell line to serum-free culture is generally required for both economical and regulatory reasons. Indeed, serum supplementation is a potential source of infectious agents like virus and prions, leads to high lot-to-lot variability, renders more difficult product purification by increasing the contaminant protein content of supernatant and finally induces higher operating costs (Froud 1999). Thus, removal of serum from the culture medium is usually a suitable step in the process development of recombinant glycoprotein production but it represents also a major change in the cell environment and consequently may affect cellular physiology and protein glycosylation. Indeed, serum contains many components (growth factors, insulin, transferrin, albumin, vitamins, etc....), which are known to be essential to several cellular functions like growth regulation or intracellular transports (Froud 1999). Thus, compared to serum-containing cultures, reduction of protein production and/or cell growth are generally observed when cells are transferred to serum-free media (Inoue et al. 1996; Lee et al. 1999; Ozturk et al. 2003). Extensive investigations for optimising medium composition to a determined cell line cultured in the desired conditions are required to recover equivalent performances (Buntemeyer et al. 1991; Hewlett 1991; Castro et al. 1992; Liu et al. 2001).
Glycan biosynthetic pathway is widely recognized to be very complex and generally results in the secretion of different glycoforms whose oligosaccharide moiety is highly heterogeneous. Resulting from sequential enzymatic reactions located in several cell compartments, glycan structures are known to be influenced by many factors. Host-cell type and culture conditions have been shown to be the most critical ones (Goto et al. 1988; Goochee and Monica 1990; Jenkins et al. 1996; Zhang et al. 2002; Tomiya et al. 2003; Yoon et al. 2005). In particular, various elements of the extracellular environment, including the configuration of the production system (Watson et al. 1994; Cabrera et al. 2005; Lipscomb et al. 2005; Spearman et al. 2005) and the composition of the culture medium like ammonia concentration (Andersen and Goochee 1995; Borys et al. 1994; Gawlitzek et al. 1998; Jenkins and Curling 1994; Grammatikos et al. 1998; Yang and Butler 2000a, b; Chen and Harcum 2005), glucosamine supplementation (Yang and Butler 2002), lipid availability (Jenkins et al. 1994), Na-butyrate addition (Lamotte et al. 1999; Sung et al. 2005) are able to affect protein glycosylation by potentially inducing changes in cell metabolism or favouring enzymatic product degradation.
Data from the literature regarding the effects of serum supplementation on protein glycosylation appear to be quite conflicting and very dependent on the investigated cell–protein system. Thus, the sialic acid content of a monoclonal IgG1 produced by murine hybridoma was reported to be clearly higher in serum-free medium than in presence of serum (Patel et al. 1992) while Lifely et al. (1995) observed only minor differences in glycosylation of an antibody produced by CHO cells in serum-free and serum-containing media. Moellering et al. (1990) did not find any significant differences in carbohydrate composition of another monoclonal antibody produced in both conditions. Concerning other recombinant glycoproteins, the glycoform distribution of interferon-γ (IFN-γ) produced by CHO cells was shown to be affected by the presence of serum, probably because of a proteolytic cleavage by serum proteases (Castro et al. 1995). Lamotte (1997) confirmed that serum could induce partial proteolysis of IFN-γ and that a decrease in sialylation and an increase in truncated glycans occurred in these culture conditions. Serum-free cultures were also reported to result in a higher level of terminal sialylation and proximal fucosylation of interleukin-2 produced by BHK-21 cells (Gawlitzek et al. 1995). Besides, the serum-free adaptation is able to influence cell physiology by inducing a self-adjustment of the cells to the environment. Improvement in cell growth has been particularly reported during the adaptation to low serum-containing medium since adapted cells showed higher growth rates than non-adapted cells in serum-containing culture. On the other hand, a loss of antibody productivity could be due to a long-term exposure to serum-free medium (Ozturk et al. 2003). In contrast, no change in glucose and glutamine uptake or lactate and ammonia production was found when hybridoma cells were adapted in serum-free conditions (Ozturk and Palsson 1991).
Erythropoietin (EPO), a plasma hormone stimulating the red blood cell production, is a particularly suitable model for studying glycosylation because of its highly glycosylated structure which represents 40% of its molecular weight (34–39 kDa according to Sasaki et al. 1987). Characterization of the glycoforms of the commercial human EPO obtained from recombinant CHO cells have been highly documented (Sasaki et al. 1987, 1988; Recny et al. 1987; Takeuchi et al. 1988; Takeuchi and Kobata 1991; Tsuda et al. 1990; Hokke et al. 1995; Stubiger et al. 2005). In contrast, data on the influence of the culture conditions on the glycosylation of this protein remain very sparse. High concentrations of ammonia have been shown to alter the glycosylation pattern of EPO by decreasing the terminal sialylation of all glycans and the content of the O-linked glycans (Yang and Butler 2000a, b) and glucosamine supplementation has been reported to increase the molecular heterogeneity of EPO regarding molecular weight and isoelectric point range (Yang and Butler 2002).
A previous paper regarding recombinant EPO produced by CHO cells in serum-free conditions deals with differences in productivity and glycosylation structure obtained in presence or in absence of serum (Le Floch et al. 2004). In fact, modifications of cell performances could result either from the serum removal or from the modification of the glycosylation mechanism during the adaptation procedure. However, to our knowledge, no information is available in the literature on the specific influence of cell adaptation procedure to serum-free conditions on protein glycosylation. In this paper, both EPO quality and quantity were analysed during serum-containing and serum-free culture processes performed with CHO cells either adapted or non-adapted to serum-free conditions. Our main objective was to determine the specific influence of the cell adaptation procedure to serum-free conditions on cellular protein glycosylation mechanism.
Materials and methods
Cell line and culture conditions
Recombinant murine EPO was produced by an α2,6 sialyltransferase (EC 2.4.99.1) expressing CHO cell line as described by Monaco et al. (1996). Briefly, the cassette bearing the cDNA of the murine EPO and a modified CMV promoter was inserted between HindIII and EcoRI restriction sites in a plasmid vector pSV40/Zeo (Invitrogen—ref V504-20) containing the zeocin resistance marker. CHO cells were then transfected by electroporation with this mammalian expression vector (4.9 kb). After zeocin selection, cells were adapted to suspension culture in a commercial serum-free medium (SFX-CHO™, HyClone—ref SH30187) supplemented with 5% serum (JRH Biosciences), leading to the first cell bank (cell bank #1). Following this first step, the adaptation to serum-free culture was carried out in Erlenmeyer agitated flasks by progressively reducing serum concentration in medium until complete elimination. The adaptation steps were monitored by following the capacity of cells to restore a doubling time of 48 h. Twelve passages (one month) were necessary to reach 0% of serum. Cells were further grown in this serum-free medium during one more month (14 passages) before constitution of the cell bank #2.
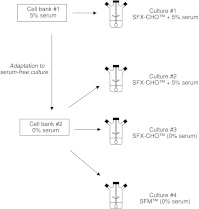
Four cultures were performed with cells in suspension in spinner flasks of 200 ml. The agitation rate was maintained at 40 rpm, temperature at 37°C and air was supplemented with 5% CO2 during culture. We used two commercial serum-free media both supplemented with 4 mM glutamine (Invitrogen), 10 mg l−1 ribonucleosides and deoxyribonucleosides (Invitrogen), 50 mg l−1 glycine (Sigma) and 500 mg l−1 zeocin (Invitrogen—ref R250-01). The SFX-CHO™ medium (containing 3 g l−1 of glucose) is characterized by its particularly low total protein content (<10 mg l−1). The other serum-free medium (SFM™, Sigma—ref C8099) contains 4.5 g l−1 of glucose and a higher protein level of 100 mg l−1. The strategy chosen to discriminate between the effects of serum component lack or of cell adaptation procedure is presented in Fig. 1. For culture #1, cells not adapted to serum-free medium (from cell bank #1) were grown in SFX-CHO™ medium supplemented with 5% serum. For culture #2, serum-free adapted cells (from cell bank #2) were grown during three passages in the same SFX-CHO™ medium, also supplemented with 5% serum before seeded. For culture 3, serum-free adapted cells (from cell bank #2) were cultivated in SFX-CHO™ medium without serum supplementation. For culture 4, the same cells (from cell bank #2) were cultivated in the second serum-free medium containing 100 mg l−1 of total proteins and 4.5 g l−1 of glucose (SFM™) after three passages in this medium. Cells were seeded between 0.2 and 0.3 × 106 cell ml−1.
Fig. 1.
Experimental strategy for discriminating between effects of serum lack and influence of cell adaptation procedure to serum-free conditions
Cell density was estimated using a haemacytometer and viability was measured by the trypan blue exclusion method. EPO concentration in the supernatant was determined by Elisa method (Quantikine IVD kit—R&D Systems—ref DEP00). The accuracy of this assay is evaluated to ± 20%. Glucose, lactate and glutamine concentrations were determined enzymatically by commercial assay kits (Sigma – ref 510-A, 735-10 and Roche—ref 102903) and ammonia concentration was determined with a selective probe (Orion). Supernatants were also collected at the beginning and at the end of the cultures, centrifuged at 800 rpm for 5 min for cell removal, and stored at −80°C until required for EPO purification and HPCE analysis.
Immuno-affinity purification of EPO
An immuno-affinity gel for the purification of EPO was prepared by coupling 1 mg of monoclonal anti-human EPO antibody (R&D Systems—ref MAB287) to 150 mg of CNBr activated sepharose 4B (Sigma) corresponding to 0.5 ml of swollen gel. Culture supernatants (containing 20–30 μg of EPO) were loaded successively three times on this immuno-affinity gel overnight at 4°C under gentle agitation. The gel was successively washed with PBS (20 volumes), PBS/Tween20 (20 volumes) and 1 M NaCl/NH4OH, pH 10.3 (2 volumes). Elution of the bound EPO was first performed with 1 M NaCl/NH4OH, pH 11.5 and then pH 11.8. The two eluant fractions were rapidly brought to neutral pH by the addition of 4 M Tris–HCl, gathered together, concentrated and dialyzed against distilled water using a Centricon YM-10® centrifugal filter unit (Millipore). Finally, the amount of purified EPO was estimated to be between 5 μg and 10 μg.
N-linked oligosaccharides analysis by HPCE
Glycans of immuno-purified EPO were analysed according to a procedure derived from the method described by Chen and Evangelista (1998) with an eCAP™ N-linked oligosaccharide profiling kit (Beckman-Coulter) including a coated capillary and reagents for enzymatic digestion (PNGase-F) of the glycoprotein, fluorescent labelling of the released glycans and HPCE analysis. The original protocol was adapted to reduce to 10 μg the amount of glycoprotein required for CE analysis (data not shown). CE separations were performed with a P/ACE 5000 (Beckman-Coulter) equipped with laser-induced fluorescence detector (excitation 488 nm/emission 520 nm) and 50 μm × 57 cm N–CHO (polyvinylalcohol) coated capillary using 25 mM acetate buffer, pH 4.75, containing 0.4% of polyethylene oxide. Equivalent quantities of N-linked glycans (approximately 0.3-0.4 nmol maltose-equivalent quantified using the maltose internal standard) were introduced into the capillary by pressure injection (0.5 psi) for 20 s. Electrophoresis was performed at 25 kV with a current of approximately 12 μA. Analysis of five profiles obtained after successive injections of the same sample showed that the mean error in the relative peak area was less than 10%. A glucose ladder of known composition was used as standard for glycan size determination based on migration time comparison.
Release of N-linked oligosaccharides from EPO by PNGase digestion
The dialyzed samples of purified EPO were concentrated to dryness on a centrifugal concentrator (Eppendorf). The residue was dissolved in a mixture containing 45 μl of enzyme buffer, 1 μl of 5% SDS and 1.5 μl of 10% β-mercapto-ethanol. The denaturation by boiling for 5 min was followed by the addition of 5 μl of NP-40 and 2 μl of peptide-N-glycosidase F (PNGase-F) solution. The mixture was incubated overnight at 37°C for digestion. Released N-glycans were separated from the EPO polypeptide by precipitation with 150 μl of cold 100% ethanol. The reaction mixture was incubated at −20°C for 1 h before being centrifuged. As labelling control, maltose (2 nmol) was added to the oligosaccharide-containing supernatant, which was then dried with a centrifugal concentrator.
Fluorescent labelling of oligosaccharides with 8-aminopyrene-1,3,6-trisulfonate (APTS)
The released glycans (0.3–0.4 nmol maltose-equivalent) were labelled by reductive amination (Chen and Evangelista 1998). The dried samples were dissolved in a mixture containing 1.5 μl APTS (100 mg ml−1) in 15% acetic acid (Sigma—ref A6283) and 1.5 μl NaBH3CN (1 M) in THF, vortexed and then incubated overnight at 37°C for derivatization. After incubation, the labelled glycans were diluted to 50 μl with distilled water. A second dilution (1:10) in distilled water was performed before analysis.
Desialylation of oligosaccharides
Removal of the terminal sialyl residues of the N-glycans was performed by mild acidic hydrolysis. Thus, 4 μl of the mixture containing the labelled oligosaccharides was mixed with 36 μl of 0.5 M acetic acid and incubated 1 h at 80°C.
Sialidase activity analysis
Sialidase activity was measured in culture supernatants at different times of culture #1, and at the end of cultures #2, #3 an #4. Cell-free supernatants were centrifuged at 10,000 g for 10 min to remove any cell fragments and then concentrated 15-fold at 4°C with a 10 kDa cut-off Nanosep® centrifugal ultra-filtration unit (Pall). Sialidase activity was determined by fluorescence according to the method described by Gramer (2000) using 4-methylumbelliferyl-α-d-N-acetylneuraminic acid (Sigma) as substrate. The standardized assay was performed at 37°C with 188 μl of 4 mM 4-methylumbelliferylyl-d-N-acetylneuraminic acid, 119 μl of 1 M phosphate buffer (pH 6.2), 119 μl of distilled water and 325 μl of 15-fold concentrated supernatant. After 30 min, 250 μl of this solution were collected and then diluted in 2250 μl of 1 M glycine/KOH buffer (pH 10.4) to stop the enzymatic reaction. Quantification was carried out with a FLX spectrofluorometer (Safas, Monaco) using 4-methylumbelliferone (Sigma) as standard. Excitation of the samples was accomplished at 360 nm and fluorescence collected at 450 nm. Enzyme activity is expressed in nanomole of substrate (4-methylumbelliferyl-α-d-N-acetylneuraminic acid) hydrolysed in one hour for one litre of supernatant (nmol h−1 l−1). The precision of the sialidase activity analysis is ±15%.
Kinetic data analysis
Specific rate of cell growth (μ) was calculated during the exponential growth phase of the batch cultures, when cell death is negligible, while specific rate of EPO production (π) was calculated all over the cultures. They were evaluated from smoothed values of viable cell densities (Xv) and EPO concentrations according to the following equations:
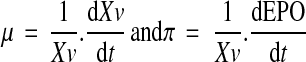 |
Results
In a previous paper (Le Floch et al. 2004) it was shown that glycosylation and especially desialylation of EPO produced by CHO cells was dependent on the presence of serum in the culture medium. As the culture of CHO cells in serum-free conditions required the adaptation of those cells, two different cell lines might emerge : (i) the original cell line growing in serum-containing medium and (ii) the adapted CHO cells capable to grow without serum in the culture medium. The discrimination between the effects of the lack of serum and the effects of the adaptation procedure to serum-free medium required a comparison of kinetic parameters and EPO glycosylation in four culture conditions (Fig. 1), combining two cell banks (cells adapted or not to serum-free medium) and different culture media (with or without serum).
Kinetics of cells adapted and non-adapted to serum-free conditions
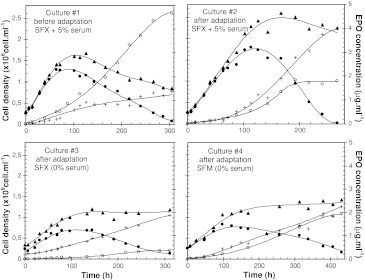
The time evolutions of the concentrations of EPO, viable, dead and total cells are presented in Fig. 2, and the kinetic parameters are given in Table 1. In a first culture, non-adapted cells were grown in the commercial serum-free medium SFX-CHO™ supplemented with 5% of serum (culture #1). A maximal cell density of 1.3 × 106 cell ml−1 was reached after 80 h of culture; then, a severe cell lysis occurred, as observed by the decreasing of the total cell density. The final titer of EPO, which increased all over the culture, was 5 μg ml−1 at the end of the culture. Maximal specific rates of growth and EPO production were respectively 0.04 h−1 and 70 μg 10−9cell h−1. It clearly appeared that EPO production occurred a long time after the maximal cell density was reached (maximal level obtained after 300 h of culture). Due to this behaviour, it was then necessary to prolong the culture duration time to harvest a maximal protein quantity.
Fig. 2.
Kinetics of EPO-producing CHO cells, adapted and non-adapted to serum-free conditions, cultivated in batch mode in different culture media: concentrations of viable cells (●), dead cells (+), total cells (▲) and EPO (○) over the process
Table 1.
Maximal concentrations and specific rates for the different culture conditions
| Culture | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Cells | Non-adapted | Adapted | Adapted | Adapted |
| Medium | SFX-CHO™ | SFX-CHO™ | SFX-CHO™ | SFM™ |
| 5% FBS | 5% FBS | 0% FBS | 0% FBS | |
| Maximal cell density (106 cells ml−1) | 1.3 | 1.8 | 0.7 | 0.8 |
| μ max (h−1) | 0.04 | 0.04 | 0.03 | 0.015 |
| Maximal EPO concentration (μg ml−1) | 5 | 2 | 0.4 | 2 |
| πmax (μg 10−9cell h−1) | 70 | 20 | 7 | 15 |
Cells from the same CHO cell line were then adapted to serum-free culture by progressively reducing serum supplementation until complete removal. The performances of those cells cultivated in a serum-free batch culture (culture #3—SFX-CHO™ medium) were clearly inferior to those obtained in serum-containing medium : the maximal cell density reached only 0.7 × 106 cell ml−1 after 100 hours and EPO production was 10-fold inferior to the titer reached in presence of serum (0.4 against 5 μg ml−1). Maximal specific rates were 0.03 h−1 for cell growth and about 7 μg 10−9cell h−1 for EPO production. As for the first culture, cell growth and EPO synthesis appeared not to be coupled. Interestingly, no significant cell lysis was found for those culture conditions.
To discriminate between the effects of the serum removal and those resulting from the serum-free adaptation procedure itself, another culture was performed in SFX-CHO™ supplemented with 5% of serum with serum-free adapted CHO cells (culture #2). A maximal cell density of 1.8 × 106 cell ml−1 was reached after 110 h and a slight cell lysis occurred for those conditions. The final titer of EPO was almost 2 μg ml−1 at the end of the culture (Fig. 2), which is twofold lower than in culture #1. Maximal specific growth and EPO production rates were respectively 0.04 h−1 and 20 μg 10−9cell h−1. Again, cell growth and EPO synthesis were not coupled.
Finally, to insure that the basic medium did not interfere in our study, a culture was performed with serum-free adapted cells in another commercial serum-free medium (SFM™ medium—culture #4) whose protein content was 10-fold higher than SFX-CHO™ medium (100 against 10 mg l−1). Similarly to the results obtained in SFX-CHO™ medium, growth performances were also clearly inferior to those obtained in serum-containing medium as the maximal cell density reached only 0.8 × 106 cell ml−1 after 100 h. On the other hand, EPO production was equivalent to that observed in culture #2 (adapted cells in presence of serum) since it reached 2 μg ml−1 at the end of the culture. Maximal specific rates were respectively 0.015 h−1 for cell growth and 15 μg 10−9 cell h−1 for EPO production and no cell lysis was observed in this culture.
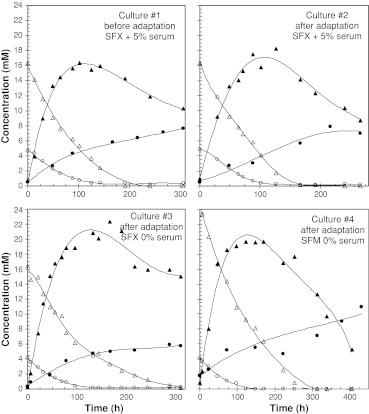
To assess the potential influences on the cell metabolism of either cell adaptation procedure to serum-free medium or of serum components removal from the culture medium, the concentrations of substrates (glucose and glutamine) and products (lactate and ammonia) were measured all over the four batch cultivations (Fig. 3). The main limiting substrate seemed to be glutamine in the four cases as cell density increase stopped when this substrate was completely exhausted from all batch cultures. Lactate production was found to be similar in the two serum-containing cultures (#1 and #2, before and after cell adaptation) but increased in the serum-free cultures (#3 and #4). In all cases lactate produced during the first 120-h period was then consumed during the cell decline phase. Besides, in the four cultures, high level of ammonia was produced compared to the glutamine uptake yielding an ammonia/glutamine ratio of 1.5–2. Final ammonia production was found to be equivalent before and after cell adaptation to serum-free medium as seen in cultures #1 and #2, but slightly different in absence of serum (lower in SFX-CHO™ medium and higher in SFM™ medium).
Fig. 3.
Kinetics of EPO-producing CHO cells, adapted and non-adapted to serum-free conditions, cultivated in batch mode in different culture media: concentrations of glucose (▵), lactate (▲), glutamine (○) and ammonia (●) over the process
Profile evolutions of N-linked glycans of EPO produced by adapted or non-adapted cells to serum-free conditions
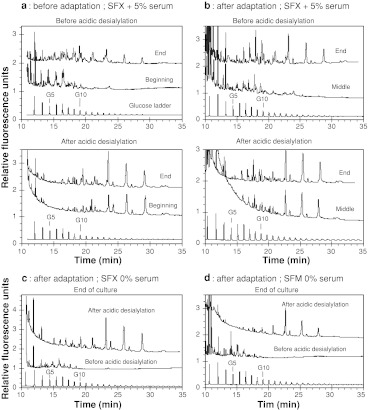
To evaluate the effects of the cell adaptation to serum-free media on the recombinant protein glycosylation, the N-glycans of EPO purified from supernatants collected at different phases of the cultures presented in Fig. 1 were analyzed by HPCE (Fig. 4). Figure 4a presents the HPCE patterns of complete and desialylated N-glycans of EPO produced at the beginning and at the end of the culture (after 40 and 300 h) performed in presence of serum, with the non-adapted cells (culture #1). As shown already (Le Floch et al. 2004), the HPCE patterns of non-desialylated and chemically desialylated N-glycans from EPO revealed that, except for the sialylation degree, glycosylation of EPO was quite constant all over the culture.
Fig. 4.
HPCE analysis of the N-linked glycans, before and after mild acidic hydrolysis, from EPO produced in the different batch CHO cell cultures presented in Fig. 2 (G5 and G10 correspond to 5 and 10 glucose units in the glucose ladder)
For the culture performed without serum and with the adapted cells (culture #3) supernatant was collected at the end of the cell decline period and the EPO glycans analyzed before or after desialylation (Fig. 4c). As already published (Le Floch et al. 2004) the EPO glycosylation patterns determined all over the process were very similar to those of EPO collected at the beginning of the previous culture meaning that desialylation observed in the previous experiment did not occur for these serum-free conditions using adapted cells.
In addition to the previously published results, we performed analysis of EPO glycans collected at the end of the cell growth phase and at the end of the culture (after 100 and 260 h) in presence of serum, but with cells previously adapted to serum-free conditions (culture #2). As can be seen in Fig. 4b, the non chemically desialylated N-glycan patterns of EPO was still greatly sialylated at the middle of the culture period, after 100 h of culture, as major peaks were located before 10 glucose units (GU); then at the end of the culture (260 h), significant amounts of non sialylated species, characterized by migration times superior to 10 GU, appeared on the surface of EPO. After mild acidic hydrolysis, the desialylated pattern remained very stable during the culture period with close similarity in the nature of major species. Therefore, a desialylation of EPO occurred during this serum-containing culture, as well as during the culture #1 performed with non-adapted cells in the same medium.
During the last culture, cells adapted to serum-free medium were cultured in another serum-free medium, containing more proteins, to observe a potential effect of the serum-free medium components (culture #4). Supernatant was collected at the end of the culture and the glycosylation patterns of EPO were analysed again before and after desialylation (Fig. 4d). The complete glycan pattern did not show any peak after 10 GU and the major species appearing after acidic desialylation have migration time close to those obtained in the other culture conditions. Thus, EPO collected at the end of this second serum-free batch culture mainly consisted in sialylated sugar chains as observed in the culture #3. Furthermore, we did not observe any differences between the two serum-free media, as N-glycan patterns of EPO produced in SFM™ medium were very similar to those obtained in SFX-CHO™ medium.
To explain the phenomenon of EPO desialylation during the serum-containing cultures, it may be postulated that an enzymatic degradation occurred. We then measured the sialidase activity in supernatants collected during and at the end of the four cultures. Clearly, we found a significant sialidase activity in the supernatants of the two serum-supplemented cultures while a negligible level was observed in the two cultures performed with serum-free media (Table 2). Interestingly, neither the adaptation of the cells nor the composition of the serum-free medium did change the level of released sialidase.
Table 2.
Sialidase activity in the culture supernatants
| Culture | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Cells | Non-adapted | Adapted | Adapted | Adapted | |
| Medium | SFX-CHO™ | SFX-CHO™ | SFX-CHO™ | SFM™ | |
| 5% FBS | 5% FBS | 0% FBS | 0% FBS | ||
| Sialidase activity (nmol h−1 l−1) | |||||
| Time (h) | |||||
| 0 | n.s. | ||||
| 100 | 250 | ||||
| 160 | 320 | ||||
| end | 430 | 425 | n.s. | n.s. | |
n.s., not significative
Discussion
Both cell growth and protein production of adapted cells to serum-free conditions significantly decreased when serum was removed from the medium (Fig. 2: cultures #3 vs. culture #1). Often reported in literature (Ozturk and Palsson 1991), these results are not surprising and seem to be extremely cell line and medium dependent. Furthermore in this paper, we have shown that, as expected, growth performances increased when serum was added again to the medium of those adapted cells, and they were even found to be improved (40%) compared to the ones initially obtained with non-adapted cells as a maximal viable cell density of 1.8 × 106 cell ml−1 was registered instead of 1.3 × 106 cell ml−1 (culture #2 vs. culture #1). In contrast, the final EPO concentration remained lower at 2 μg ml−1, meaning that serum addition after serum-free adaptation did not allow to completely recover the level of EPO production (5 μg ml−1) observed with non-adapted cells. In fact, this reduced EPO production by adapted cells resulted from a lower specific production rate (Table 1). However, when adapted cells were grown in the second serum-free medium (SFM™) which had a higher protein content, though cell growth was still limited, the same final EPO titer was reached compared to the culture performed with adapted cells in SFX-CHO™ + 5% FBS (culture #4 vs. culture #2). Therefore, rich media with serum or high protein content seems to be required to partly restore the EPO production capacity compromised by cell adaptation. Regarding global process optimization, this could be of particular importance and will have to be balanced with the downstream processing requirements. Besides, the serum-free medium chosen for our study (SFX-CHO™) was not specifically optimized for our cell line. Serum or nutrient starvation during the adaptation procedure might have led to the enrichment of a particular sub-population of lower EPO-producing CHO cells. Therefore, serum-free adaptation procedure, which is often based on selection of the best growing sub-clone, may need to be more tightly controlled to evaluate the nutrient limitations leading to the selection of clones with irreversible lower producing capacities.
Although cell physiology was probably modified during serum-free adaptation, uptakes of glucose and glutamine as well as production of lactate and ammonia were found to be quite similar for the different culture conditions tested. This is in good agreement with the results reported by Ozturk and Palsson (1991) who did not observe a significant influence of the serum level on cell metabolism for two hybridoma cell lines. Interestingly, while lactate accumulation occurred during the exponential growth phase, we observed a decrease of lactate concentration during the cell decline phase. When glutamine and glucose became limiting after about 150 h of culture, the cells probably metabolized lactate as an energy/carbon source. A high quantity of ammonia was produced during the four cultures with final concentrations ranging from 5 mM to 10 mM compared to the 4 mM of glutamine consumed. This indicates that additional nitrogen-containing components were probably consumed in the two commercial media whose compositions remain confidential. Thus, contrary to the EPO production rate, neither the serum-free adaptation nor the absence of serum induced significant changes in the glucose and glutamine metabolisms.
An important concern for therapeutic recombinant proteins is a possible change of their structure during the production processes. Therefore, to discriminate between the potential effect of serum starvation and cell adaptation procedure to serum-free medium, we have used the previously adapted HPCE-LIF technique (Le Floch et al. 2004) to monitor EPO glycosylation during the course of the different batch cultures. A dramatical EPO desialylation was registered when produced by non-adapted cells cultivated with serum (Fig. 4a) but this phenomenon was not observed when the protein was produced by adapted cells cultivated in serum-free conditions (Fig. 4c, d). Nevertheless, the non-sialylated glycan profiles obtained after mild acidic hydrolysis remained very similar during the course of the different processes. Therefore, except for the sialylation degree, EPO glycosylation was not significantly modified by the serum removal from the culture medium, which suggests that the intracellular process of EPO glycosylation involving numerous glycosyltransferases and glycan precursors seems to be not compromised. This phenomenon was already reported in the literature as serum was shown to affect the sugar content of the glycans of glycoproteins, particularly by decreasing the sialylation degree which is known to play a critical role in their biological activity (Patel et al. 1992; Megaw and Johnson 1979; Maiorella et al. 1993; Lamotte 1997; Gawlitzek et al. 1995). However, these results seem to be closely dependent on the molecule investigated and of the culture conditions used (Gawlitzek et al. 1995) as other papers reported a minor effect of the serum on the glycosylation pattern of recombinant proteins (Lifely et al. 1995; Moellering et al. 1990; Kopp et al. 1996).
As lower sialylation of EPO during the culture processes containing serum could result either from the serum removal (inducing a different metabolic behaviour of the cells) or from the cell clone selection during the adaptation procedure, EPO was produced in the same serum-containing medium by either non-adapted or adapted cells and the protein glycosylation patterns were analysed. As protein desialylation occurred in the course of both cultures, while the asialoglycans were not modified, these results clearly suggest that the adaptation protocol did not affect the intracellular EPO glycosylation capacities of the selected CHO clone. Moreover, the cell adaptation to serum-free conditions did not avoid the desialylation phenomenon which occurred when serum is added again. Therefore, sialylation changes observed after cell adaptation in serum-free medium were likely to be due to the absence of serum. This hypothesis is supported by the fact that EPO HPCE profiles from cultures performed with the same adapted cells in SFMTM medium (Fig. 4d) and in SFX-CHOTM medium (Fig. 4c) are very similar, showing an absence of EPO desialylation.
One hypothesis to explain the progressive desialylation observed during the cultures performed in presence of serum could be a change in protein sialylation reaction induced by the evolution of the cell environment. The wide source of nutrients, hormones or other molecules provided by serum supplementation obviously represents a strong change in cell environment which is known to be able to affect oligosaccharide biosynthesis and consequently to induce variability in the sialylation status of glycoproteins (Goochee and Monica 1990). Moreover, variations in culture conditions may induce not only potential changes in glycan biosynthesis but also enzymatic degradation. For example, ammonia is capable to affect the glycosylation profile and the sialylation of therapeutic glycoproteins (Kopp et al. 1996; Yang and Butler 2000a, b, c, 2002), and has been shown to increase the secretion rate of various glycosidase activities (Gramer et al. 1995). Nevertheless, in our case, ammonia is probably not a major factor responsible for the desialylation of EPO in presence of serum, as the highest titer (11 mM) was measured during the SFM™ serum-free culture.
Desialylation in presence of serum could also be due to an enzymatic degradation of protein glycans by serum enzymes or by cytoplasmic enzymes released during cell lysis (Gramer and Goochee 1993). In the case of sialidase activities present in serum preparations (Gawlitzek et al. 1995), a protein desialylation should have appeared from the early beginning of the culture. In fact, desialylation occurred only during the late phases of the CHO-EPO cultures and no significant level of sialidase activities was present in the serum-containing fresh media and at the culture beginning (Table 2). Desialylation is more probably due to the release of degradative enzymes upon cell lysis. Indeed, we found that sialidase activity accumulated in the culture medium during the serum-containing cultures (Table 2) concomitantly to cell lysis, whereas no activity could be detected in the culture medium devoid of serum, even after 400 h of culture. In addition, the sialidase release was restored when the serum-independent cells were recultured with serum. Therefore, sialidase activity release following cell lysis, in serum-containing conditions, was certainly responsible for the cleavage of terminal sialyl residues. This hypothesis was confirmed by the cell lysis occurring at the end of the two serum-containing cultures, particularly with non-adapted cells. This phenomenon was not observed for serum-free conditions as the total maximal cell densities remained stable for both SFM™ and SFX-CHO™ media. Even if the number of lysed cells seemed to be inferior in culture #2 compared to culture #1, cell lysis could have released the same amount of sialidase activity: indeed, as the EPO production capacity seemed to be altered after the serum-free adaptation, this phenomenon may also have induced a higher intra-cellular content of sialidase. Because most of the EPO production by our CHO cell line occurred after the maximal cell density was reached, cultures had to be maintained for prolonged periods; therefore, serum-containing conditions could be detrimental to the protein quality as enzymes released upon cell lysis could affect the structure of the recombinant protein. In fact, when serum is added to the culture medium of the adapted cells, a maximal cell density 2.5-fold higher is observed. Then a substrate not measured in the present study could be limiting only for these high cell densities and thus responsible for a higher fragility of the cell and therefore a cell lysis upon starvation of this nutrient. This lysis phenomenon was found to be less severe after serum-free adaptation, probably because of a lower nutrient requirement of the sub-population selected during the procedure.
In conclusion, the main objective of this work was to determine the specific influence of the cell adaptation procedure to serum-free conditions on recombinant EPO glycosylation/sialylation by CHO cells. Our results show that the cell adaptation process did not significantly affect the EPO desialylation mechanism observed in serum-containing medium as this phenomenon occurred in the same manner when adapted or non-adapted cells were cultivated in presence of serum. The EPO desialylation, which was not observed in two different serum-free media, was restored when the serum-independent cells were recultured with serum. This desialylation mechanism seems mainly result from the release of a sialidase activity in the supernatant by cell lysis occurring in serum-containing cultures at the same time of the EPO secretion in culture medium. The reason of the higher level of cell lysis in presence of serum remains to be investigated.
Acknowledgments
The F. Le Floch fellowship and the project were financed by the Agence Nationale pour la Recherche Technique and Aventis Pharma (Paris, France). The authors are grateful to Dr. Abderrahim Mahfoudi (Aventis Pharma, Paris) for donating the cDNA coding for the EPO cassette, Fabrice Blanchard (LSGC, Nancy) for his technical support, and Professor Jean Straczek and Pascal Perrin (University H. Poincaré, Nancy) for their expert scientific assistance in capillary electrophoresis analysis.
References
- Andersen DC, Goochee CF. The effect of ammonia on the O-linked glycosylation of granulocyte colony-stimulating factor produced by Chinese hamster ovary cells. Biotechnol Bioeng. 1995;47:96–105. doi: 10.1002/bit.260470112. [DOI] [PubMed] [Google Scholar]
- Borys MC, Linzer DIH, Papoutsakis ET. Ammonia affects the glycosylation patterns of recombinant mouse placental lactogen-I by Chinese hamster ovary cells in a pH-dependant manner. Biotechnol Bioeng. 1994;43:505–514. doi: 10.1002/bit.260430611. [DOI] [PubMed] [Google Scholar]
- Buntemeyer H, Lutkemeyer D, Lehmann J. Optimization of serum-free fermentation processes for antibody production. Cytotechnology. 1991;5:57–67. doi: 10.1007/BF00365534. [DOI] [PubMed] [Google Scholar]
- Cabrera G, Crema JA, Valdes R, Garcia R, Gonzales Y, Montesino R, Gomez H, Gonzales M. Influence of culture conditions on the N-glycosylation of a monoclonal antibody specific for recombinant hepatis B surface antigen. Biotechnol Appl Biochem. 2005;41:67–76. doi: 10.1042/BA20040032. [DOI] [PubMed] [Google Scholar]
- Castro PM, Hayter PM, Ison AP, Bull AT. Application of a statistical design to the optimization of culture medium for recombinant interferon-γ production by Chinese hamster ovary cells. Appl Microbiol Biotechnol. 1992;38:84–90. doi: 10.1007/BF00169424. [DOI] [PubMed] [Google Scholar]
- Castro PML, Ison AP, Hayter PM, Bull AT. The macroheterogeneity of recombinant human interferon-γ produced by Chinese hamster ovary cells is affected by the protein and lipid content of the culture medium. Biotechnol Appl Biochem. 1995;21:87–100. [PubMed] [Google Scholar]
- Chen AFT, Evangelista RA. Profiling glycoprotein N-linked oligosaccharide by capillary electrophoresis. Electrophoresis. 1998;19:2639–2644. doi: 10.1002/elps.1150191512. [DOI] [PubMed] [Google Scholar]
- Chen P, Harcum SW. Effects of amino acid additions on ammonium stressed CHO cells. J Biotechnol. 2005;117:277–286. doi: 10.1016/j.jbiotec.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Dordal MS, Wang FF, Goldwasser E. The role of carbohydrate in erythropoietin action. Endocrinology. 1985;116:2293–2299. doi: 10.1210/endo-116-6-2293. [DOI] [PubMed] [Google Scholar]
- Elliott S, Egrie J, Browne J, Lorenzini T, Busse L, Rogers N, Ponting I. Control oh rHuEPO biological activity: the role of carbohydrate. Exp Hematol. 2004;32:1146–1155. doi: 10.1016/j.exphem.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Froud SJ. The development, benefits and disadvantages of serum-free media. Dev Biol Stand. 1999;99:157–166. [PubMed] [Google Scholar]
- Gawlitzek M, Valley U, Nimtz M, Wagner R, Conradt HS. Characterization of changes in the glycosylation pattern of recombinant proteins from BHK-21 cells due to different culture conditions. J Biotechnol. 1995;42:117–131. doi: 10.1016/0168-1656(95)00065-X. [DOI] [PubMed] [Google Scholar]
- Gawlitzek M, Valley U, Wagner R. Ammonium ion and glucosamine dependent increases of oligosaccharide complexity in recombinant glycoproteins secreted from cultivated BHK-21 cells. Biotechnol Bioeng. 1998;57:518–528. doi: 10.1002/(SICI)1097-0290(19980305)57:5<518::AID-BIT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Goochee CF, Monica T. Environmental effects on protein glycosylation. Bio/Technology. 1990;8:421–426. doi: 10.1038/nbt0590-421. [DOI] [PubMed] [Google Scholar]
- Goto M, Akai K, Murakami C, Hashimoto C, Tsuda E, Ueda M, Kawanishi G, Takahashi N, Ishimoto A, Chiba H, Sasaki R. Production of recombinant human erythropoietin in mammalian cells: host-cell dependency of the biological activity of the cloned glycoprotein. Bio/Technology. 1988;6:67–71. doi: 10.1038/nbt0188-67. [DOI] [Google Scholar]
- Gramer MJ, Goochee CF. Glycosidase activities in Chinese hamster ovary cell lysate and cell culture supernatant. Biotechnol Prog. 1993;9:366–373. doi: 10.1021/bp00022a003. [DOI] [PubMed] [Google Scholar]
- Gramer MJ, Goochee CF, Chock VY, Brousseau DT, Sliwkowski MB. Removal of sialic acid from a glycoprotein in CHO cell culture supernatant by action of an extracellular CHO cell sialidase. Bio/Technology. 1995;13:692–698. doi: 10.1038/nbt0795-692. [DOI] [PubMed] [Google Scholar]
- Gramer MJ. Detecting and minimizing glycosidase activities that can hydrolyze sugars from cell culture-produced glycoproteins. Mol Biotechnol. 2000;15:69–75. doi: 10.1385/MB:15:1:69. [DOI] [PubMed] [Google Scholar]
- Grammatikos SI, Valley U, Nimtz M, Conradt HS, Wagner R. Intracellular UDP-N-acetylhexosamine pool affects N-glycan complexity: a mechanism of ammonium action on protein glycosylation. Biotechnol Prog. 1998;14:410–419. doi: 10.1021/bp980005o. [DOI] [PubMed] [Google Scholar]
- Hewlett G. Strategies for optimising serum-free media. Cytotechnology. 1991;5:3–14. doi: 10.1007/BF00365530. [DOI] [PubMed] [Google Scholar]
- Hokke CH, Bergwerff AA, Dedem GW, Kamerling JP, Vliegenthart JF. Structural analysis of the sialylated N- and O-linked carbohydrate chains of recombinant human erythropoietin expressed in Chinese hamster ovary cells. Sialylation patterns and branch location of dimeric N-acetyllactosamine units. Eur J Biochem. 1995;228:981–1008. doi: 10.1111/j.1432-1033.1995.tb20350.x. [DOI] [PubMed] [Google Scholar]
- Imai N, Higuchi M, Kawamura A, Tomonoh K, Oh-Eda M, Fujiwara M, Shimonaka Y, Ochi N. Physicochemical and biological characterization of asialoerythropoietin. Suppressive effects of sialic acid in the expression of biological activity of human erythropoietin in vitro. Eur J Biochem. 1990;194:457–462. doi: 10.1111/j.1432-1033.1990.tb15639.x. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Lopez LB, Kawamoto S, Arita N, Teruya K, Seki K, Shoji M, Kamei M, Hashizume S, Shiozawa Y, Tachibana H, Ohashi H, Yasumoto K, Nagashima A, Nakahashi H, Suzuki T, Imai T, Nomoto K, Takenoyama M, Katakura Y, Shirahata S. Production of recombinant human monoclonal antibody using ras-amplified BHK-21 cells in a protein-free medium. Biosci Biotech Biochem. 1996;60:811–817. doi: 10.1271/bbb.60.811. [DOI] [PubMed] [Google Scholar]
- Jenkins N, Curling EMA. Glycosylation of recombinant proteins: problems and prospects. Enzyme Microb Technol. 1994;16:354–364. doi: 10.1016/0141-0229(94)90149-X. [DOI] [PubMed] [Google Scholar]
- Jenkins N, Castro P, Menon S, Ison A, Bull A. Effect of lipid supplements on the production and glycosylation of recombinant interferon-gamma expressed in CHO cells. Cytotechnology. 1994;15:209–215. doi: 10.1007/BF00762395. [DOI] [PubMed] [Google Scholar]
- Jenkins N, Parekh RB, James DC. Getting the glycosylation right: implications for the biotechnology industry. Nat Biotechnol. 1996;14:975–981. doi: 10.1038/nbt0896-975. [DOI] [PubMed] [Google Scholar]
- Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur J Biochem. 1992;209:483–501. doi: 10.1111/j.1432-1033.1992.tb17313.x. [DOI] [PubMed] [Google Scholar]
- Kopp K, Schluter M, Werner R. Monitoring the glycosylation pattern of recombinant interferon-gamma with high-pH anion-exchange chromatography and capillary electrophoresis. Arzneim-Forsch/Drug Res. 1996;46:1191–1196. [PubMed] [Google Scholar]
- Koury MJ. Sugar coating extends half-lives and improves effectiveness of cytokine hormones. Trends Biotechnol. 2003;21:462–464. doi: 10.1016/j.tibtech.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Lamotte D (1997) Production et glycosylation de l’interféron-γ humain par des cellules CHO cultivées en bioréacteurs discontinus et perfusés. Influence des conditions opératoires et du potentiel de glycosylation des cellules. Ph.D. thesis, INPL, Nancy, pp 133–142
- Lamotte D, Buckberry L, Monaco L, Soria M, Jenkins N, Engasser JM, Marc A. Na-butyrate increases the production and alpha 2,6-sialylation of recombinant interferon-gamma expressed by alpha 2,6-sialyltransferase engineered CHO cells. Cytotechnology. 1999;29:55–64. doi: 10.1023/A:1008080432681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GM, Kim EJ, Kim NS, Yoon SK, Ahn YH, Song JY. Development of serum-free medium for the production of EPO by suspension culture of recombinant CHO cells using a statistical design. J Biotechnol. 1999;69:85–93. doi: 10.1016/S0168-1656(99)00004-8. [DOI] [PubMed] [Google Scholar]
- Le Floch F, Tessier B, Chenuet S, Guillaume JM, Cans P, Marc A, Goergen JL. HPCE-monitoring of the N-glycosylation pattern and sialylation of murine erythropoietin produced by CHO cells in batch processes. Biotechnol Prog. 2004;20:864–871. doi: 10.1021/bp0343211. [DOI] [PubMed] [Google Scholar]
- Lifely MR, Hale C, Boyce S, Keen MJ, Phillips J. Glycosylation and biological activity of CAMPATH-1H expressed in different cell lines and grown under different culture conditions. Glycobiology. 1995;5:813–822. doi: 10.1093/glycob/5.8.813. [DOI] [PubMed] [Google Scholar]
- Lipscomb ML, Palomares LA, Hernandez V, Ramirez OT, Kompala DS. Effect of production method and gene amplification on the glycosylation pattern of a secreted reporter protein in CHO cells. Biotechnol Prog. 2005;21:40–49. doi: 10.1021/bp049761m. [DOI] [PubMed] [Google Scholar]
- Liu C, Chu I, Hwang S. Factorial designs combined with the steepest ascent method to optimize serum-free media for CHO cells. Enzyme Microb Technol. 2001;28:314–321. doi: 10.1016/S0141-0229(00)00346-X. [DOI] [PubMed] [Google Scholar]
- Maiorella BL, Winkelhake J, Young J, Moyer B, Bauer R, Hora M, Andya J, Thomson J, Patel T, Parekh R. Effect of culture conditions on IgM antibody structure, pharmacokinetics and activity. Bio/Technology. 1993;11:387–392. doi: 10.1038/nbt0393-387. [DOI] [PubMed] [Google Scholar]
- Megaw JM, Johnson LD. Glycoprotein synthesized by cultured cells: effects of serum concentrations and buffers on sugar content. Proc Soc Exp Biol Med. 1979;161:60–65. doi: 10.3181/00379727-161-40490. [DOI] [PubMed] [Google Scholar]
- Misaizu T, Matsuki S, Strickland TW, Takeuchi M, Kobata A, Takasaki S. Role of antennary structure of N-linked sugar chains in renal handling of recombinant human erythropoietin. Blood. 1995;86:4097–4104. [PubMed] [Google Scholar]
- Moellering BJ, Tedesco JL, Townsend R, Hardy MR, Scott RS, Prior CP. Electrophoretic differences in a MAb expressed in three media. BioPharm. 1990;3:30–38. [Google Scholar]
- Monaco L, Marc A, Eon-Duval A, Acerbis G, Distefano G, Lamotte D, Engasser JM, Soria M, Jenkins N. Genetic engineering of alpha2,6-sialyltransferase in recombinant CHO cells and its effect on the sialylation of recombinant interferon-gamma. Cytotechnology. 1996;22:197–203. doi: 10.1007/BF00353939. [DOI] [PubMed] [Google Scholar]
- Ozturk SS, Palsson BO. Physiological changes during the adaptation of hybridoma cells to low serum and serum-free media. Biotechnol Bioeng. 1991;37:35–46. doi: 10.1002/bit.260370107. [DOI] [PubMed] [Google Scholar]
- Ozturk S, Kaseko G, Mahaworasilpa T, Coster HG. Adaptation of cell lines to serum-free culture medium. Hybrid Hybridomics. 2003;22:267–272. doi: 10.1089/153685903322329009. [DOI] [PubMed] [Google Scholar]
- Patel TP, Parekh RB, Moellering B, Prior CP. Different culture methods lead to differences in glycosylation of a murine IgG monoclonal antibody. Biochem J. 1992;285:839–845. doi: 10.1042/bj2850839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recny MA, Scoble HA, Kim Y. Structural characterization of natural human urinary and recombinant DNA-derived erythropoietin. Identification of des-arginine 166 erythropoietin. J Biol Chem. 1987;262:17156–17163. [PubMed] [Google Scholar]
- Sasaki H, Bothner B, Dell A, Fukuda M. Carbohydrate structure of erythropoietin expressed in CHO cells by a human erythropoietin cDNA. J Biol Chem. 1987;262:12059–12076. [PubMed] [Google Scholar]
- Sasaki H, Ochi N, Dell A, Fukuda M. Site-specific glycosylation of human recombinant erythropoietin: analysis of glycopeptides or peptides at each glycosylation site by FAB-MS. Biochemistry. 1988;27:8618–8626. doi: 10.1021/bi00423a017. [DOI] [PubMed] [Google Scholar]
- Schauer R. Sialic acids as antigenic determinants of complex carbohydrates. Adv Exp Med Biol. 1988;228:47–72. doi: 10.1007/978-1-4613-1663-3_2. [DOI] [PubMed] [Google Scholar]
- Spearman M, Rodriguez J, Huzel N, Butler M. Production and glycosylation of recombinant beta-interferon in suspension and cytopore microcarrier cultures of CHO cells. Biotechnol Prog. 2005;21:31–39. doi: 10.1021/bp0498084. [DOI] [PubMed] [Google Scholar]
- Stubiger G, Marchetti M, Nagano M, Grimm R, Gmeiner G, Reichel C, Allmaier G. Characterization of N- and O- glycopeptides of recombinant human erythropoietins as potential biomarkers for doping analysis by means of microscale sample purification combined with MALDI-TOF and quadrupole IT/RTOF mass spectrometry. J Sep Sci. 2005;28:1764–1778. doi: 10.1002/jssc.200500148. [DOI] [PubMed] [Google Scholar]
- Sung YH, Song YJ, Lim SW, Chung JY, Lee GM. Effect of sodium butyrate on the production, heterogeneity and biological activity of human thrombopoietin by recombinant Chinese hamster ovary cells. J Biotechnol. 2005;112:323–335. doi: 10.1016/j.jbiotec.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Takasaki S, Miyazaki H, Kato T, Hoshi S, Kochibe N, Kobata A. Comparative study of the asparagine-linked sugar chains of human erythropoietins purified from urine and the culture medium of recombinant CHO cells. J Biol Chem. 1988;263:3657–3663. [PubMed] [Google Scholar]
- Takeuchi M, Inoue N, Strikland TW, Kubota M, Wada M, Shimizu R, Hoshi S, Kozutsumi H, Takasaki S, Kobata A. Relationship between sugar chain structure and biological activity of recombinant human erythropoietin produced in Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1989;86:7819–7822. doi: 10.1073/pnas.86.20.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Kobata A. Structures and functional roles of the sugar chains of human erythropoietins. Glycobiology. 1991;1:337–346. doi: 10.1093/glycob/1.4.337. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Betenbaugh MJ, Lee YC. Humanization of lepidopteran insect-cell-produced glycoproteins. Acc Chem Res. 2003;36:613–620. doi: 10.1021/ar020202v. [DOI] [PubMed] [Google Scholar]
- Tsuda E, Kawanishi G, Ueda M, Masuda S, Sasaki R. The role of carbohydrate in recombinant human erythropoietin. Eur J Biochem. 1990;188:405–411. doi: 10.1111/j.1432-1033.1990.tb15417.x. [DOI] [PubMed] [Google Scholar]
- Watson E, Bhide A, Halbeek H. Structure determination of the intact major sialylated oligosaccharide chains of recombinant human erythropoietin expressed in CHO cells. Glycobiology. 1994;4:227–237. doi: 10.1093/glycob/4.2.227. [DOI] [PubMed] [Google Scholar]
- Yang M, Butler M. Effects of ammonia on CHO cell growth, erythropoietin production and glycosylation. Biotechnol Bioeng. 2000;68:370–380. doi: 10.1002/(SICI)1097-0290(20000520)68:4<370::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yang M, Butler M. Effect of ammonia on the glycosylation of human recombinant erythropoietin in culture. Biotechnol Prog. 2000a;16:751–759. doi: 10.1021/bp000090b. [DOI] [PubMed] [Google Scholar]
- Yang M, Butler M. Enhanced erythropoietin in a CHO culture is caused by proteolytic degradation and can be eliminated by a high glutamine level. Cytotechnology. 2000b;34:83–89. doi: 10.1023/A:1008137712611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Butler M. Effects of ammonia and glucosamine on the heterogeneity of erythropoietin glycoforms. Biotechnol Prog. 2002;18:129–138. doi: 10.1021/bp0101334. [DOI] [PubMed] [Google Scholar]
- Yoon SK, Choi SL, Song JY, Lee GM. Effect of culture pH on EPO production by CHO cells grown in suspension at 32.5 and 37.0 degrees C. Biotechnol Bioeng. 2005;89:345–356. doi: 10.1002/bit.20353. [DOI] [PubMed] [Google Scholar]
- Yuen CT, Storring PL, Tiplady RJ, Izquierdo M, Wait R, Gee CK, Gerson P, Lloyd P, Cremata JA. Relationships between the N-glycan structures and biological activities of recombinant human erythropoietins produced using different culture conditions and purification procedures. Br J Haematol. 2003;121:511–526. doi: 10.1046/j.1365-2141.2003.04307.x. [DOI] [PubMed] [Google Scholar]
- Yusa A, Kitajima K, Habuchi O. N-linked oligosaccharides are required to produce and stabilize the active form of chondroitin 4-sulphotransferase-1. Biochem J. 2005;388:115–121. doi: 10.1042/BJ20041573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Murhammer DW, Linhardt RJ. Enzyme kinetics and glycan structural characterization of secreted alkaline phosphatase prepared using the baculovirus expression vector system. Appl Biochem Biotechnol. 2002;101:197–210. doi: 10.1385/ABAB:101:3:197. [DOI] [PubMed] [Google Scholar]