Abstract
Significance: Among different forms of oxidative stress, lipid peroxidation comprises the interaction of free radicals with polyunsaturated fatty acids, which in turn leads to the formation of highly reactive electrophilic aldehydes. Among these, the most abundant aldehydes are 4-hydroxy-2-nonenal (HNE) and malondialdehyde, while acrolein is the most reactive. HNE is considered a robust marker of oxidative stress and a toxic compound for several cell types. Proteins are particularly susceptible to modification caused by HNE, and adduct formation plays a critical role in multiple cellular processes. Recent Advances: With the outstanding progress of proteomics, the identification of putative biomarkers for neurodegenerative disorders has been the main focus of several studies and will continue to be a difficult task. Critical Issues: The present review focuses on the role of lipid peroxidation, particularly of HNE-induced protein modification, in neurodegenerative diseases. By comparing results obtained in different neurodegenerative diseases, it may be possible to identify both similarities and specific differences in addition to better characterize selective neurodegenerative phenomena associated with protein dysfunction. Results obtained in our laboratory and others support the common deregulation of energy metabolism and mitochondrial function in neurodegeneration. Future Directions: Research towards a better understanding of the molecular mechanisms involved in neurodegeneration together with identification of specific targets of oxidative damage is urgently required. Redox proteomics will contribute to broaden the knowledge in regard to potential biomarkers for disease diagnosis and may also provide insight into damaged metabolic networks and potential targets for modulation of disease progression. Antioxid. Redox Signal. 17, 1590–1609.
Introduction
The brain is particularly susceptible to lipid peroxidation since the composition of neuronal tissue makes the brain vulnerable to chain reactions mediated by free radicals and leading to products of lipid peroxidation. The brain contains high levels of polyunsaturated fatty acids (PUFAs) and high levels of redox transition metal ions in addition to its high oxygen consumption. On the other hand, levels of lower molecular weight and enzymatic antioxidants are relatively low and might contribute to the accumulation of oxidative damage. In recent years, numerous studies have suggested that free radical–mediated oxidative damage occurs early in the pathogenesis of neurodegenerative disorders (121, 127, 132, 199).
Lipid peroxidation is one of the major sources of free radical–mediated injury that directly damages membranes and generates a number of secondary products. In particular, markers of lipid peroxidation have been found to be elevated in brain tissues and body fluids in several neurodegenerative diseases, and the role of lipid peroxidation has been widely investigated in the pathogenesis of Alzheimer disease (AD), Parkinson disease (PD), amyotrophic lateral sclerosis (ALS), Huntington disease (HD), and Down syndrome (DS) (32, 111, 175, 176, 185).
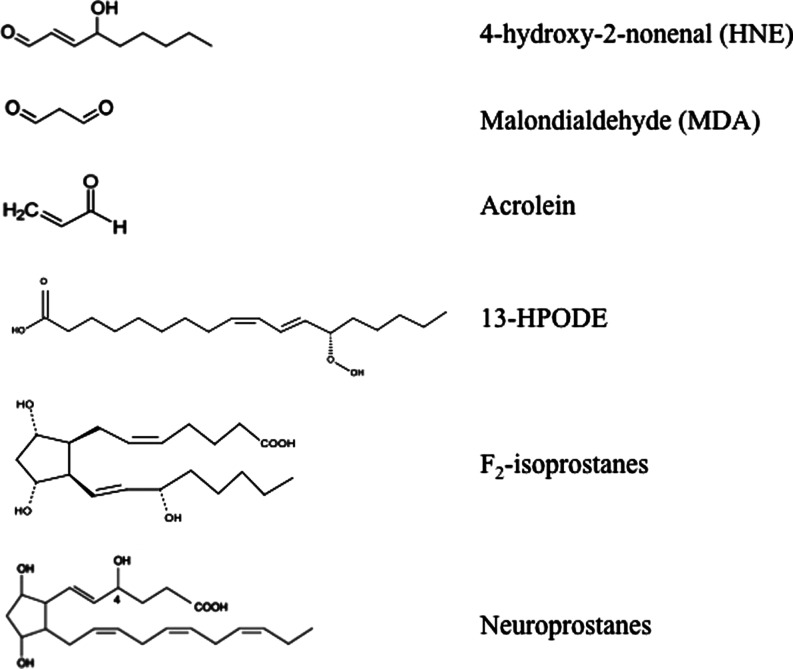
This complex process involves the interaction of oxygen-derived free radicals with PUFAs resulting in a variety of highly reactive electrophilic aldehydes, including malondialdehyde (MDA), 4-hydroxy-2-nonenal (HNE), and acrolein (Fig. 1) (70, 118, 163). Following lipid peroxidation, HNE and MDA are the most abundant aldehydes produced, while acrolein is the most reactive. In addition, lipid hydroperoxyl radicals undergo endocyclization to produce fatty acids esters; two classes of these cyclized fatty acids are ispoprostanes and neuroprostanes (Fig. 1) (133, 134). Free radical damage to arachidonic acid leads to the formation F2-isoprostanes (F2-IsoPs), while F4-neuroprostanes (F4-NPs) are the stable product of free radical damage to docosahexanoic acid (DHA). Once formed, F2-NPs and F4-NPs can undergo hydrolysis to liberate free iso- and neuroprostanes that are detectable in body fluids (170).
FIG. 1.
Products of lipid peroxidation. MDA, malondialdehyde; HNE, 4-hydroxy-2-trans-nonenal; acrolein; 13-HPODE, 3-hydroperoxy-9Z,11E-octadecadienoic acid; F2-isoprostanes; neuroprostanes.
PUFAs and their metabolites display important physiological functions such as maintenance of membrane structure and fluidity, cell signaling, energy production, and regulation of gene expression. Among PUFAs, arachidonic acid (AA) and DHA are the major constituents of the brain phospholipid bilayer and are particularly vulnerable to free radical attack due to the presence of double bonds and associated allylic H atoms in their structure. In fact, the C–H bonds on the allylic carbon atom adjacent to the double bond become more labile and it results in a facile H atom abstraction from a methylene group (-CH2-) (86, 155). These reactions are self-propagating and proceed until substrate is consumed or termination occurs. In this way, lipid peroxidation is a self-sustaining process, which amplifies the effects of the original free radical and leads to the activation of a cascade of toxic reactions resulting in extensive tissue damage. Structural damage to membranes cause subsequent generation of oxidized products that, in turn, are chemically reactive and can covalently modify most biological macromolecules. Peroxidation of membrane lipids can have numerous effects: increased membrane rigidity, decreased activity of membrane-bound enzymes (e.g., sodium pump), altered activity of membrane receptors, and altered permeability (9, 213). In addition to effects on phospholipids, radicals can also directly attack membrane proteins and induce lipid–lipid, lipid–protein, and protein–protein cross-linking, all of which obviously have effects on membrane function (71). Several efforts have been devoted to understanding the role of toxic aldehydes in contributing to neuronal dysfunction in neurodegenerative diseases. Numerous studies have documented increased levels of reactive products of lipid peroxidation in diseased regions of brain in different brain disorders (32, 72, 194). Consistent with the importance of lipid peroxidation in neurodegenerative diseases, proteins modified by lipid peroxidation products were present in diseased regions of the brain but not in regions uninvolved in the disease (32).
Lipid Peroxidation: Chemistry and Products
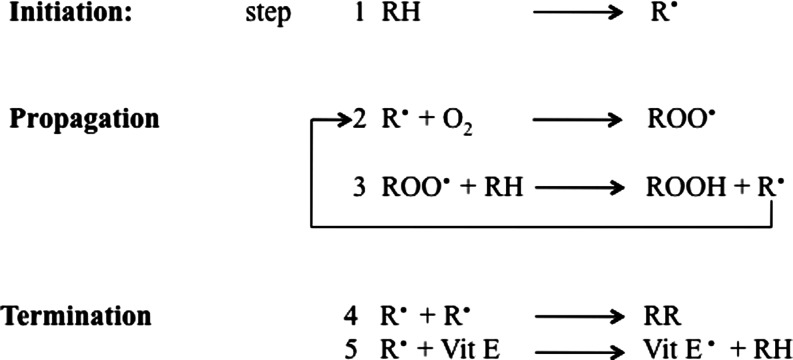
Lipid peroxidation refers to the oxidative degradation of lipids which can be described in a five-step sequence (Fig. 2).
FIG. 2.
Mechanism of lipid peroxidation (five steps).
Step 1 involves initiation, in which the free radical (hydroxyl HO·, alkoxyl RO·, peroxyl ROO·, and possibly HO2· but not H2O2 or O2−·) attacks a methylene group in the PUFAs, leading to an allylic H atom abstraction with rearrangement of the double bonds to the conjugated diene form, and simultaneously producing a carbon-centered alkyl radical. In step 2, the alkyl radical reacts with paramagnetic molecular oxygen to form a peroxyl radical. Propagation occurs in step 3, in which the peroxyl radical abstracts another allylic H atom, thereby initiating a self-perpetuating chain reaction in which most of the membrane lipids are converted to a variety of hydroperoxides and cyclic peroxides. The hydroperoxides can be further degraded to hydrocarbons, alcohols, ether, epoxides, and aldehydes. Of these products, MDA, HNE, and acrolein can cause irreversible modification of phospholipids, proteins, and DNA resulting in impaired function (69). In step 4, termination occurs, in which direct interaction between different types of radicals block the chain reaction cascade. Finally, in step 5, there is termination by interaction between the radicals and antioxidants, giving rise to nonradical products or unreactive radicals.
Both exogenous and endogenous antioxidants such as vitamin E and vitamin C prevent the propagation of lipid peroxidation at the early stages of free radical attack (21, 56). Vitamin E (α-tocopherol) acts as a “chain-breaking” antioxidant and can terminate propagation steps of lipid peroxidation. When the hydrogen is abstracted in step 1, an α-tocopherol radical forms (step 5) that can be reverted back to vitamin E by the potent antioxidants vitamin C (ascorbic acid) and glutathione (GSH). Cadenas et al. (40) in 1983 proposed this mechanism by comparing the effects of toxic concentrations of HNE on rat hepatocytes with and without pretreatment with Vitamin E. The protective effects exerted by antioxidant towards HNE and other toxic aldehydes have been investigated by many groups to test the possibility of a therapeutic use of free radical scavengers and antioxidants against lipid peroxidation–mediated toxicity. In addition to small molecules, antioxidant enzymes such as heme oxygenase-1, catalase, superoxide dismutase, peroxiredoxin, and GSH reductase have been shown to lead to a significant decrease in lipid peroxidation products (189, 204). Lipid peroxidation also has been reported in phospholipids, specifically in the form of oxidized phosphatidylcholine, which is considered a marker for inflammation as observed in both stroke and multiple sclerosis (2, 164).
HNE
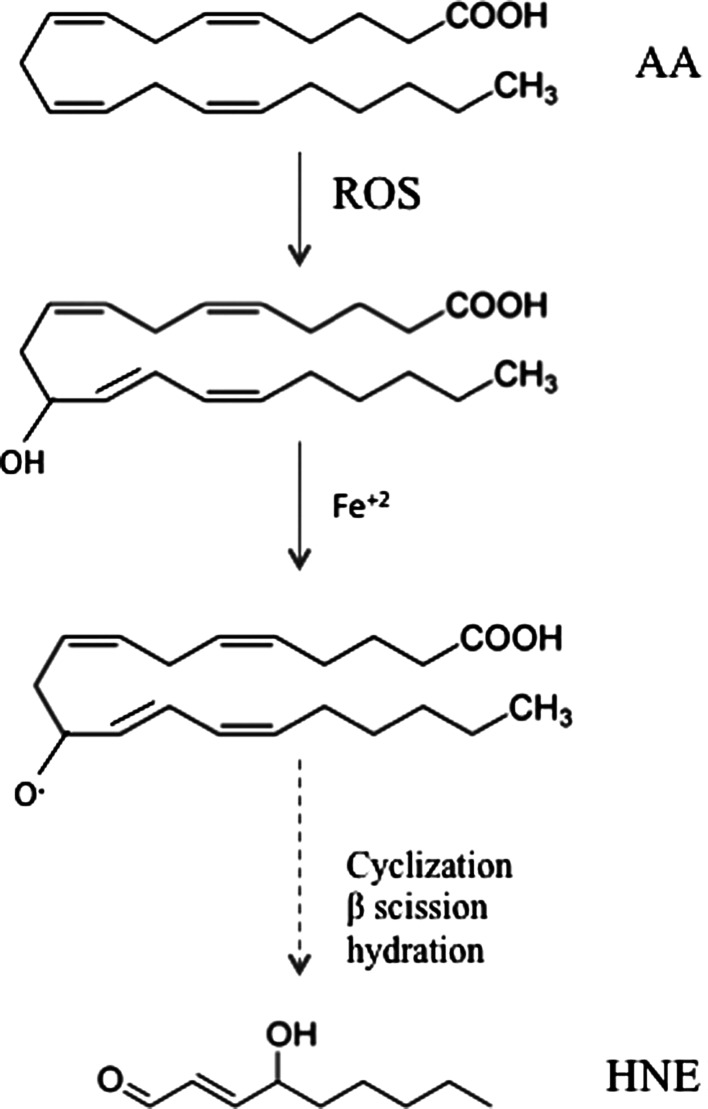
HNE is an α,β-unsaturated aldehyde that is formed by peroxidation of ω-6 PUFAs such as linoleic acid, linolenic acid, and mostly AA (Fig. 3). Although the precise steps of formation may be multifactorial, it is generally assumed that HNE is the product of a nonenzymatic process in which an initial hydroperoxide undergoes fragmentation to form HNE (161). However, evidence by Esterbauer's group showed that the formation of HNE from AA is greater in the presence of NADPH-dependent microsomal enzymes (68). In addition, iron ions also promote the formation of HNE by PUFAs.
FIG. 3.
Formation of HNE by arachidonic acid (AA).
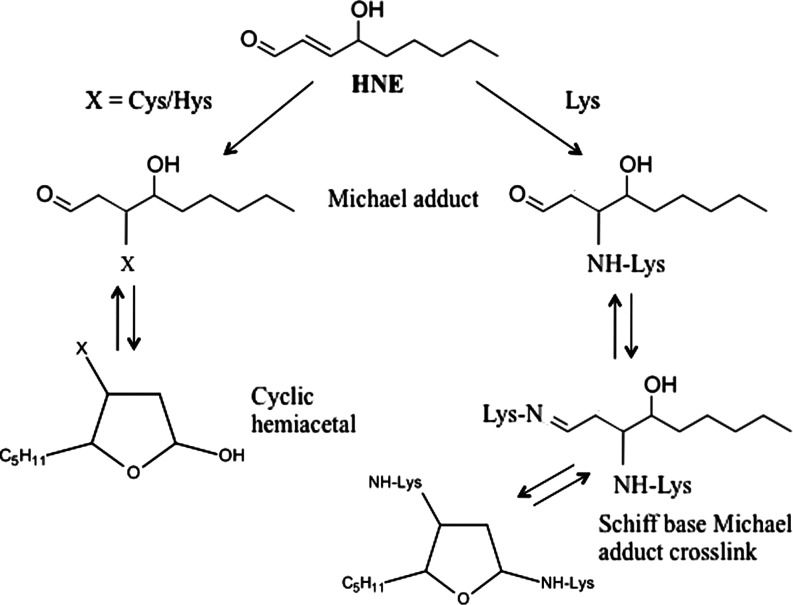
PUFAs undergo free radical–mediated mechanisms by which a lipid peroxyl radical is formed (182). Ultimately, the peroxyl radical is converted to an allylic carbocation via β-scission of the PUFA derivative. The peroxyl radical is further oxidized to a lipid peroxide. A hydration reaction breaks the C–O bond, resulting the a 9-carbon alkenal HNE. Once formed, HNE can covalently attach to proteins by Michael addition to Cys, His, and Lys residues, which can alter protein structure and cause a loss of protein function and activity (195, 202). HNE is not only a potent electrophile reacting with a variety of nucleophilic compounds, but also may act as a stress signaling molecule (70). HNE can accumulate at concentrations of 10 μM to 5 mM in response to oxidative insults and invokes a wide range of biological activities, including the selective suppression of basal and inducible nuclear factor-kappa B (NF-κB) activity (211). The transcription factor NF-κB can prevent neuronal death in experimental models of neurodegenerative disorders by inducing the expression of antiapoptotic proteins. HNE can disrupt ions homeostasis such as Ca2+, impair Na+/K+ATPase activity, disrupt the microtubule structure, and activate the caspase pathways leading to cell death (70). This reactive alkenal also causes an impairment of glucose transport in cultured rat hippocampal neurons and an alteration of the glutamate transport in rat neocortical synaptosomes (146). Therefore, uncontrolled and/or excessive production of HNE could interfere with normal cellular signaling and lead to development of pathological conditions as occur in several neurodegenerative diseases (129).
Reaction of HNE with proteins: biological consequences
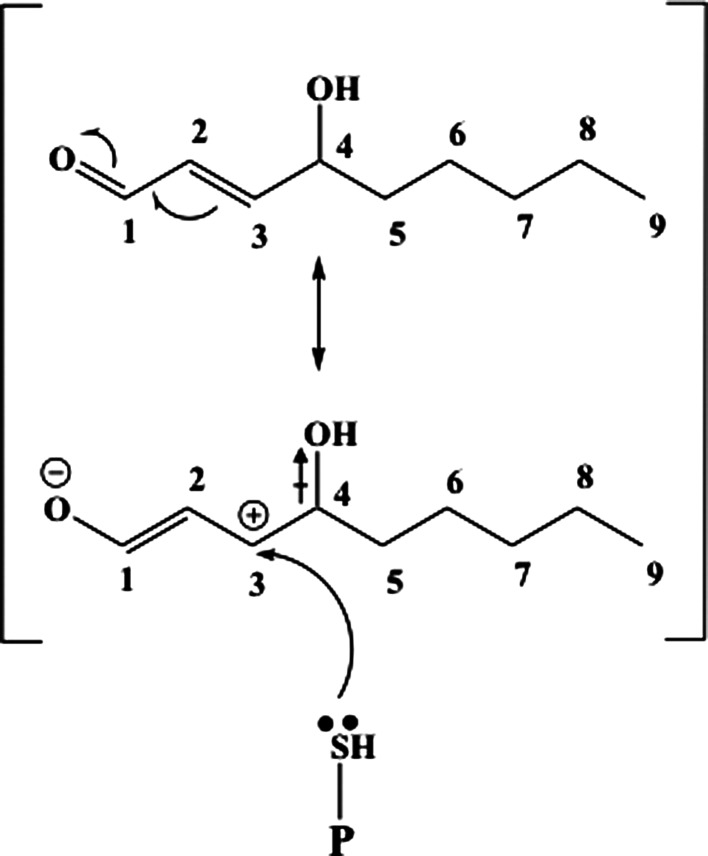
HNE is an amphiphilic compound, with water-soluble, but much stronger lipophilic, properties that make HNE remain associated with the membranes where it is generated; however, HNE can also diffuse to different cellular compartments and interact with many different substrates. The high reactivity of HNE is due to the presence of three functional groups that frequently act synergistically (156). First a conjugated system of a C=C double bond and a CO carbonyl group provide a partial positive charge to carbon 3. This positive charge is further increased by the inductive effect of the hydroxy group at carbon 4 (Fig. 4). Therefore, nucleophilic attack, for example, by thiol or amino groups, occurs primarily at carbon 3 and second at the carbonyl carbon 1 (155). Besides biochemical modifications, biophysical changes of protein and lipid membrane properties also have to be considered. Alteration of membrane fluidity or loss of phospholipid asymmetry occurs in a dose-dependent manner (195). A physiologically relevant concentration of 1 mM HNE was found to cause significant protein conformational changes in synaptosomal membranes, while membrane fluidity increased only at a much higher concentration (50 mM) (195). In addition, HNE was found to modulate phospholipid asymmetry in synaptosomal membranes (46). This latter effect may have resulted from the formation of HNE adduct with the Cys residues of the enzyme flippase, which functions to maintain asymmetry of membrane lipid bilayer (46).
FIG. 4.
The first step of Michael addition. C-3 has electron density moved toward O atom of OH on C-4 due to inductive effect ( ). At the same time, resonance between the double bond between C-3 and C-2 and the aldehyde dumps electron density away from C-3. The net effect is a partially positive C-3 that becomes a target for electrophilic reaction of lone pair of electrons on the S atom of cysteine.
). At the same time, resonance between the double bond between C-3 and C-2 and the aldehyde dumps electron density away from C-3. The net effect is a partially positive C-3 that becomes a target for electrophilic reaction of lone pair of electrons on the S atom of cysteine.
Although proteins are the major targets of HNE reactivity, a great variety of biomolecules has been identified to form covalent adducts with HNE, including lipids, nucleic acids, and also cofactors and vitamins (39).
Proteins are particularly exposed to modification caused by HNE, and it is estimated that 1%–8% of the HNE formed in cells will modify proteins (101, 119). It is well established that HNE forms adducts with three different side chains in proteins, namely Cys, His, and Lys (Fig. 5). It can interact with thiol (SH) and amino (NH2) groups of these amino acids via Michael addition resulting in a covalent, bond between HNE and the amino acid. The differential reactivity of these residues compared with those of Arg and Glu for adduct formation showed that Cys residues displayed the highest reactivity, with Cys>His>Lys. This order does not imply that Cys residues are always the preferential targets of HNE in proteins. The tertiary structure of the protein determines the accessibility, and therefore reactivity, of an amino acid residue towards exogenous chemicals. No reactions of HNE were detected with Glu (60). Numerous proteins have been shown to be modified by HNE, including plasma membrane ion and nutrient transporters; receptors for growth factors and neurotransmitters; mitochondrial electron transport chain proteins; protein chaperones; proteasomal proteins; and cytoskeletal proteins (152, 155).
FIG. 5.
Michael addition reaction between HNE and Lys, Hys, and Cys.
HNE-induced modification could lead to the alteration of both secondary and tertiary structure of proteins together with altered subunit association. Low levels of HNE modification appear sufficient to increase susceptibility of proteins to proteolysis and removal by the proteasomal system. But, while under normal conditions the proteasomal system is able to remove the majority of oxidatively damaged and modified proteins, under severe oxidative stress conditions accumulation of modified proteins occurs. This could take place either because of protein cross-linking or malfunction of the proteolytic machinery of the cell. Several other factors besides reactivity may determine the adduction site, such as polarity of the microenvironment and accessibility in the tertiary/quaternary structure (156).
Within this frame, reactivity of HNE towards amyloid beta-peptide (Aβ), the major component of senile plaques (SPs), has to be mentioned. Similar to proteins, Aβ possesses a nucleophilic side chain that can react with HNE. HNE covalently modifies Aβ(1–40) via 1,4-conjugate addition and hastens Aβ aggregation and toxicity, likely by altering the oligomers formed, which in turn causes oxidative stress that leads to the formation of even more lipid peroxidation products, such as HNE and more toxic Aβ oligomers (187). Interestingly, in AD brain the major efflux protein for Aβ, LRP-1, is covalently modified by HNE, and the consequent dysfunction of this efflux protein conceivably may contribute to accumulation of this neurotoxic peptide (144).
Cells are endowed with several mechanisms to detoxify HNE and thereby prevent its potentially damaging actions. GSH is the most important defense against HNE, and it has the ability to efficiently bind HNE through its Cys residue. Also glutathione S-transferases (GSTs), participate and contribute to regulate the intracellular level of HNE together with the multidrug resistant protein (MRP-1). The latter catalyzes the export the GSH conjugate of HNE out of neurons. In addition to GSH, the dipeptide carnosine (β-alanyl-L-histidine) can quench HNE via intramolecular Michael addition (84). Proteins present in high amounts within or outside of cells may also play important roles in binding and thereby quenching HNE; examples include albumin (3) and apolipoprotein.
Lipid Peroxidation and Neurodegenerative Disorders: A Redox Proteomics Overview
The involvement of lipid peroxidation in neurodegenerative processes is widespread (32, 168). The number of people expected to suffer brain disorders—mainly AD and PD—is rapidly increasing as the worldwide population ages. Oxidative stress occurs in specific brain regions affected in the respective neurodegenerative disorders (130), and membrane lipid peroxidation and HNE are believed to contribute to neuronal dysfunction and death. However, the evidence that lipid peroxidation is a crucial factor is strongest for AD, while more detailed studies are lacking for PD, HD, and ALS. Proteomics analysis is a powerful tool for the identification of putative biomarkers of neurodegenerative diseases, including proteins on which HNE adduction has occurred (32, 168). These modifications yield a loss or reduction of enzyme activity and alterations in protein structure. By using proteomics to investigate these protein changes, several molecular mechanisms involved in degeneration of neuronal cells have been elucidated and likely will contribute to the establishment of prospective biomarkers to be used in clinical diagnosis.
The present review summarizes current knowledge on the role of HNE-induced protein modification and its effects on protein function. However, most of the studies have been performed for AD, while intriguing data are still lacking for many other neurodegenerative diseases. Further studies are needed to give a more detailed picture of the effects of lipid peroxidation in the pathogenesis of neuronal damage and to possibly establish a link between protein oxidation/dysfunction and neuronal death.
Redox proteomics
Proteomics involves the systematic study and identification of the entire set of proteins that are expressed within the cell. The term proteomics was first coined as an analogy to genomics in 1995 (209), but proteomics is much more complicated than genomics. While the genome is a relatively constant entity, the proteome continuously varies in response to both endogenous and exogenous stimuli. Protein expression is expected to be radically different in different cell types and in different stages of development. These differences become particularly intriguing when they are associated with a disease process. Thus, expression of specific proteins is often altered in disease conditions, and proteomics analysis is a powerful tool to help decipher biological processes and phenotypes of both normal and diseased cells.
Redox proteomics, arguably pioneered in the Butterfield laboratory (44, 50), is the branch of proteomics that allows the identification of oxidatively modified proteins, most often by coupling two-dimensional polyacrylamide gel electrophoresis, Western blot analyses, and mass spectrometry (MS) (50). The most common and abundant types of oxidative modifications to proteins are protein carbonylation, 3-nitrotyrosine formation, binding of HNE, and glutathionylation. Redox proteomics can be applied to study all the previously mentioned modifications, and several studies have been performed in our laboratory by following this approach. However, other non–gel-based approaches that utilize liquid or affinity chromatography in combination with MS have also been developed for these modifications by other groups (50).
In the following section, the most relevant results on HNE-modified proteins in neurodegenerative diseases are reported. By comparing results obtained in different forms of neurodegeneration, it is reasonable to hypothesize that HNE modification may be not a random event but affects specific proteins, which, in turn, display altered functions. The biological effects of such modifications are also discussed.
Amyotrophic Lateral Sclerosis
ALS is a progressive neurodegenerative disorder that affects motor neurons of the cerebral cortex, brainstem, and the anterior horn of the spinal cord (49). Clinical manifestations of the disease are muscle weakness, wasting, spasticity, and weight loss. Death usually occurs within 2–5 years after the onset of symptoms. While the majority of cases are sporadic (sALS), about 5%–10% of patients have a positive family history (fALS) with genetic mutations of Cu/Zn superoxide dismutase (SOD1), an enzyme that is part of the cellular antioxidant defense systems. Among the proposed hypothesis of motor neuron degeneration in ALS, accumulating data suggest a role for oxidative stress–mediated cell injury and excitotoxicity. Considering that glutamate excitotoxicity is associated with increased oxidative stress and that failure of antioxidant defense systems can lead to excitotoxicity, a vicious cycle is initiated, thus suggesting common final pathways to motor neuron degeneration. Over 100 SOD1 mutations (7) have been identified in fALS patients, most of which result from substitution of one single amino acid, such as SOD1G85R, SOD1G37R, and SOD1G93A. Interestingly, it became clear that SOD1-mediated toxicity in ALS is due to a “gain” of toxic properties that are independent of the levels of SOD1 activity (73). mSOD1 proteins are or become misfolded and consequently oligomerize to form intracellular aggregates (49, 203), which also embody other essential proteins that once adducted cannot perform their proper function. In addition, toxicity is also caused by aberrant chemistry of the active Cu/Zn sites of the misfolded enzyme (20), which contributes to further exacerbate oxidative stress conditions by increasing the levels of reactive oxygen species (ROS) within the cell (20, 115). This latter mechanism participates in misfolding of SOD1 (165). Elevated levels of ROS and the formation of insoluble protein complexes of mSOD1 protein have been shown in spinal cords of the G93A transgenic mice and precede motor neuron degeneration (116, 117). Reasonably, protein aggregation and oxidative stress simultaneously occur, and this is consistent with the finding that SOD1 itself is oxidatively modified in the G93A-SOD1 transgenic mice (158).
Interestingly, recent studies showed that treatment with hydrogen peroxide or another oxidant induced wtSOD1 misfolding (24), which resulted in the toxic gain of functions similar to mSOD1. Following insult with an oxidizing agent wtSOD1, dimer conformation is altered, Cu/Zn ions are scattered, and finally dissociation into monomeric units occurs. Misfolded wtSOD1 displayed many properties that were thought to be characteristic of the mutant protein such as ubiquitinylation, association with chaperones, insolubility, and formation of protein aggregates. In addition, misfolded/oxidized wtSOD1 may be released outside the cell where it can induce other molecular pathways that lead to motor neurons degeneration, as it does mSOD1 (24). All these data support the view that oxidative damage, including mSOD itself, plays a central role in the pathogenesis and progression of ALS. Multiple pathological studies have reported evidence of increased oxidative stress in ALS post mortem tissue compared to control samples (17). Elevated protein carbonyl levels have been shown in the spinal cord (183) and motor cortex (72) from sALS cases, and increased 3-nitrotyrosine levels were found within spinal cord motor neurons in both SOD1–fALS and fALS patients (1). Markers for lipid peroxidation were detected in spinal cord from sALS patients but were absent in control spinal cords (184). Additionally, elevated levels of HNE (190) were observed in CSF samples from ALS patients. Transgenic mouse and cell culture models of ALS showed a similar pattern of oxidation, which was confirmed by the findings of increased levels of oxidative damage to protein, lipid, and DNA observed in the human disease (8, 72, 73, 117).
Redox proteomics studies from our laboratory identified proteins that were significantly modified by HNE in the spinal cord tissue of a model of fALS, G93A-SOD1 transgenic mice, including collapsin response mediator protein-2 (CRMP-2), heat shock protein (HSP)70, and possibly α-enolase (Table 1) (148). CRMP-2 is a member of the dihydropyrimidinase-related protein (DRP) gene family involved in axonal outgrowth and pathfinding through the transmission and modulation of extracellular signals (82). CRMP-2 colocalizes in the neurofibrillary tangles (NFTs) of AD human brain, suggesting that CRMP-2 plays a role in neuronal degeneration (214). CRMP-2 displays repair activity in the brain, and CRMP-2 oxidation may be responsible for loss of such function. The finding of increased level of HNE-modified CRMP-2 in G93A-SOD1 transgenic mice could provide a link between oxidation-mediated loss in protein function and neuritic regeneration and plasticity known to be altered in ALS (87).
Table 1.
HNE-Modified Proteins Identified in the Spinal Cord of G93A-SOD1 Tg Mice As a Model of Amyotrophic Lateral Sclerosis
| Protein | Function |
|---|---|
| ATP synthase | Energy metabolism/mitochondrial function |
| Collapsin response mediator protein-2 | Neuronal communication |
| α-Enolase | Energy metabolism |
HSP response is activated under stress condition (157), and SOD1 is aggregated with HSP70, HSP40, and α-crystallin in transfected cells (186). HSPs comprise a family of chaperone proteins that assist correct folding and transport of newly synthesized proteins (41). SOD1-immunoreactive inclusions in spinal cord of ALS patients and transgenic mice were found to be positive after staining with antibody against heat-shock cognate HSC70 (207). Moreover, overexpression of Hsp70 played a defensive role against protein aggregation and protected cell viability of G93A-SOD1 overexpressed motor neurons (29). Drug candidates with the ability to induce the heat shock response are currently investigated as potential anti-ALS therapy (105), supporting the role of HSP70 in the folding of SOD1 and prevention of aggregate formation. We suggested that diminished degradation of mSOD1 is possibly due to inactivation of HSP70, which is impaired by covalent binding by HNE. These data demonstrated that lipid peroxidation plays a crucial role in the pathophysiology of motor neuron disease, in particular in the context of mutations of SOD1.
Alzheimer Disease
AD, the most common form of dementia in the elderly, is characterized by the degeneration of neurons in brain regions involved in cognition (hippocampus, entorhinal and frontal cortex) and emotional behaviors (amygdala, prefrontal cortex, hypothalamus) (162). A definitive diagnosis of AD can only be made by post mortem brain evaluation and includes the detection of two major hallmarks: extracellular SPs and intracellular NFTs. SPs consist mostly of Aβ, a 40–42 amino acid peptide that derives from the proteolysis of the amyloid precursor protein (APP) catalyzed by beta- and gamma-secretases. NFTs are composed of hyperphosphorylated tau protein (64, 83). Synapse loss is also a characteristic feature of the disease with memory loss and cognitive decline.
AD has at least three stages: mild cognitive impairment (MCI), early-stage Alzheimer disease (EAD), and late-stage Alzheimer disease (LAD). Some patients show a presymptomatic phase before MCI, namely preclinical Alzheimer disease (PCAD). Due to the progressive nature of AD, patients are usually diagnosed on the basis of the severity of symptoms during the transition into each progressing stage. Braak staging (scoring) characterizes the severity of this disease based on the distribution of NFTs and neurophil threads; there are six levels of Braak staging (the higher the stage, the greater the severity of AD).
The identification of PCAD is a very recent finding and describes individuals who do not show memory deficits but have pronounced AD neuropathology (208). Braak scores are III or higher based on post mortem NFT levels (208). Due to current difficulties in collecting PCAD samples, experimental data on these subjects are still poor and will be available in the near future. However, our laboratory has described proteomics changes in PCAD brain (4) and in the transition from PCAD to amnestic MCI, including identification of proteins with elevated protein carbonylation that conceivably may be involved in memory loss of amnestic MCI compared with PCAD (5).
More detailed data are available for MCI, which is considered a prodromal phase of AD. Though MCI is considered the first clinical stage of AD, not all AD patients are diagnosed with MCI. Criteria for MCI include (a) no dementia, (b) slight cognitive deficit corroborated by a second person, and (c) undisturbed activities of daily living. MCI patients can be classified as having amnestic (memory-affecting) MCI or nonamnestic MCI (65, 153). Braak staging for MCI is typically III or IV, as NFTs are beginning to form in the hippocampus and neocortex. Pathologically, MCI brain shows mild degradation of the hippocampus, entorhinal cortex, sulci, and gyri as evaluated by using magnetic resonance imaging (MRI) technology (54), while early and late stage AD patients have considerably greater degradation in these areas (61). It is estimated that the rate of conversion of amnestic MCI to AD is roughly 10%–15% per year (173); however, in some cases individuals with MCI can revert to normal (153).
EAD, an intermediate stage between MCI and LAD, is characterized by increased frontal lobe atrophy, ventricular widening with progressive brain deterioration. Braak scores for this stage typically are between IV and V. As expected, EAD pathology shows decreased hippocampal volume that correlates with memory decline. There is a significant increase in the NFT load in EAD subjects compared with MCI subjects in both the frontal and the temporal lobes (126), which correlates with impairments in verbal abilities, visuo-spatial functions, and attention and executive functions. Similar to PCAD, EAD sample availability is very rare and therefore experimental evidence is still limited.
LAD is the final stage of the disease, and such patients show consistent symptom severity including memory loss, dementia, behavioral changes, and significant impairment of activities of daily living. Braak staging for these patients is approximately IV–VI, as NFTs have caused substantial neuronal death throughout the brain regions responsible for memory and learning, the hippocampus, and neocortex. Accordingly, synapse loss, Aβ accumulation, and SPs are profound (179). Markers of oxidative stress including DNA oxidation, protein oxidation, and lipid peroxidation are significantly higher in these patients. In contrast, levels of the antioxidant enzymes such as catalase, superoxide dismutase, GST, and GSH reductase are significantly decreased in LAD subjects.
The increased levels of lipid peroxidation throughout the progression of this dementing disorder highlights the great importance of this phenomenon in AD pathogenesis and progression.
Protein-bound HNE: from MCI to late stage AD
Lipid peroxidation is an early event in MCI as indexed by increased levels of HNE and acrolein (38, 103, 210). Elevated levels of F2-IsoPs and F4-NPs have also been observed in the frontal, parietal, and occipital lobes, which could contribute to the slight memory deficits associated with this stage of the disease (159). An important role for HNE in AD is suggested by data from human patients and animal and cell culture models. Biochemical and immunohistochemical analysis of brain tissue samples from AD patients and age-matched neurologically normal control subjects have documented elevated levels of HNE in association with Aβ plaques and NFTs (125). Moreover, HNE levels are increased in the cerebrospinal fluid in AD demonstrating a robust ongoing process and also suggesting HNE as a potential biomarker of the disease (120). Both HNE and acrolein were found to be increased in a disease progression-dependent manner with statistically significant elevations in MCI and EAD (210).
Redox proteomics studies from our laboratory allowed the identification of HNE-modified proteins in MCI, EAD, and LAD, while redox proteomics data on identification of HNE-modified proteins are not yet available for PCAD. Two brain regions were investigated for MCI and late AD; namely, the inferior parietal lobule (IPL) and hippocampus (HP). Studies for EAD were performed only on IPL. The principal aims of our investigation were to establish a link between lipid peroxidation and both pathogenesis and progression of AD and to better understand the molecular mechanisms involved in degeneration of neuronal cells.
By following this approach, this section of the current review is designed to offer the reader the insight on the role of HNE modification in AD by the detailed analysis of the proteins identified in the three different stages of the disease. First, proteins oxidized in common in the three stages of the disease (MCI, EAD, and LAD) will be discussed; second, proteins at least in common for two stages (EAD and late AD); and ultimately proteins modified only in one of the three stages. Based on our findings, we suggest that MCI could represent the most “deregulated” stage; i.e., a condition in which the patient is at high risk to develop AD due to dysfunction of multiple proteins. However, other factors have to be involved in driving the progression to AD. Our data are also supported by studies from Moreira et al. (132), who suggested that oxidative damage is most pronounced early in AD.
As shown in Table 2 two proteins were found to be HNE-modified in the three stages of AD: ATP synthase and α-enolase. In addition, SOD2 and CRMP-2 were found to be HNE-modified in both EAD and late AD.
Table 2.
HNE-Modified Proteins Identified in Human Hippocampus and Inferior Parietal Lobule in Mild Cognitive Impairment, Early Alzheimer Disease, and Late-Stage Alzheimer Disease
| AD Stage | HNE-modified proteins in HP | Function | HNE-modified proteins in IPL | Function |
|---|---|---|---|---|
| MCI | Nh3 | Neuronal communication | β-action | Cytoskeletal integrity |
| CRI | Antioxidant defence | PK | Energy metabolism | |
| LDH | Energy metabolism | ATP synthasea | Energy metabolism/mitochondrial functiona | |
| PGK1 | Energy metabolism | eIFα | Protein synthesis | |
| HSP70 | Stress response | EF-Tu | Protein synthesis | |
| ATP synthasea | Energy metabolism/mitochondrial functiona | |||
| α-enolasea | Energy metabolisma | |||
| EAD | N.A. | SOD2 | Antioxidant defense | |
| α-enolasea | Energy metabolisma | |||
| ATP synthasea | Energy metabolism/mitochondrial functiona | |||
| CRMP-2b | Neuronal communicationb | |||
| TPI | Energy metabolism | |||
| MDH | Energy metabolism | |||
| LAD | Aconitase | Energy metabolism | ATP synthasea | Energy metabolisma |
| ALDOI | Energy metabolism | GS | Excitotoxicity | |
| Prx VI | Antioxidant defence | SOD1 | Antioxidant defense | |
| α-Tubulin | Cytoskeletal integrity | CRMP-2b | Neuronal communicationb | |
| α-enolasea | Energy metabolisma |
Proteins oxidized in all the three stages of AD.
Proteins found to be oxidized in both EAD and LAD (150, 167, 169). AD, Alzheimer disease; EAD, early Alzheimer disease; GS, glutamine synthase; HP, hippocampus; IPL, inferior parietal lobule; LAD, late-stage Alzheimer disease; MCI, mild cognitive impairment; MDH, malate dehydrogenase; NA, not available.
Energy dysfunction: ATP synthase and α-enolase
Although the brain is only 5% in weight compared with the rest of the body, it consumes 30% of total oxygen and depends almost exclusively on glucose metabolism for efficient ATP production. Glucose is the primary source of energy, and glucose metabolism is essential for proper brain function; a minimum interruption of glucose metabolism causes brain dysfunction and memory loss (91, 131). Positron emission tomography scanning shows a consistent pattern of reduced cerebral glucose utilization in AD brain (94, 166). Decreased ATP production could lead eventually to cellular impairment. Using redox proteomics we identified two crucial proteins essential for maintenance of ATP homeostasis: ATP synthase and α-enolase.
ATP synthase is a mitochondrial regulating subunit of complex V that plays a key role in energy production. ATP synthase goes through a sequence of coordinated conformational changes in its major subunits (α, β) to produce ATP. The oxidation of ATP synthase leads to the inactivation of this mitochondrial complex and is consistent with decreased enzymatic activity (197). Failure of ATP synthase could contribute to decreased activity of the entire electron transport chain (ETC) and impaired ATP production, resulting in possible electron leakage from their carrier molecules to generate ROS, suggesting an alternative rationalization for the well-documented existence of oxidative stress in AD (200). Altered expression of mitochondrial proteins, functional deficits, and lowered activity in various complexes of the ETC are seen in AD (12). These changes, coupled with the changes in complexes I, III, and IV, may cause electron leakage from the mitochondria to produce ROS.
α-Enolase catalyzes the conversion of 2-phosphoenolpyruvate to phosphoenolpyruvate in glycolysis, a 10-step process in which one molecule of glucose is converted to two molecules of ATP in the cytoplasm. α-Enolase demonstrates increased oxidation in AD and models of AD (36). HNE modification of this protein leads to lowered enzymatic activity, which has been reported in MCI (167), EAD (169), and AD (150). ATP is extremely important at nerve terminals for normal neural communication and decreased levels may be involved in loss of synapses and synaptic function. Both consequences can affect the propagation of action potentials, which may ultimately contribute to memory loss, a characteristic feature of AD pathology. Although the main function of enolase is its role in glycolysis, enolase can regulate by plasminogen (leading to plasmin that can degrade Aβ) and activate the MEK/ERK pro-survival pathway (36). Oxidative modification and dysfunction of α-enolase would disrupt neuronal energy metabolism and ion homeostasis, thereby impairing the function of membrane ion-motive ATPases and glucose and glutamate transporters and causing loss of membrane asymmetry and signal transduction, and could lead to diminution of both degradation of Aβ and activation of prosurvival pathways. Such oxidative and metabolic compromise may thereby render neurons vulnerable to excitotoxicity and apoptosis.
Interestingly, two other proteins were found to be significantly HNE-modified both in EAD and LAD: MnSOD (SOD2) and CRMP-2.
SOD catalyzes the conversion of superoxide anion and two protons to hydrogen peroxide and oxygen. Maintenance of this enzyme is critical to achieving oxidative balance; otherwise, the cell would be in a continual state of oxidative stress. There are four distinct forms of SOD, including Cu/ZnSOD (SOD1), MnSOD (SOD2), NiSOD, and FeSOD. SOD2 is located primarily in the mitochondria. Based on its location, oxidative impairment of mitochondrial resident SOD2 is likely a contributing factor to the mitochondrial dysfunction associated with AD. The activity of SOD2 is significantly reduced in EAD brain (169) and CSF compared with age-matched controls, which is consistent with the concept of mitochondrial dysfunction being a factor in the progression of AD. SOD2 was also found to be nitrated and subsequently inactivated in mice by peroxynitrite (6, 53, 75, 123). Overexpression of SOD2 increases Aβ degradation while partial deficiency promotes Aβ deposition, thereby likely contributing to cognitive decline observed in a transgenic mouse model of AD (63).
CRMP-2 has been reported to be HNE-modified in the spinal cord of ALS transgenic mice. As already discussed, CRMP-2 is a crucial adaptor protein that regulates cytoskeletal dynamics, microtubule organization, axonal transport, and other critical functions. CRMP-2 binds and stabilizes tubulin at the end of microtubules, thus promoting axon extension (82). Thus, overexpressing CRMP-2 in neuronal cells causes an increase of neurite length and can result in supernumerary axons (11, 97). CRMP-2 itself has no known enzymatic activity but interacts with several binding partners to affect microtubule dynamics, neurite outgrowth, neural differentiation, kinesin-dependent axonal transport, Ca2+ homeostasis, and neurotransmitter release, and other essential neurophysiology still being elucidated (93).
Although plaques and tangles are histological hallmarks of AD pathology, synapse loss is significantly correlated to clinical features of dementia (10). Synapse loss is evident even in MCI (181), in which loss of synapses generally correlates with local NFT density. Interestingly, CRMP-2 was found to be hyperphosphorylated in the AD brain by the same CDK5 and GSK3β kinases responsible for pathological tau phosphorylation (113). In fact, CRMP-2 phosphorylation in AD is so pronounced that it has been used as a marker of NFTs. Based on the role of CRMP-2 in maintaining neurite cytoskeletal integrity, it is likely that Aβ and tau pathways might converge to cause synapse loss through a CRMP-2–dependent mechanism (93). This hypothesis reasonably correlates with the three neuropathologic features of AD: Aβ, NFTs, and synapse loss. The processes responsible for co-aggregation of CRMP-2 with its binding partners within NFTs may be related to the redox status of the cell stress because oxidative stress is a well-known factor that can initiate/propagate protein aggregation phenomena through covalent cross-linkage and hydrophobic protein–protein interactions (93). Consistent with such a possibility, our studies showed that CRMP-2 is HNE-modified in both EAD and LAD, thus suggesting that its impairment has a crucial pathogenic role.
Although the following proteins have not been found to be specifically modified in each stage of the disease, it is possible to outline at least an altered “function” in common as a consequence of specific protein oxidation. In fact, we identified phosphoglycerate kinase (PGK)1, pyruvate kinase (PK), and lactate dehydrogenase (LDH) as HNE-modified in MCI, trioso phosphate isomerase (TPI) in EAD, and aldolase (ALDO1) in LAD. These proteins are all members of the glycolytic pathway, thus supporting a general trend of glycolysis impairment in the three stages of the disease.
PGK catalyzes the dephosphorylation of 1,3-bisphosphoglycerate to 3-phosphoglycerate. This reaction undergoes substrate level phosphorylation by phosphoryl transfer from 1,3-bisphosphoglycerate to ADP to produce one molecule of ATP. Impairment of this glycolytic enzyme results in decreased energy production and irreversible downstream effects, such as multidrug resistance (62). This result could conceivably be related to the identification of MRP-1 as a protein with elevated HNE binding in AD (198).
PK catalyzes the final step in glycolysis, the conversion of phosphoenolpyruvate to pyruvate, with the concomitant transfer of the high-energy phosphate group from phosphoenolpyruvate to ADP, thereby generating ATP. Under aerobic conditions, pyruvate can be transported to the mitochondria, where it enters the tricarboxylic acid (TCA) cycle and is further metabolized to produce considerably more ATP through oxidative phosphorylation. Anaerobically, pyruvate can be reduced to lactate in mitochondria-deficient cells or under hypoxic conditions, such as those found in active muscle tissues or brain ischemia. Additionally, enzymatic activity is reduced, thus suggesting that oxidative modification leads to impairment of protein function (167).
LDH anaerobically reduces pyruvate to lactate through lactic acid fermentation using NADH as a cofactor. The NAD+ generated in this process is used in glycolysis to oxidize glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate. Lactate is a substrate for gluconeogenesis and given that glucose is the major supplier of energy to the brain, proper lactate production is crucial (104). LDH enzymatic activity is significantly reduced in MCI hippocampus (167), which provides supplemental evidence for the correlation between protein oxidative modification by HNE and enzyme activity impairment. Dysfunction of this enzyme could yield excess pyruvate and a reduction in the production of glucose.
ALDO1 cleaves fructose 1,6-bisphosphate and produces the two glycolytic intermediates, glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. Fructose 1,6-bisphosphate is neuroprotective and preserves GSH in cortical neurons during oxidative stress conditions (206). ALDO1 catalyzes a critical step as it generates two substrates that are used to eventually produce two molecules of ATP and more in the TCA cycle and ETC. Consequently, HNE modification results in decreased energy metabolism. Levels of ALDO1 are significantly decreased in AD hippocampus (22) and PD (81). Enzyme activity is reduced (22) and impairment can cause increased levels of fructose 1,6-bisphosphate, inhibition of complete glycolysis, and ATP depletion.
Similar comments could also be extended to TCA cycle proteins because we identified malate dehydrogenase (MDH) in EAD and aconitase in LAD to be HNE-modified.
MDH catalyzes the reversible oxidation of malate to oxaloacetate by NAD+ in the TCA cycle. MDH links glycolysis to the electron transport chain by transferring NADH to NADH dehydrogenase (Complex I) through the malate-aspartate shuttle resulting in the production of ATP. In contrast to elevated HNE binding to MDH, MDH levels were increased in AD patients, but the level of protein oxidation of MDH was not significant, which possibly reflects a compensatory mechanism in response to oxidative stress (107). Activity of MDH increases during aging (30, 141), which further supports the hypothesis of mitochondrial dysfunction in AD.
Aconitase catalyzes the isomerization of citrate to isocitrate in the TCA cycle. As an iron-sulfur protein, its Fe-S cluster participates in the hydration–dehydration reaction that occurs. The three cysteine residues in the Fe-S core can undergo Michael addition and form acrolein-, HNE-, and MDA-conjugated adducts, thereby increasing lipid peroxidation markers (114, 188). Enzymatic activity of this enzyme is significantly reduced in AD, consonant with protein dysfunction (150). The TCA cycle takes place in the mitochondria; therefore, aconitase impairment results in mitochondrial dysfunction, a common theme of neurodegenerative diseases (178, 212). As noted above, decreased ATP production can lead to voltage-gated channel and ion-motive pump disruption as well as synapse loss, an early event in Alzheimer's disease pathology (180).
The proteins we identified to be HNE-modified exclusively in MCI included actin, carbonyl reductase (CR1), elongation factor Tu (EF-Tu), initiation factor 1 alpha (eIF-α), neuropolypeptide h3 (Nh3), and HSP70.
Actin is a key protein that plays a central role in maintaining structural integrity, cell morphology, and structure of the plasma membrane (18). In the central nervous system, actin is distributed widely in neurons, astrocytes, and blood vessels. It is particularly concentrated in growth cones, dendritic spines, and presynaptic terminals. HNE conjugation of actin can lead to loss of membrane cytoskeletal structure, decreased membrane fluidity, and trafficking of synaptic proteins and mitochondria. Moreover, actin is involved in the elongation of the growth cone, and loss of function of actin could play a role in the loss of synapses and neuronal communication documented in AD (128, 180).
CR1 is an enzyme that reduces carbonyl-containing compounds to their resultant alcohols, thereby reducing the level of protein carbonyls. Subsequent malfunction or down-regulation of this enzyme would be consistent with increased protein carbonyls in AD (92) and amnestic MCI (167), which, because of the polarity of the carbonyl moiety, could expose normally buried hydrophobic amino acids to the protein surface resulting in a disruption of conformation. CR has been shown to reduce the levels of HNE (59, 142). CR expression is altered in DS and AD subjects (15). This enzyme was found to be modified in persons with corticobasal degeneration, a neurological disorder whose symptoms closely mirror that of PD (48). The gene for CR is located in close proximity to the gene for the antioxidant enzyme, SOD1 (112); these genes, in addition to APP, are located on chromosome 21, which is trisomic in DS patients (77, 106). A potential link between DS and AD by irregular meiotic recombination in chromosome 21 (154) has been postulated. Current research supports a possible relationship among CR, DS, and Aβ in neurodegeneration.
EF-Tu and eIF-α are intimately involved in protein synthesis machinery. Human mitochondrial EF-Tu is a nuclear-encoded protein that functions in the translational apparatus of mitochondria. Mammalian EF-Tu acts as a GTPase by hydrolyzing one molecule of GTP for each A site amino-acylated tRNA of the ribosome. As discussed before, mitochondria play pivotal roles in eukaryotic cells in producing cellular energy and essential metabolites as well as in controlling apoptosis by integrating various death signals (143). Mitochondrial protein synthesis inhibition, either by deleting mtDNA or by blocking translation in the organelle, is associated with the impairment of differentiation in different cell types, including neurons (205). Loss of neuronal differentiation can lead to an incomplete development of the neuron, which would result in reduced neurotransmission.
eIF-α, which binds aminoacyl-tRNA to acceptor sites of ribosomes in a GTP-dependent manner (151), is involved in cytoskeletal organization by bundling and binding actin filaments and microtubules. The expression level of eIF-α is regulated in aging, transformation, and growth arrest. Because of eIF-α regulation in differing states of cell life and its key position in protein synthesis and cytoskeletal organization, this protein is an important determinant of cell proliferation and senescence (201). Inhibition of eIF-α promotes apoptosis (27, 151), indicating that eIF-α activity is critical to normal cell function.
Taken together, increased levels of HNE-bound eIF-α and EF-Tu suggest an impairment of protein synthesis machinery, either in cytosol or mitochondria, associated with an impairment of the rate and specificity of ribosome functions. Numerous studies have provided indirect evidence that suggests alterations in protein synthesis may occur in AD (58, 74, 176). The dysfunction of the protein synthesis apparatus, mediated in part by redox proteomics identified oxidatively dysfunctional EF-Tu and eIF-α, secondary to oxidative stress–induced lipid peroxidation and formation of HNE, could compromise the ability of brain cells to generate the countless factors needed to regulate cell homeostasis, thus contributing to impaired neuronal function and to the development of neuropathology in AD.
Nh3 is critical for modulation of choline acetyltransferase, an enzyme essential in the synthesis of acetylcholine. The loss of choline acetyltransferase leads to reduced levels of acetylcholine causing poor neurotransmission (51, 140). N-methyl D-aspartic acid (NMDA) receptors activate the production of this enzyme, and modulation of the NMDA receptor mediates cholinergic deficits (99). AD patients have considerable cholinergic deficits, consistent with dysregulation of acetylcholine levels and loss of cholinergic neurons (51, 52, 172). The oxidative modification of this protein further supports the involvement of cholinergic neurons in AD, an early hypothesis of this disorder (78). Nh3 undergoes HNE modification in MCI hippocampus and nitration in LAD (33). Nh3 has several other names, including phosphatidylethanolamine binding protein (PEBP), hippocampal cholinergic neurostimulating peptide, and Raf kinase inhibitor protein. As a phosphatidylethanolamine binding protein, PEBP may be important in phospholipid asymmetry. Apoptosis is initiated when phosphatidylserine resides on the outer leaflet of the membrane. Loss of function and changes in conformation of PEBP conceivably could lead to loss of phospholipid asymmetry, a signal for neuronal apoptosis, which further supports the role of PEBP as a parapoptosis inhibitor (191). HNE modification of PEBP may impact lipid asymmetry as loss of activity is observed in AD and MCI and mouse models of familial AD (13, 14, 80) and can potentially disrupt cellular homeostasis. PEBP levels are decreased in AD, which promotes amyloid beta accumulation in the Tg2576 transgenic mouse model of AD (80). RAF kinases are serine/threonine protein kinases involved in cell signaling in the mitogen activated protein cascade and NF-κB. As demonstrated by the various functions through its numerous monikers, Nh3 is a highly important protein and oxidative modification likely is detrimental to neurons.
As discussed previously, HSPs serve as molecular chaperones and help guide damaged proteins to the proteasome. HSPs vary in size and are named according to their molecular weight. HSP70 has been reported as being oxidized in AD by using redox proteomics (45). Several other HSPs have been found to be oxidatively modified in AD (45) and HD (67), including HSP90 and HSP60, whereas other heat shock proteins, HSP27 and HSP32, are induced in amnestic MCI (57). Impairment of this protein may exacerbate protein misfolding and aggregation and eventual proteasomal overload and dysfunction known to occur in AD (102). Aβ-treated synaptosomes show that HPSs are oxidatively modified (25), further illustrating the importance of Aβ in inducing lipid peroxidation in AD brain (110) and the importance of proper functioning of HSPs in the cell.
Redox proteomics-identified proteins that are HNE-modified exclusively in LAD among the various stages of AD include α-tubulin, glutamine synthase (GS), and peroxiredoxin VI (Prx VI) (150).
α-Tubulin is an isoform of tubulin that alternates with β-tubulin to form a prominent cytoskeletal structure, the microtubule. Microtubules are used to transport cargo (i.e., vesicles and organelles) from the cell body to the periphery and vice versa. Upon HNE modification, α- tubulin is structurally altered, and microtubules depolymerize (79). Therefore, cargo cannot reach its destination and the cytoskeleton is altered (136, 137). This could contribute to the notion that synaptic domains are the first to be damaged in AD neurons (109).
Peroxiredoxins are a family of antioxidant enzymes that are pivotal in antioxidant defense as already discussed. Prx VI is a 1-Cys peroxiredoxin that plays a role as a second messenger for growth factors and cytokines. Prx VI, a GSH peroxidase that exhibits Ca2+-independent phospholipase A2 activity (177), is cytosolic and is expressed in astrocytes and in neurons at low levels (47). In addition, the decrease in the activity of this enzyme may also lead to decreased phospholipase A2 activity. Phospholipase A2 is a target for regulation by Pin1, which has been reported to be oxidatively modified and down-regulated and to have decreased activity in both AD and MCI brain (37, 196, 197). Prx VI has been found to be protective against mitochondrial dysfunction, a feature that pinpoints its effectiveness as an antioxidant (66). Prx VI also plays important roles in cell differentiation and apoptosis. Consequently, HNE modification may lead to tau hyperphosphorylation and NFT formation, in addition to development of oxidative stress.
GS is an important enzyme in maintaining the glutamate–glutamine cycle. GS converts the acidic amino acid glutamate to the basic amino acid glutamine. Glutamate is taken up from the extracellular fluid of neuronal tissues via glutamate transporters so that levels are upheld. Once GS is conjugated with HNE, it becomes structurally altered and can no longer preserve glutamate levels, resulting in possible excitotoxicity and neurodegeneration (35). In addition, GS levels are significantly decreased in AD brain (92) and contribute to neuronal excitotoxic death.
Down Syndrome
DS is the most common genetic cause of mental retardation and results from trisomy of chromosome 21. DS should be considered a multifactorial disease in which an abnormal expression of trisomic genes arises not only from genetic, but also environmental factors (89). Thus, trisomy also affects disomic genes, which ultimately results in a deregulation of a plethora of functions with a high variability within the DS population (171). Neuropathological features of DS include decreased brain weight and neuronal number, abnormal neuronal differentiation, and structural changes in synapses (16). Most DS patients (aged above 40 years) show progressive cognitive decline, dementia, and neuropathological hallmarks of older AD patients (124). NFTs are detected both in DS and AD brains as is Aβ deposition. There is accumulating evidence that neuronal cell death may be induced by oxidative stress in DS (95). Although oxidative stress has been demonstrated to contribute to the DS phenotype (43, 100, 145, 215), a direct link between the accumulation of oxidative damage and clinical features of DS has not yet been elucidated. Chronic oxidative injury in the brain could represent one of the major risk factors for subsequent neurodegeneration in aged DS patients (95, 138, 193). Overexpression of some of the genes located on chromosome 21 results in increased conditions of oxidative stress. SOD1 is at the top of the list: increased levels of SOD1 leads to overproduction of H2O2, which is in turn a source of other ROS. In addition, overexpression of SOD1 in DS subjects has been found to alter the levels of other antioxidant enzymes, such as catalase and GSH peroxidase, which may induce oxidative damage if dysfunctional. Accordingly, Busciglio and Yankner (31) demonstrated increased production of ROS and elevated levels of lipid peroxidation in neurons of DS fetuses. A proteomics study from Gulesserian et al. (85) showed that oxidative stress in fetal DS did not result from overexpression of SOD1 protein but appeared to be the consequence of low levels of reducing agents and enzymes involved in removal of hydrogen peroxide, such as GSTs and thioredoxin peroxidases.
Included in the list of potential chromosome 21 gene candidates leading to DS, another important player is APP. Its overexpression leads to overproduction of Aβ(1–42), which is proved to induce oxidative stress (34, 42, 98, 160), and post mortem studies on DS brain evidenced accumulation of Aβ(1–42) that correlated with age (90).
Elevated levels of thiobarbituric acid reactive substances were demonstrated in the cortex from DS fetal brain compared with controls (139). Further, increased levels of isoprostane (8,12-iso-iPF2α) have been measured in urine samples from adults with DS (160).
Ishihara et al. (98) reported increased level of ROS and mitochondrial dysfunction in primary cultured astrocytes and neurons from Ts1Cje transgenic mice. The authors also identified by a redox proteomics approach the putative target proteins that were modified by lipid peroxidation-derived products (98). ATP synthase mitochondrial F1 complex b subunit, α-enolase and TPI were identified as proteins modified by 3-hydroperoxy-9Z,11E-octadecadienoic acid (13-HPODE). Neurofilament light polypeptide, internexin neuronal intermediate filament α, neuron specific enolase, Prx VI, PGK1, and TPI were shown to be HNE-modified proteins (Table 3). Thus, dysfunction of these proteins as a consequence of oxidative damage may affect ATP production, the neuronal cytoskeleton system and antioxidant network function. Interestingly, redox proteomics studies from our laboratory identified α-enolase, Prx VI, PGK1, and TPI to be HNE-modified in AD and MCI brain (32, 150, 167), suggesting that these proteins might contribute to cognitive dysfunction and neurodegenerative processes occurring in DS. These findings are consistent with the notion that DS and AD may share common pathways of neurodegeneration, a hypothesis that needs to be further elucidated.
Table 3.
Proteins in Ts1Cje Transgenic Mice (DS Model) Modified by HNE and 3-Hydroperoxy-9Z,11E-Octadecadienoic Acid
| Protein | Function | |
|---|---|---|
| Proteins HNE-bound | Neurofilament light polypeptide | Cytoskeletal integrity |
| Internexin neuronal filament | Cytoskeletal integrity | |
| Prx VI | Antioxidant defense | |
| PGK1 | Energy metabolism | |
| TPI | Energy metabolism | |
| Proteins 13-HPODE-modified | TPI | Energy metabolism |
| α-Enolase | Energy metabolism | |
| ATP synthase | Energy metabolism | |
| Mitochondrial function |
13-HPODE, 3-hydroperoxy-9Z,11E-octadecadienoic acid.
A recent study from our laboratory, investigated the levels of oxidative stress in the amniotic fluid from DS-affected pregnancies compared with healthy controls. Amniotic fluid, similar to CSF for the brain, is in direct contact with the fetus and can be considered a reliable index of the physiological condition of the fetus. Therefore, amniotic fluid could be used for the identification of disease biomarkers to be coupled with current genetic screening. We evaluated a set of oxidative stress biomarkers in DS amniotic and found increased levels of oxidative stress, as indexed by increased protein oxidation, lipid peroxidation, reduction of GSH and Trx levels and induction of the HSP response. By a redox proteomics approach, we identified selective proteins that showed increased oxidation in DS fetuses compared with healthy controls. Our results demonstrated that oxidative stress is an early event in the pathogenesis of DS (147).
Parkinson Disease
PD, the second most common form of dementia in the elderly, is pathologically characterized by a decline in motor function in the form of resting tremors, muscle rigidity, akinesia, and bradykinesia. PD, similar to several other age-related neurodegenerative diseases, is considered a “protein misfolding disease.” In the case of PD, protein aggregates of α-synuclein, a protein that is important for proper mitochondrial function and synaptic vesicle formation (23), are found. These aggregates are the major component of Lewy bodies located primarily in the putamen and substantia nigra. These brain regions are largely involved in learning and motor control. Evidence of the involvement of α-synuclein in neurodegeneration came from studies showing that three independent mutations in this synaptic protein lead to the development of familial PD (175). Additional studies demonstrated that duplications and triplications of the α-synuclein gene also cause an early onset of PD (96). α-Synuclein is a small, unfolded cytoplasmic protein that is highly expressed in the CNS, which has the ability to bind lipids, including lipid membranes (108, 192) and fatty acids (122). This binding activity is thought to be responsible for its neuroprotective role, specifically in response to oxidative stress, and it was also demonstrated to prevent oxidation of membrane PUFAs only in the monomeric, but not fibrillar, form of the protein (217). The first evidence to show the involvement of lipid peroxidation in PD as a cause of nigral cell death was from Dexter and co-workers (55). It is well documented that HNE and MDA adducts accumulate in Lewy bodies in neocortical and brain stem neurons (216). HNE was also found to alter dopamine transport (76). These findings may contribute to the loss of dopamine, which is paramount to the PD pathogenesis. Indeed, under conditions of elevated lipid peroxidation, dopamine, in addition to other catecholamines, is oxidatively converted to the corresponding o-quinone, which may initiate a cascade of spontaneous reactions, including intramolecular cyclization and interaction with molecular targets ultimately resulting in cytotoxic responses and altered cellular functions. Further, dopamine may interact with products of lipid peroxidation, notably glyoxal and other aldehydes, leading for example to tetrahydroisoquinolines via Pictet-Spengler chemistry (135). Dopamine loss coupled with protein aggregation and HNE elevation causes a profound effect on the learning and physical capabilities of persons with PD.
Besides these findings, redox proteomics data on HNE-modified proteins have not been currently undertaken. Further studies will be valuable towards this direction.
Huntington Disease
HD is a progressive neurodegenerative disorder in which mutation in the huntingtin protein (Htt) occurs via multiple CAG (Gln) repeats. The CAG repeat region shows a range of 11–35 repeats in normal individuals, while a repeat number greater than 35 indicates a high probability of developing HD (174). Although the function of the Htt protein is not clearly understood, it has been shown to interact in cell signaling and vesicle transport (88). HD was first described by George Huntington (1872) as chorea, to indicate a movement disorder characterized by involuntary jerky limb movements. Although progressive, this neurological disease takes approximately 10 years to fully manifest into rigidity and bradykinesia. HD is inherited in an autosomal dominant manner, which indicates that each offspring of a parent with HD has a 50% chance of inheriting this devastating disease. As observed in some other neurodegenerative diseases, movement decline is also associated with cognitive decline and psychiatric problems. Neuronal degeneration is evidenced throughout the basal ganglia, thalamus, brain stem, and mostly in striatum and cortex, which demonstrate consistent protein aggregation and cell death. Accordingly, the polyQ expansion can cause a conformational change in the Htt mutant protein leading to intranuclear and intracytoplasmic aggregates, which are a pathological hallmark in the brains of both HD patients and mouse models thereof (19). The mechanisms by which mutant Htt causes neuronal dysfunction and degeneration are not fully understood. It is not unreasonable to propose that the HD mutation may increase ROS, resulting in a primary defect associated with oxidative stress (28). As such, a significant body of evidence from studies in both HD patients and experimental models of HD support a role for oxidative stress and mitochondrial dysfunction in mediating the neuronal degeneration observed in HD brain (19). Increased levels of oxidative damage indices, including protein nitration, lipid peroxidation, DNA oxidation, and exacerbated lipofuscin accumulation, occur in HD. Strong evidence exists for early oxidative stress in HD, coupled with mitochondrial dysfunction, each exacerbating the other and leading to an energy deficit (26). A significant increase in HNE adducts is observed in the striatum and found to be colocalized in Htt inclusions and also MDA has also been found to be elevated in HD brain (28, 111). It is worthy to note that HNE immunoreactivity was colocalized with mtHtt inclusions in the striatal neurons of R6/2 HD mice. Administration of the antioxidant compound nordihydroguaiaretic acid (NDGA) markedly reduced HNE adduct formation in the nuclear inclusions of R6/2 striatal neurons. NDGA also protected cultured neurons against oxidative stress-induced cell death by improving ATP generation and mitochondrial morphology and function (111). As indicated for PD, redox proteomics studies in HD models have thus far focused on protein carbonylation (149). Specific redox proteomics studies for the identification of HNE-modified proteins have not yet been performed, and adding new data for this important issue is desirable.
Concluding Remarks
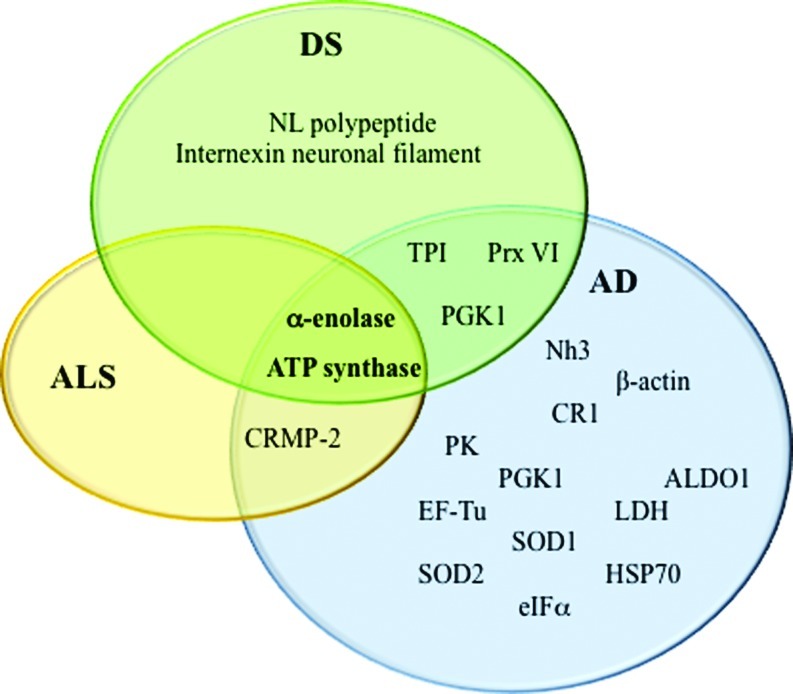
Many studies support a fundamental role of lipid peroxidation in neurodegenerative diseases. HNE is the most abundant by-product of lipid peroxidation, and its toxic properties have been extensively demonstrated for AD, PD, HD, ALS, and DS. The precise mechanisms of HNE neurotoxicity remains to be established. These mechanisms involve a cascade of chain reactions initiated by the covalent interaction with a nucleophilic compound. Among several substrates, proteins are major targets of HNE reactivity resulting in impairment of protein function. In the last decade, redox proteomics studies from our laboratory contributed to better understanding of the biological effects of these complex processes. Identification of specific HNE-modified proteins in the brain of diseased subjects has led to the determination of which selective cellular functions are altered, how they possibly translate into clinical symptoms, and how they relate to pathology of these disorders. By comparing results obtained in different neurodegenerative diseases, it potentially could be possible to identify both similarities and specific differences in addition to better characterize selective neurodegenerative phenomena that involve protein dysfunction. So far, results obtained by following this approach in our laboratory and others suggest that lipid peroxidation is an early event in the pathogenesis of neurodegenerative diseases and that therapeutic strategies towards prevention of lipid peroxidation reactions could be neuroprotective. As show in Figure 6, α-enolase and ATP synthase are the only HNE-modified proteins that overlap AD, DS, and ALS. However, most of the proteins identified in the diseases discussed in this review, though not the same, often share at least the same functions, consistent with the hypothesis that altered energy metabolism, reduced antioxidant defense, and mitochondrial dysfunction are characteristic hallmarks of neurodegenerative disorders and provide support for the physical impairments and progressive memory loss associated with the diseases. Proteomics and redox proteomics in particular have contributed significantly to broaden the knowledge in regard to potential biomarkers for disease diagnosis and may also provide insight into damaged metabolic networks and potential targets for modulation of disease progression.
FIG. 6.
Venn diagram of HNE modified proteins identified in ALS, AD, and Down syndrome (DS). ALDO1, aldolase; CR1, carbonyl reductase 1; CRMP-2, collapsin response mediator protein-2; EF-Tu, elongation factor Tu; eIF-α, initiation factor 1 alpha; HSP70, heat shock protein70; LDH, lactate dehydrogenase; Nh3, neuropolypeptide h3; NL, neurofilament light polypeptide; PGK1, phosphoglycerate kinase 1; PK, pyruvate kinase; Prx VI, peroxiredoxin VI; SOD1, Cu/Zn superoxide dismutase; SOD2, Mn superoxide dismutase; TPI, triose phosphate isomerase. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Abbreviations used
- 13-HPODE
3-hydroperoxy-9Z,11E-octadecadienoic acid
- Aβ
amyloid beta-peptide
- AA
arachidonic acid
- AD
Alzheimer disease
- ALDO1
aldolase
- ALS
amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- CR1
carbonyl reductase 1
- CRMP-2
collapsin response mediator protein-2
- DHA
docosahexanoic acid
- DS
Down syndrome
- EAD
early Alzheimer disease
- EF-Tu
elongation factor Tu
- eIF-α
initiation factor 1 alpha
- ETC
electron transport chain
- F2-IsoP
F2 isoprostane
- F4-NP
F4-neuroprostane
- fALS
familial amyotrophic lateral sclerosis
- GS
glutamine synthase
- GSH
glutathione
- GST
glutathione S-transferase
- HD
Huntington disease
- HNE
4-hydroxy-2-trans-nonenal
- HSP
heat shock protein
- IPL
inferior parietal lobule
- LAD
late-stage Alzheimer disease
- LDH
lactate dehydrogenase
- MCI
mild cognitive impairment
- MDA
malondialdehyde
- MDH
malate dehydrogenase
- MRP-1
multidrug resistance protein-1
- MS
mass spectrometry
- NDGA
nordihydroguaiaretic acid
- NF-κB
nuclear factor-kappa B
- NFT
neurofibrillary tangle
- Nh3
neuropolypeptide h3
- NMDA
N-methyl D-aspartic acid
- O2-·
superoxide
- PCAD
preclinical Alzheimer disease
- PD
Parkinson disease
- PEBP
phosphatidyloethanolamine binding protein
- PGK1
phosphoglycerate kinase1
- PK
pyruvate kinase
- Prx VI
peroxiredoxin VI
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- sALS
sporadic amyotrophic lateral sclerosis
- SOD1
Cu/Zn superoxide dismutase
- SOD2
Mn superoxide dismutase
- SP
senile plaque
- TCA
tricarboxylic acid
- TPI
triose phosphate isomerase
Acknowledgments
This work was supported in part by NIH grants to D.A.B. [AG-05119; AG-029839]. We thank former Ph.D. students and postdoctoral scholars in the Butterfield laboratory for their excellent research cited in this review.
Disclosure Statement
No competing financial interests exist.
References
- 1.Abe K. Pan LH. Watanabe M. Kato T. Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci Lett. 1995;199:152–154. doi: 10.1016/0304-3940(95)12039-7. [DOI] [PubMed] [Google Scholar]
- 2.Adibhatla RM. Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008;41:560–567. doi: 10.5483/bmbrep.2008.41.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldini G. Vistoli G. Regazzoni L. Gamberoni L. Facino RM. Yamaguchi S. Uchida K. Carini M. Albumin is the main nucleophilic target of human plasma: a protective role against pro-atherogenic electrophilic reactive carbonyl species? Chem Res Toxicol. 2008;21:824–835. doi: 10.1021/tx700349r. [DOI] [PubMed] [Google Scholar]
- 4.Aluise CD. Robinson RA. Beckett TL. Murphy MP. Cai J. Pierce WM. Markesbery WR. Butterfield DA. Preclinical Alzheimer disease: brain oxidative stress, Abeta peptide and proteomics. Neurobiol Dis. 2010;39:221–228. doi: 10.1016/j.nbd.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aluise CD. Robinson RA. Cai J. Pierce WM. Markesbery WR. Butterfield DA. Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer's disease: insights into memory loss in MCI. J Alzheimers Dis. 2011;23:257–269. doi: 10.3233/JAD-2010-101083. [DOI] [PubMed] [Google Scholar]
- 6.Anantharaman M. Tangpong J. Keller JN. Murphy MP. Markesbery WR. Kiningham KK. St Clair DK. Beta-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer's disease. Am J Pathol. 2006;168:1608–1618. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen PM. Genetic factors in the early diagnosis of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(Suppl 1):S31–42. doi: 10.1080/14660820052415899. [DOI] [PubMed] [Google Scholar]
- 8.Andrus PK. Fleck TJ. Gurney ME. Hall ED. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1998;71:2041–2048. doi: 10.1046/j.1471-4159.1998.71052041.x. [DOI] [PubMed] [Google Scholar]
- 9.Anzai K. Ogawa K. Goto Y. Senzaki Y. Ozawa T. Yamamoto H. Oxidation-dependent changes in the stability and permeability of lipid bilayers. Antioxid Redox Signal. 1999;1:339–347. doi: 10.1089/ars.1999.1.3-339. [DOI] [PubMed] [Google Scholar]
- 10.Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- 11.Arimura N. Inagaki N. Chihara K. Menager C. Nakamura N. Amano M. Iwamatsu A. Goshima Y. Kaibuchi K. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem. 2000;275:23973–23980. doi: 10.1074/jbc.M001032200. [DOI] [PubMed] [Google Scholar]
- 12.Atamna H. Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion. 2007;7:297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Bader Lange ML. Cenini G. Piroddi M. Abdul HM. Sultana R. Galli F. Memo M. Butterfield DA. Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol Dis. 2008;29:456–464. doi: 10.1016/j.nbd.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bader Lange ML. St Clair D. Markesbery WR. Studzinski CM. Murphy MP. Butterfield DA. Age-related loss of phospholipid asymmetry in APP(NLh)/APP(NLh) x PS-1(P264L)/PS-1(P264L) human double mutant knock-in mice: relevance to Alzheimer disease. Neurobiol Dis. 2010;38:104–115. doi: 10.1016/j.nbd.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balcz B. Kirchner L. Cairns N. Fountoulakis M. Lubec G. Increased brain protein levels of carbonyl reductase and alcohol dehydrogenase in Down syndrome and Alzheimer's disease. J Neural Transm Suppl. 2001;61:193–201. doi: 10.1007/978-3-7091-6262-0_15. [DOI] [PubMed] [Google Scholar]
- 16.Ball MJ. Nuttall K. Neurofibrillary tangles, granulovacuolar degeneration, and neuron loss in Down syndrome: quantitative comparison with Alzheimer dementia. Ann Neurol. 1980;7:462–465. doi: 10.1002/ana.410070512. [DOI] [PubMed] [Google Scholar]
- 17.Barber SC. Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Battaini F. Pascale A. Lucchi L. Pasinetti GM. Govoni S. Protein kinase C anchoring deficit in postmortem brains of Alzheimer's disease patients. Exp Neurol. 1999;159:559–564. doi: 10.1006/exnr.1999.7151. [DOI] [PubMed] [Google Scholar]
- 19.Beal MF. Neurochemistry and toxin models in Huntington's disease. Curr Opin Neurol. 1994;7:542–547. doi: 10.1097/00019052-199412000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JS. Chen J. Crow JP. Ye YZ. Reactions of nitric oxide, superoxide and peroxynitrite with superoxide dismutase in neurodegeneration. Prog Brain Res. 1994;103:371–380. doi: 10.1016/s0079-6123(08)61151-6. [DOI] [PubMed] [Google Scholar]
- 21.Behl C. Vitamin E and other antioxidants in neuroprotection. Int J Vitam Nutr Res. 1999;69:213–219. doi: 10.1024/0300-9831.69.3.213. [DOI] [PubMed] [Google Scholar]
- 22.Bigl M. Bruckner MK. Arendt T. Bigl V. Eschrich K. Activities of key glycolytic enzymes in the brains of patients with Alzheimer's disease. J Neural Transm. 1999;106:499–511. doi: 10.1007/s007020050174. [DOI] [PubMed] [Google Scholar]
- 23.Bonini NM. Giasson BI. Snaring the function of alpha-synuclein. Cell. 2005;123:359–361. doi: 10.1016/j.cell.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Bosco DA. Morfini G. Karabacak NM. Song Y. Gros-Louis F. Pasinelli P. Goolsby H. Fontaine BA. Lemay N. McKenna-Yasek D. Frosch MP. Agar JN. Julien JP. Brady ST. Brown RH., Jr. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd-Kimball D. Castegna A. Sultana R. Poon HF. Petroze R. Lynn BC. Klein JB. Butterfield DA. Proteomic identification of proteins oxidized by Abeta(1–42) in synaptosomes: implications for Alzheimer's disease. Brain Res. 2005;1044:206–215. doi: 10.1016/j.brainres.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 26.Browne SE. Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 27.Browne SE. Bowling AC. MacGarvey U. Baik MJ. Berger SC. Muqit MM. Bird ED. Beal MF. Oxidative damage and metabolic dysfunction in Huntington's disease: selective vulnerability of the basal ganglia. Ann Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 28.Browne SE. Ferrante RJ. Beal MF. Oxidative stress in Huntington's disease. Brain Pathol. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruening W. Roy J. Giasson B. Figlewicz DA. Mushynski WE. Durham HD. Up-regulation of protein chaperones preserves viability of cells expressing toxic Cu/Zn-superoxide dismutase mutants associated with amyotrophic lateral sclerosis. J Neurochem. 1999;72:693–699. doi: 10.1046/j.1471-4159.1999.0720693.x. [DOI] [PubMed] [Google Scholar]
- 30.Bubber P. Haroutunian V. Fisch G. Blass JP. Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 31.Busciglio J. Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 32.Butterfield DA. Bader Lange ML. Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim Biophys Acta. 2010;1801:924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butterfield DA. Castegna A. Proteomics for the identification of specifically oxidized proteins in brain: technology and application to the study of neurodegenerative disorders. Amino Acids. 2003;25:419–425. doi: 10.1007/s00726-003-0027-7. [DOI] [PubMed] [Google Scholar]
- 34.Butterfield DA. Galvan V. Lange MB. Tang H. Sowell RA. Spilman P. Fombonne J. Gorostiza O. Zhang J. Sultana R. Bredesen DE. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic Biol Med. 2010;48:136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butterfield DA. Hensley K. Cole P. Subramaniam R. Aksenov M. Aksenova M. Bummer PM. Haley BE. Carney JM. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer's disease. J Neurochem. 1997;68:2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- 36.Butterfield DA. Lange ML. Multifunctional roles of enolase in Alzheimer's disease brain: beyond altered glucose metabolism. J Neurochem. 2009;111:915–933. doi: 10.1111/j.1471-4159.2009.06397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butterfield DA. Poon HF. St Clair D. Keller JN. Pierce WM. Klein JB. Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiol Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Butterfield DA. Reed T. Perluigi M. De Marco C. Coccia R. Cini C. Sultana R. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci Lett. 2006;397:170–173. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Butterfield DA. Stadtman ER. Protein Oxidation processes in aging brain. Adv Cell Aging Gerontol. 1997;2:161–191. [Google Scholar]
- 40.Cadenas E. Muller A. Brigelius R. Esterbauer H. Sies H. Effects of 4-hydroxynonenal on isolated hepatocytes. Studies on chemiluminescence response, alkane production and glutathione status. Biochem J. 1983;214:479–487. doi: 10.1042/bj2140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calabrese V. Scapagnini G. Ravagna A. Colombrita C. Spadaro F. Butterfield DA. Giuffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech Ageing Dev. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Campos C. Guzman R. Lopez-Fernandez E. Casado A. Evaluation of urinary biomarkers of oxidative/nitrosative stress in adolescents and adults with Down syndrome. Biochim Biophys Acta. 2011;1812:760–768. doi: 10.1016/j.bbadis.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Capone GT. Down syndrome: advances in molecular biology and the neurosciences. J Dev Behav Pediatr. 2001;22:40–59. doi: 10.1097/00004703-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Castegna A. Aksenov M. Aksenova M. Thongboonkerd V. Klein JB. Pierce WM. Booze R. Markesbery WR. Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002;33:562–571. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- 45.Castegna A. Aksenov M. Thongboonkerd V. Klein JB. Pierce WM. Booze R. Markesbery WR. Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 46.Castegna A. Lauderback CM. Mohmmad-Abdul H. Butterfield DA. Modulation of phospholipid asymmetry in synaptosomal membranes by the lipid peroxidation products, 4-hydroxynonenal and acrolein: implications for Alzheimer's disease. Brain Res. 2004;1004:193–197. doi: 10.1016/j.brainres.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 47.Caudle WM. Pan S. Shi M. Quinn T. Hoekstra J. Beyer RP. Montine TJ. Zhang J. Proteomic identification of proteins in the human brain: Towards a more comprehensive understanding of neurodegenerative disease. Proteomics Clin Appl. 2008;2:1484–1497. doi: 10.1002/prca.200800043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen ZH. Saito Y. Yoshida Y. Sekine A. Noguchi N. Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 49.Cleveland DW. Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 50.Dalle Donne I. Scaloni A. Butterfield DA. Redox Proteomics: From Protein Modifications to Cellular Dysfunction and Diseases. Hoboken, NJ: John Wiley and Sons; 2006. [DOI] [PubMed] [Google Scholar]
- 51.Davies P. Challenging the cholinergic hypothesis in Alzheimer disease. JAMA. 1999;281:1433–1434. doi: 10.1001/jama.281.15.1433. [DOI] [PubMed] [Google Scholar]
- 52.Davis BM. Mohs RC. Greenwald BS. Mathe AA. Johns CA. Horvath TB. Davis KL. Clinical studies of the cholinergic deficit in Alzheimer's disease. I. Neurochemical and neuroendocrine studies. J Am Geriatr Soc. 1985;33:741–748. doi: 10.1111/j.1532-5415.1985.tb04184.x. [DOI] [PubMed] [Google Scholar]
- 53.Demicheli V. Quijano C. Alvarez B. Radi R. Inactivation and nitration of human superoxide dismutase (SOD) by fluxes of nitric oxide and superoxide. Free Radic Biol Med. 2007;42:1359–1368. doi: 10.1016/j.freeradbiomed.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 54.Devanand DP. Pradhaban G. Liu X. Khandji A. De Santi S. Segal S. Rusinek H. Pelton GH. Honig LS. Mayeux R. Stern Y. Tabert MH. de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 55.Dexter D. Carter C. Agid F. Agid Y. Lees AJ. Jenner P. Marsden CD. Lipid peroxidation as cause of nigral cell death in Parkinson's disease. Lancet. 1986;2:639–640. doi: 10.1016/s0140-6736(86)92471-2. [DOI] [PubMed] [Google Scholar]
- 56.Dhitavat S. Rivera ER. Rogers E. Shea TB. Differential efficacy of lipophilic and cytosolic antioxidants on generation of reactive oxygen species by amyloid-beta. J Alzheimers Dis. 2001;3:525–529. [PubMed] [Google Scholar]
- 57.Di Domenico F. Sultana R. Tiu GF. Scheff NN. Perluigi M. Cini C. Butterfield DA. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res. 2010;1333:72–81. doi: 10.1016/j.brainres.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding Q. Markesbery WR. Cecarini V. Keller JN. Decreased RNA, and increased RNA oxidation, in ribosomes from early Alzheimer's disease. Neurochem Res. 2006;31:705–710. doi: 10.1007/s11064-006-9071-5. [DOI] [PubMed] [Google Scholar]
- 59.Doorn JA. Maser E. Blum A. Claffey DJ. Petersen DR. Human carbonyl reductase catalyzes reduction of 4-oxonon-2-enal. Biochemistry. 2004;43:13106–13114. doi: 10.1021/bi049136q. [DOI] [PubMed] [Google Scholar]
- 60.Doorn JA. Petersen DR. Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem Biol Interact. 2003;143–144:93–100. doi: 10.1016/s0009-2797(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 61.Du AT. Schuff N. Amend D. Laakso MP. Hsu YY. Jagust WJ. Yaffe K. Kramer JH. Reed B. Norman D. Chui HC. Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duan Z. Lamendola DE. Yusuf RZ. Penson RT. Preffer FI. Seiden MV. Overexpression of human phosphoglycerate kinase 1 (PGK1) induces a multidrug resistance phenotype. Anticancer Res. 2002;22:1933–1941. [PubMed] [Google Scholar]
- 63.Dumont M. Wille E. Stack C. Calingasan NY. Beal MF. Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duyckaerts C. Delatour B. Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 65.Economou A. Papageorgiou SG. Karageorgiou C. Vassilopoulos D. Nonepisodic memory deficits in amnestic MCI. Cogn Behav Neurol. 2007;20:99–106. doi: 10.1097/WNN.0b013e31804c6fe7. [DOI] [PubMed] [Google Scholar]
- 66.Eismann T. Huber N. Shin T. Kuboki S. Galloway E. Wyder M. Edwards MJ. Greis KD. Shertzer HG. Fisher AB. Lentsch AB. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G266–274. doi: 10.1152/ajpgi.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eliuk SM. Renfrow MB. Shonsey EM. Barnes S. Kim H. active site modifications of the brain isoform of creatine kinase by 4-hydroxy-2-nonenal correlate with reduced enzyme activity: mapping of modified sites by Fourier transform-ion cyclotron resonance mass spectrometry. Chem Res Toxicol. 2007;20:1260–1268. doi: 10.1021/tx7000948. [DOI] [PubMed] [Google Scholar]
- 68.Esterbauer H. Benedetti A. Lang J. Fulceri R. Fauler G. Comporti M. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochim Biophys Acta. 1986;876:154–166. doi: 10.1016/0005-2760(86)90329-2. [DOI] [PubMed] [Google Scholar]
- 69.Esterbauer H. Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 70.Esterbauer H. Schaur RJ. Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 71.Farooqui AA. Horrocks LA. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell Mol Neurobiol. 1998;18:599–608. doi: 10.1023/A:1020625717298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferrante RJ. Browne SE. Shinobu LA. Bowling AC. Baik MJ. MacGarvey U. Kowall NW. Brown RH Jr. Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 73.Ferrante RJ. Shinobu LA. Schulz JB. Matthews RT. Thomas CE. Kowall NW. Gurney ME. Beal MF. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann Neurol. 1997;42:326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- 74.Ferrer I. Differential expression of phosphorylated translation initiation factor 2 alpha in Alzheimer's disease and Creutzfeldt-Jakob's disease. Neuropathol Appl Neurobiol. 2002;28:441–451. doi: 10.1046/j.1365-2990.2002.t01-1-00410.x. [DOI] [PubMed] [Google Scholar]
- 75.Filipovic MR. Stanic D. Raicevic S. Spasic M. Niketic V. Consequences of MnSOD interactions with nitric oxide: nitric oxide dismutation and the generation of peroxynitrite and hydrogen peroxide. Free Radic Res. 2007;41:62–72. doi: 10.1080/10715760600944296. [DOI] [PubMed] [Google Scholar]
- 76.Fleuranceau-Morel P. Barrier L. Fauconneau B. Piriou A. Huguet F. Origin of 4-hydroxynonenal incubation-induced inhibition of dopamine transporter and Na+/K+adenosine triphosphate in rat striatal synaptosomes. Neurosci Lett. 1999;277:91–94. doi: 10.1016/s0304-3940(99)00652-7. [DOI] [PubMed] [Google Scholar]
- 77.Forrest GL. Gonzalez B. Carbonyl reductase. Chem Biol Interact. 2000;129:21–40. doi: 10.1016/s0009-2797(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 78.Francis PT. Palmer AM. Snape M. Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gadoni E. Olivero A. Miglietta A. Bocca C. Gabriel L. Cytoskeletal modifications induced by 4-hydroxynonenal. Cytotechnology. 1993;11(Suppl 1):S62–64. doi: 10.1007/BF00746057. [DOI] [PubMed] [Google Scholar]
- 80.George AJ. Holsinger RM. McLean CA. Tan SS. Scott HS. Cardamone T. Cappai R. Masters CL. Li QX. Decreased phosphatidylethanolamine binding protein expression correlates with Abeta accumulation in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Aging. 2006;27:614–623. doi: 10.1016/j.neurobiolaging.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 81.Gomez A. Ferrer I. Increased oxidation of certain glycolysis and energy metabolism enzymes in the frontal cortex in Lewy body diseases. J Neurosci Res. 2009;87:1002–1013. doi: 10.1002/jnr.21904. [DOI] [PubMed] [Google Scholar]
- 82.Goshima Y. Nakamura F. Strittmatter P. Strittmatter SM. Collapsin-Induced Growth Cone Collapse Mediated by an Intracellular Protein Related to Unc-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 83.Grundke-Iqbal I. Iqbal K. Quinlan M. Tung YC. Zaidi MS. Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 84.Guiotto A. Calderan A. Ruzza P. Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–2315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- 85.Gulesserian T. Engidawork E. Fountoulakis M. Lubec G. Antioxidant proteins in fetal brain: superoxide dismutase-1 (SOD-1) protein is not overexpressed in fetal Down syndrome. J Neural Transm. 2001;Suppl:71–84. doi: 10.1007/978-3-7091-6262-0_6. [DOI] [PubMed] [Google Scholar]
- 86.Halliwell B. Gutteridge J. Free Radicals in Biology and Medicine. New York: Oxford University Press; 2007. [Google Scholar]
- 87.Hand CK. Rouleau GA. Familial amyotrophic lateral sclerosis. Muscle Nerve. 2002;25:135–159. doi: 10.1002/mus.10001. [DOI] [PubMed] [Google Scholar]
- 88.Harjes P. Wanker EE. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem Sci. 2003;28:425–433. doi: 10.1016/S0968-0004(03)00168-3. [DOI] [PubMed] [Google Scholar]
- 89.Hayes A. Batshaw ML. Down syndrome. Pediatr Clin North Am. 1993;40:523–35. doi: 10.1016/s0031-3955(16)38548-0. [DOI] [PubMed] [Google Scholar]
- 90.Head E. Lott IT. Down syndrome and beta-amyloid deposition. Curr Opin Neurol. 2004;17:95–100. doi: 10.1097/00019052-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 91.Heller SR. Macdonald IA. The measurement of cognitive function during acute hypoglycaemia: experimental limitations and their effect on the study of hypoglycaemia unawareness. Diabet Med. 1996;13:607–615. doi: 10.1002/(SICI)1096-9136(199607)13:7<607::AID-DIA159>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 92.Hensley K. Hall N. Subramaniam R. Cole P. Harris M. Aksenov M. Aksenova M. Gabbita SP. Wu JF. Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 93.Hensley K. Venkova K. Christov A. Gunning W. Park J. Collapsin response mediator protein-2: an emerging pathologic feature and therapeutic target for neurodisease indications. Mol Neurobiol. 2011;43:180–191. doi: 10.1007/s12035-011-8166-4. [DOI] [PubMed] [Google Scholar]
- 94.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 95.Iannello RC. Crack PJ. de Haan JB. Kola I. Oxidative stress and neural dysfunction in Down syndrome. J Neural Transm Suppl. 1999;57:257–267. doi: 10.1007/978-3-7091-6380-1_17. [DOI] [PubMed] [Google Scholar]
- 96.Ibanez P. Bonnet AM. Debarges B. Lohmann E. Tison F. Pollak P. Agid Y. Durr A. Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 97.Inagaki N. Chihara K. Arimura N. Menager C. Kawano Y. Matsuo N. Nishimura T. Amano M. Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- 98.Ishihara K. Amano K. Takaki E. Ebrahim AS. Shimohata A. Shibazaki N. Inoue I. Takaki M. Ueda Y. Sago H. Epstein CJ. Yamakawa K. Increased lipid peroxidation in Down's syndrome mouse models. J Neurochem. 2009;110:1965–1976. doi: 10.1111/j.1471-4159.2009.06294.x. [DOI] [PubMed] [Google Scholar]
- 99.Jouvenceau A. Dutar P. Billard JM. Alteration of NMDA receptor-mediated synaptic responses in CA1 area of the aged rat hippocampus: contribution of GABAergic and cholinergic deficits. Hippocampus. 1998;8:627–637. doi: 10.1002/(SICI)1098-1063(1998)8:6<627::AID-HIPO5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 100.Jovanovic SV. Clements D. MacLeod K. Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic Biol Med. 1998;25:1044–1048. doi: 10.1016/s0891-5849(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 101.Kehrer JP. Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 102.Keller JN. Hanni KB. Markesbery WR. Impaired proteasome function in Alzheimer's disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 103.Keller JN. Schmitt FA. Scheff SW. Ding Q. Chen Q. Butterfield DA. Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 104.Kida K. Nishio T. Nagai K. Matsuda H. Nakagawa H. Gluconeogenesis in the kidney in vivo in fed rats. Circadian change and substrate specificity. J Biochem (Tokyo) 1982;91:755–760. doi: 10.1093/oxfordjournals.jbchem.a133762. [DOI] [PubMed] [Google Scholar]
- 105.Kieran D. Kalmar B. Dick JR. Riddoch-Contreras J. Burnstock G. Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- 106.Korenberg JR. Bradley C. Disteche CM. Down syndrome: molecular mapping of the congenital heart disease and duodenal stenosis. Am J Hum Genet. 1992;50:294–302. [PMC free article] [PubMed] [Google Scholar]
- 107.Korolainen MA. Goldsteins G. Nyman TA. Alafuzoff I. Koistinaho J. Pirttila T. Oxidative modification of proteins in the frontal cortex of Alzheimer's disease brain. Neurobiol Aging. 2006;27:42–53. doi: 10.1016/j.neurobiolaging.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 108.Kruger R. Muller T. Riess O. Involvement of alpha-synuclein in Parkinson's disease and other neurodegenerative disorders. J Neural Transm. 2000;107:31–40. doi: 10.1007/s007020050002. [DOI] [PubMed] [Google Scholar]
- 109.LaFontaine MA. Mattson MP. Butterfield DA. Oxidative stress in synaptosomal proteins from mutant presenilin-1 knock-in mice: implications for familial Alzheimer's disease. Neurochem Res. 2002;27:417–421. doi: 10.1023/a:1015560116208. [DOI] [PubMed] [Google Scholar]
- 110.Lauderback CM. Hackett JM. Huang FF. Keller JN. Szweda LI. Markesbery WR. Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1–42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 111.Lee J. Kosaras B. Del Signore SJ. Cormier K. McKee A. Ratan RR. Kowall NW. Ryu H. Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington's disease mice. Acta Neuropathol. 2011;121:487–498. doi: 10.1007/s00401-010-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lemieux N. Malfoy B. Forrest GL. Human carbonyl reductase (CBR) localized to band 21q22.1 by high-resolution fluorescence in situ hybridization displays gene dosage effects in trisomy 21 cells. Genomics. 1993;15:169–172. doi: 10.1006/geno.1993.1024. [DOI] [PubMed] [Google Scholar]
- 113.Li T. Hawkes C. Qureshi HY. Kar S. Paudel HK. Cyclin-dependent protein kinase 5 primes microtubule-associated protein tau site-specifically for glycogen synthase kinase 3beta. Biochemistry. 2006;45:3134–3145. doi: 10.1021/bi051635j. [DOI] [PubMed] [Google Scholar]
- 114.Li YF. Wang Y. Channon KM. Schultz HD. Zucker IH. Patel KP. Manipulation of neuronal nitric oxide synthase within the paraventricular nucleus using adenovirus and antisense technology. Methods Mol Med. 2005;112:59–79. doi: 10.1385/1-59259-879-x:059. [DOI] [PubMed] [Google Scholar]
- 115.Liochev SI. Fridovich I. Copper- and zinc-containing superoxide dismutase can act as a superoxide reductase and a superoxide oxidase. J Biol Chem. 2000;275:38482–38485. doi: 10.1074/jbc.M007891200. [DOI] [PubMed] [Google Scholar]
- 116.Liu D. Wen J. Liu J. Li L. The roles of free radicals in amyotrophic lateral sclerosis: reactive oxygen species and elevated oxidation of protein, DNA, and membrane phospholipids. FASEB J. 1999;13:2318–2328. doi: 10.1096/fasebj.13.15.2318. [DOI] [PubMed] [Google Scholar]
- 117.Liu R. Althaus JS. Ellerbrock BR. Becker DA. Gurney ME. Enhanced oxygen radical production in a transgenic mouse model of familial amyotrophic lateral sclerosis. Ann Neurol. 1998;44:763–770. doi: 10.1002/ana.410440510. [DOI] [PubMed] [Google Scholar]
- 118.Loidl-Stahlhofen A. Hannemann K. Spiteller G. Generation of alpha-hydroxyaldehydic compounds in the course of lipid peroxidation. Biochim Biophys Acta. 1994;1213:140–148. doi: 10.1016/0005-2760(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 119.LoPachin RM. Barber DS. Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Toxicol Sci. 2006;94:240–255. doi: 10.1093/toxsci/kfl066. [DOI] [PubMed] [Google Scholar]
- 120.Lovell MA. Ehmann WD. Mattson MP. Markesbery WR. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer's disease. Neurobiol Aging. 1997;18:457–461. doi: 10.1016/s0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 121.Lovell MA. Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lucke C. Gantz DL. Klimtchuk E. Hamilton JA. Interactions between fatty acids and alpha-synuclein. J Lipid Res. 2006;47:1714–1724. doi: 10.1194/jlr.M600003-JLR200. [DOI] [PubMed] [Google Scholar]
- 123.Macmillan-Crow LA. Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 124.Mann DM. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev. 1988;43:99–136. doi: 10.1016/0047-6374(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 125.Markesbery WR. Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 126.Markesbery WR. Schmitt FA. Kryscio RJ. Davis DG. Smith CD. Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 127.Martinez A. Portero-Otin M. Pamplona R. Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010;20:281–297. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Masliah E. Mallory M. Hansen L. DeTeresa R. Alford M. Terry R. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci Lett. 1994;174:67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 129.Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mattson MP. Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meier-Ruge W. Bertoni-Freddari C. Iwangoff P. Changes in brain glucose metabolism as a key to the pathogenesis of Alzheimer's disease. Gerontology. 1994;40:246–252. doi: 10.1159/000213592. [DOI] [PubMed] [Google Scholar]
- 132.Moreira PI. Santos MS. Oliveira CR. Shenk JC. Nunomura A. Smith MA. Zhu X. Perry G. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets. 2008;7:3–10. doi: 10.2174/187152708783885156. [DOI] [PubMed] [Google Scholar]
- 133.Morrow JD. Awad JA. Kato T. Takahashi K. Badr KF. Roberts LJ., 2nd Burk RF. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992;90:2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Musiek ES. Yin H. Milne GL. Morrow JD. Recent advances in the biochemistry and clinical relevance of the isoprostane pathway. Lipids. 2005;40:987–994. doi: 10.1007/s11745-005-1460-7. [DOI] [PubMed] [Google Scholar]
- 135.Napolitano A. Manini P. d'Ischia M. Oxidation chemistry of catecholamines and neuronal degeneration: an update. Curr Med Chem. 2011;18:1832–1845. doi: 10.2174/092986711795496863. [DOI] [PubMed] [Google Scholar]
- 136.Neely MD. Boutte A. Milatovic D. Montine TJ. Mechanisms of 4-hydroxynonenal-induced neuronal microtubule dysfunction. Brain Res. 2005;1037:90–98. doi: 10.1016/j.brainres.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 137.Neely MD. Sidell KR. Graham DG. Montine TJ. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J Neurochem. 1999;72:2323–2333. doi: 10.1046/j.1471-4159.1999.0722323.x. [DOI] [PubMed] [Google Scholar]
- 138.Nunomura A. Perry G. Aliev G. Hirai K. Takeda A. Balraj EK. Jones PK. Ghanbari H. Wataya T. Shimohama S. Chiba S. Atwood CS. Petersen RB. Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 139.Odetti P. Angelini G. Dapino D. Zaccheo D. Garibaldi S. Dagna-Bricarelli F. Piombo G. Perry G. Smith M. Traverso N. Tabaton M. Early glycoxidation damage in brains from Down's syndrome. Biochem Biophys Res Commun. 1998;243:849–851. doi: 10.1006/bbrc.1998.8186. [DOI] [PubMed] [Google Scholar]
- 140.Ojika K. Tsugu Y. Mitake S. Otsuka Y. Katada E. NMDA receptor activation enhances the release of a cholinergic differentiation peptide (HCNP) from hippocampal neurons in vitro. Brain Res Dev Brain Res. 1998;106:173–180. doi: 10.1016/s0165-3806(98)00014-5. [DOI] [PubMed] [Google Scholar]
- 141.Op den Velde W. Stam FC. Some cerebral proteins and enzyme systems in Alzheimer's presenile and senile dementia. J Am Geriatr Soc. 1976;24:12–16. doi: 10.1111/j.1532-5415.1976.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 142.Oppermann U. Carbonyl reductases: the complex relationships of mammalian carbonyl- and quinone-reducing enzymes and their role in physiology. Annu Rev Pharmacol Toxicol. 2007;47:293–322. doi: 10.1146/annurev.pharmtox.47.120505.105316. [DOI] [PubMed] [Google Scholar]
- 143.Orrenius S. Burgess DH. Hampton MB. Zhivotovsky B. Mitochondria as the focus of apoptosis research. Cell Death Differ. 1997;4:427–428. doi: 10.1038/sj.cdd.4400272. [DOI] [PubMed] [Google Scholar]
- 144.Owen JB. Sultana R. Aluise CD. Erickson MA. Price TO. Bu G. Banks WA. Butterfield DA. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free Radic Biol Med. 2010;49:1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pallardo FV. Degan P. d'Ischia M. Kelly FJ. Zatterale A. Calzone R. Castello G. Fernandez-Delgado R. Dunster C. Lloret A. Manini P. Pisanti MA. Vuttariello E. Pagano G. Multiple evidence for an early age pro-oxidant state in Down syndrome patients. Biogerontology. 2006;7:211–220. doi: 10.1007/s10522-006-9002-5. [DOI] [PubMed] [Google Scholar]
- 146.Pedersen WA. Cashman NR. Mattson MP. The lipid peroxidation product 4-hydroxynonenal impairs glutamate and glucose transport and choline acetyltransferase activity in NSC-19 motor neuron cells. Exp Neurol. 1999;155:1–10. doi: 10.1006/exnr.1998.6890. [DOI] [PubMed] [Google Scholar]
- 147.Perluigi M. di Domenico F. Fiorini A. Cocciolo A. Giorgi A. Foppoli C. Butterfield DA. Giorlandino M. Giorlandino C. Schinina ME. Coccia R. Oxidative stress occurs early in Down syndrome pregnancy: a redox proteomics analysis of amniotic fluid. Proteomics Clin Appl. 2011;5:167–178. doi: 10.1002/prca.201000121. [DOI] [PubMed] [Google Scholar]
- 148.Perluigi M. Fai Poon H. Hensley K. Pierce WM. Klein JB. Calabrese V. De Marco C. Butterfield DA. Proteomic analysis of 4-hydroxy-2-nonenal-modified proteins in G93A-SOD1 transgenic mice—a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;38:960–968. doi: 10.1016/j.freeradbiomed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 149.Perluigi M. Poon HF. Maragos W. Pierce WM. Klein JB. Calabrese V. Cini C. De Marco C. Butterfield DA. Proteomic analysis of protein expression and oxidative modification in r6/2 transgenic mice: a model of Huntington disease. Mol Cell Proteomics. 2005;4:1849–1861. doi: 10.1074/mcp.M500090-MCP200. [DOI] [PubMed] [Google Scholar]
- 150.Perluigi M. Sultana R. Cenini G. Di Domenico F. Memo M. Pierce WM. Coccia R. Butterfield DA. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer's disease: role of lipid peroxidation in Alzheimer's disease pathogenesis. Proteomics Clin Appl. 2009;3:682–693. doi: 10.1002/prca.200800161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pestova TV. Hellen CU. The structure and function of initiation factors in eukaryotic protein synthesis. Cell Mol Life Sci. 2000;57:651–674. doi: 10.1007/PL00000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Petersen DR. Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 153.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 154.Petronis A. Alzheimer's disease and down syndrome: from meiosis to dementia. Exp Neurol. 1999;158:403–413. doi: 10.1006/exnr.1999.7128. [DOI] [PubMed] [Google Scholar]
- 155.Poli G. Biasi F. Leonarduzzi G. 4-Hydroxynonenal-protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol Aspects Med. 2008;29:67–71. doi: 10.1016/j.mam.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 156.Poli G. Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 157.Poon HF. Calabrese V. Scapagnini G. Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J Gerontol A Biol Sci Med Sci. 2004;59:478–493. doi: 10.1093/gerona/59.5.m478. [DOI] [PubMed] [Google Scholar]
- 158.Poon HF. Hensley K. Thongboonkerd V. Merchant ML. Lynn BC. Pierce WM. Klein JB. Calabrese V. Butterfield DA. Redox proteomics analysis of oxidatively modified proteins in G93A-SOD1 transgenic mice—a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;39:453–462. doi: 10.1016/j.freeradbiomed.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 159.Pratico D. Clark CM. Liun F. Rokach J. Lee VY. Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 160.Pratico D. Iuliano L. Amerio G. Tang LX. Rokach J. Sabatino G. Violi F. Down's syndrome is associated with increased 8,12-iso-iPF2alpha-VI levels: evidence for enhanced lipid peroxidation in vivo. Ann Neurol. 2000;48:795–798. [PubMed] [Google Scholar]
- 161.Pratt DA. Tallman KA. Porter NA. Free radical oxidation of polyunsaturated lipids: New mechanistic insights and the development of peroxyl radical clocks. Acc Chem Res. 2011;44:458–467. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Price DL. New perspectives on Alzheimer's disease. Annu Rev Neurosci. 1986;9:489–512. doi: 10.1146/annurev.ne.09.030186.002421. [DOI] [PubMed] [Google Scholar]
- 163.Pryor WA. Porter NA. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic Biol Med. 1990;8:541–543. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- 164.Qin J. Goswami R. Balabanov R. Dawson G. Oxidized phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J Neurosci Res. 2007;85:977–984. doi: 10.1002/jnr.21206. [DOI] [PubMed] [Google Scholar]
- 165.Rakhit R. Cunningham P. Furtos-Matei A. Dahan S. Qi XF. Crow JP. Cashman NR. Kondejewski LH. Chakrabartty A. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. J Biol Chem. 2002;277:47551–47556. doi: 10.1074/jbc.M207356200. [DOI] [PubMed] [Google Scholar]
- 166.Rapoport SI. In vivo PET imaging and postmortem studies suggest potentially reversible and irreversible stages of brain metabolic failure in Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(Suppl 3):46–55. doi: 10.1007/pl00014174. [DOI] [PubMed] [Google Scholar]
- 167.Reed T. Perluigi M. Sultana R. Pierce WM. Klein JB. Turner DM. Coccia R. Markesbery WR. Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer's disease. Neurobiol Dis. 2008;30:107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 168.Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 169.Reed TT. Pierce WM. Markesbery WR. Butterfield DA. Proteomic identification of HNE-bound proteins in early Alzheimer disease: insights into the role of lipid peroxidation in the progression of AD. Brain Res. 2009;1274:66–76. doi: 10.1016/j.brainres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 170.Roberts LJ., 2nd Montine TJ. Markesbery WR. Tapper AR. Hardy P. Chemtob S. Dettbarn WD. Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 171.Roper RJ. Reeves RH. Understanding the basis for Down syndrome phenotypes. PLoS Genet. 2006;2:e50. doi: 10.1371/journal.pgen.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rossor MN. Iversen LL. Johnson AJ. Mountjoy CQ. Roth M. Cholinergic deficit in frontal cerebral cortex in Alzheimer's disease is age dependent. Lancet. 1981;2:1422. doi: 10.1016/s0140-6736(81)92836-1. [DOI] [PubMed] [Google Scholar]
- 173.Rozzini L. Chilovi BV. Conti M. Bertoletti E. Delrio I. Trabucchi M. Padovani A. Conversion of amnestic Mild Cognitive Impairment to dementia of Alzheimer type is independent to memory deterioration. Int J Geriatr Psychiatry. 2007;22:1217–1222. doi: 10.1002/gps.1816. [DOI] [PubMed] [Google Scholar]
- 174.Rubinsztein DC. Leggo J. Coles R. Almqvist E. Biancalana V. Cassiman JJ. Chotai K. Connarty M. Crauford D. Curtis A. Curtis D. Davidson MJ. Differ AM. Dode C. Dodge A. Frontali M. Ranen NG. Stine OC. Sherr M. Abbott MH. Franz ML. Graham CA. Harper PS. Hedreen JC. Hayden MR, et al. Phenotypic characterization of individuals with 30–40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36–39 repeats. Am J Hum Genet. 1996;59:16–22. [PMC free article] [PubMed] [Google Scholar]
- 175.Ruiperez V. Darios F. Davletov B. Alpha-synuclein, lipids and Parkinson's disease. Prog Lipid Res. 2010;49:420–428. doi: 10.1016/j.plipres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 176.Sajdel-Sulkowska EM. Marotta CA. Alzheimer's disease brain: alterations in RNA levels and in a ribonuclease-inhibitor complex. Science. 1984;225:947–949. doi: 10.1126/science.6206567. [DOI] [PubMed] [Google Scholar]
- 177.Salmon M. Dedessus Le Moutier J. Wenders F. Chiarizia S. Eliaers F. Remacle J. Royer V. Pascal T. Toussaint O. Role of the PLA2-independent peroxiredoxin VI activity in the survival of immortalized fibroblasts exposed to cytotoxic oxidative stress. FEBS Lett. 2004;557:26–32. doi: 10.1016/s0014-5793(03)01437-6. [DOI] [PubMed] [Google Scholar]
- 178.Schapira AH. Mitochondrial involvement in Parkinson's disease, Huntington's disease, hereditary spastic paraplegia and Friedreich's ataxia. Biochim Biophys Acta. 1999;1410:159–170. doi: 10.1016/s0005-2728(98)00164-9. [DOI] [PubMed] [Google Scholar]
- 179.Scheff SW. DeKosky ST. Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 180.Scheff SW. Price DA. Alzheimer's disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis. 2006;9:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 181.Scheff SW. Price DA. Schmitt FA. DeKosky ST. Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 182.Schneider C. Porter NA. Brash AR. Autoxidative transformation of chiral omega6 hydroxy linoleic and arachidonic acids to chiral 4-hydroxy-2E-nonenal. Chem Res Toxicol. 2004;17:937–941. doi: 10.1021/tx049913n. [DOI] [PubMed] [Google Scholar]
- 183.Shaw PJ. Ince PG. Falkous G. Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann Neurol. 1995;38:691–695. doi: 10.1002/ana.410380424. [DOI] [PubMed] [Google Scholar]
- 184.Shibata N. Nagai R. Uchida K. Horiuchi S. Yamada S. Hirano A. Kawaguchi M. Yamamoto T. Sasaki S. Kobayashi M. Morphological evidence for lipid peroxidation and protein glycoxidation in spinal cords from sporadic amyotrophic lateral sclerosis patients. Brain Res. 2001;917:97–104. doi: 10.1016/s0006-8993(01)02926-2. [DOI] [PubMed] [Google Scholar]
- 185.Shichiri M. Yoshida Y. Ishida N. Hagihara Y. Iwahashi H. Tamai H. Niki E. alpha-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Radic Biol Med. 2011;50:1801–1811. doi: 10.1016/j.freeradbiomed.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 186.Shinder GA. Lacourse MC. Minotti S. Durham HD. Mutant Cu/Zn-superoxide dismutase proteins have altered solubility and interact with heat shock/stress proteins in models of amyotrophic lateral sclerosis. J Biol Chem. 2001;276:12791–12796. doi: 10.1074/jbc.M010759200. [DOI] [PubMed] [Google Scholar]
- 187.Siegel SJ. Bieschke J. Powers ET. Kelly JW. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry. 2007;46:1503–1510. doi: 10.1021/bi061853s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Singh AK. Gupta S. Jiang Y. Oxidative stress and protein oxidation in the brain of water drinking and alcohol drinking rats administered the HIV envelope protein, gp120. J Neurochem. 2008;104:1478–1493. doi: 10.1111/j.1471-4159.2007.05094.x. [DOI] [PubMed] [Google Scholar]
- 189.Siow RC. Ishii T. Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- 190.Smith RG. Henry YK. Mattson MP. Appel SH. Presence of 4-hydroxynonenal in cerebrospinal fluid of patients with sporadic amyotrophic lateral sclerosis. Ann Neurol. 1998;44:696–699. doi: 10.1002/ana.410440419. [DOI] [PubMed] [Google Scholar]
- 191.Sperandio S. Poksay KS. Schilling B. Crippen D. Gibson BW. Bredesen DE. Identification of new modulators and protein alterations in non-apoptotic programmed cell death. J Cell Biochem. 2010;111:1401–1412. doi: 10.1002/jcb.22870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stockl M. Fischer P. Wanker E. Herrmann A. Alpha-synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J Mol Biol. 2008;375:1394–1404. doi: 10.1016/j.jmb.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 193.Subba Rao K. Mechanisms of disease: DNA repair defects and neurological disease. Nat Clin Pract Neurol. 2007;3:162–172. doi: 10.1038/ncpneuro0448. [DOI] [PubMed] [Google Scholar]
- 194.Subbarao KV. Richardson JS. Ang LC. Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. J Neurochem. 1990;55:342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- 195.Subramaniam R. Roediger F. Jordan B. Mattson MP. Keller JN. Waeg G. Butterfield DA. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J Neurochem. 1997;69:1161–1169. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- 196.Sultana R. Boyd-Kimball D. Poon HF. Cai J. Pierce WM. Klein JB. Markesbery WR. Zhou XZ. Lu KP. Butterfield DA. Oxidative modification and down-regulation of Pin1 in Alzheimer's disease hippocampus: A redox proteomics analysis. Neurobiol Aging. 2006;27:918–925. doi: 10.1016/j.neurobiolaging.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 197.Sultana R. Boyd-Kimball D. Poon HF. Cai J. Pierce WM. Klein JB. Merchant M. Markesbery WR. Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 198.Sultana R. Butterfield DA. Oxidatively modified GST and MRP1 in Alzheimer's disease brain: implications for accumulation of reactive lipid peroxidation products. Neurochem Res. 2004;29:2215–2220. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- 199.Sultana R. Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J Alzheimers Dis. 2010;19:341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 200.Sultana R. Perluigi M. Butterfield DA. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer's disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal. 2006;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- 201.Thompson JE. Hopkins MT. Taylor C. Wang TW. Regulation of senescence by eukaryotic translation initiation factor 5A: implications for plant growth and development. Trends Plant Sci. 2004;9:174–179. doi: 10.1016/j.tplants.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 202.Uchida K. Stadtman ER. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra- and intermolecular cross-linking reaction. J Biol Chem. 1993;268:6388–6393. [PubMed] [Google Scholar]
- 203.Valentine JS. Do oxidatively modified proteins cause ALS? Free Radic Biol Med. 2002;33:1314–1320. doi: 10.1016/s0891-5849(02)01080-8. [DOI] [PubMed] [Google Scholar]
- 204.Vander Jagt DL. Hunsaker LA. Vander Jagt TJ. Gomez MS. Gonzales DM. Deck LM. Royer RE. Inactivation of glutathione reductase by 4-hydroxynonenal and other endogenous aldehydes. Biochem Pharmacol. 1997;53:1133–1140. doi: 10.1016/s0006-2952(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 205.Vayssiere JL. Cordeau-Lossouarn L. Larcher JC. Basseville M. Gros F. Croizat B. Participation of the mitochondrial genome in the differentiation of neuroblastoma cells. In Vitro Cell Dev Biol. 1992;28A:763–772. doi: 10.1007/BF02631065. [DOI] [PubMed] [Google Scholar]
- 206.Vexler ZS. Wong A. Francisco C. Manabat C. Christen S. Tauber M. Ferriero DM. Gregory G. Fructose-1,6-bisphosphate preserves intracellular glutathione and protects cortical neurons against oxidative stress. Brain Res. 2003;960:90–98. doi: 10.1016/s0006-8993(02)03777-0. [DOI] [PubMed] [Google Scholar]
- 207.Watanabe M. Dykes-Hoberg M. Culotta VC. Price DL. Wong PC. Rothstein JD. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol Dis. 2001;8:933–941. doi: 10.1006/nbdi.2001.0443. [DOI] [PubMed] [Google Scholar]
- 208.West MJ. Kawas CH. Stewart WF. Rudow GL. Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer's disease. Neurobiol Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 209.Wilkins MR. Sanchez JC. Williams KL. Hochstrasser DF. Current challenges and future applications for protein maps and post-translational vector maps in proteome projects. Electrophoresis. 1996;17:830–838. doi: 10.1002/elps.1150170504. [DOI] [PubMed] [Google Scholar]
- 210.Williams TI. Lynn BC. Markesbery WR. Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 211.Yang Y. Sharma R. Sharma A. Awasthi S. Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- 212.Yarian CS. Rebrin I. Sohal RS. Aconitase and ATP synthase are targets of malondialdehyde modification and undergo an age-related decrease in activity in mouse heart mitochondria. Biochem Biophys Res Commun. 2005;330:151–156. doi: 10.1016/j.bbrc.2005.02.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yehuda S. Rabinovitz S. Carasso RL. Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging. 2002;23:843–853. doi: 10.1016/s0197-4580(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 214.Yoshida H. Watanabe A. Ihara Y. Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer's disease. J Biol Chem. 1998;273:9761–9768. doi: 10.1074/jbc.273.16.9761. [DOI] [PubMed] [Google Scholar]
- 215.Zana M. Janka Z. Kalman J. Oxidative stress: a bridge between Down's syndrome and Alzheimer's disease. Neurobiol Aging. 2007;28:648–676. doi: 10.1016/j.neurobiolaging.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 216.Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003;24:293–303. doi: 10.1016/s0098-2997(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 217.Zhu M. Qin ZJ. Hu D. Munishkina LA. Fink AL. Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry. 2006;45:8135–8142. doi: 10.1021/bi052584t. [DOI] [PubMed] [Google Scholar]