Abstract
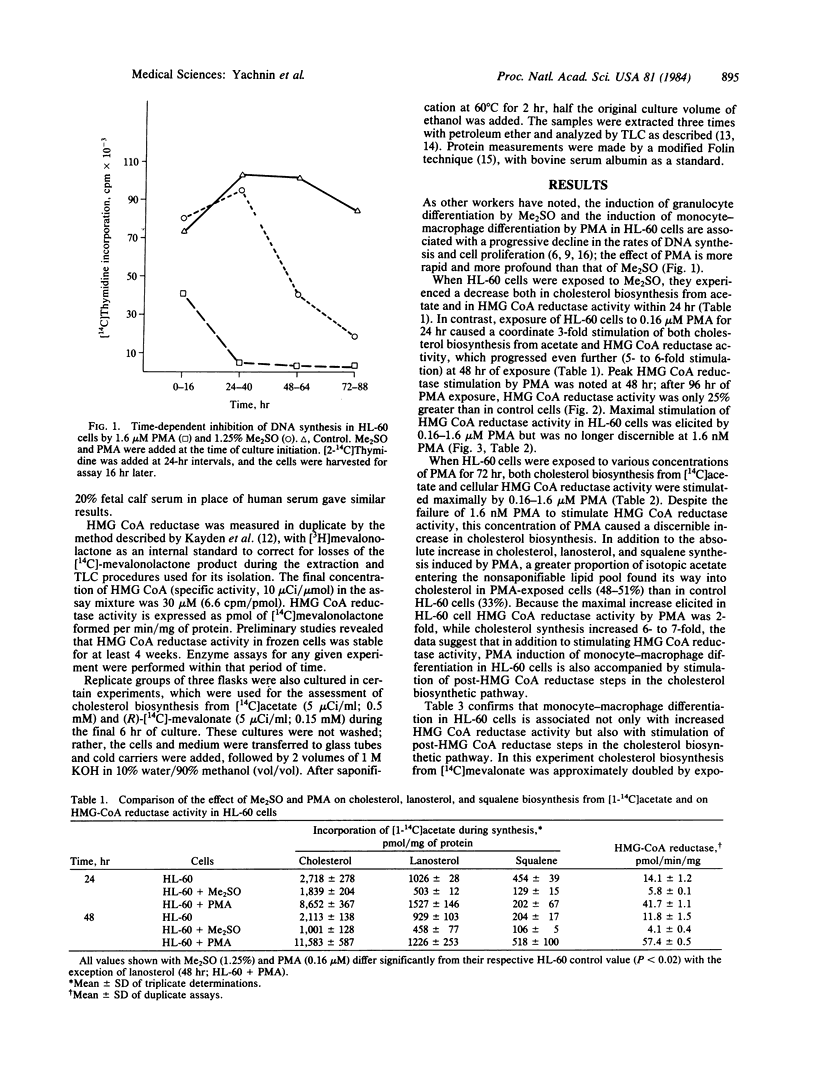
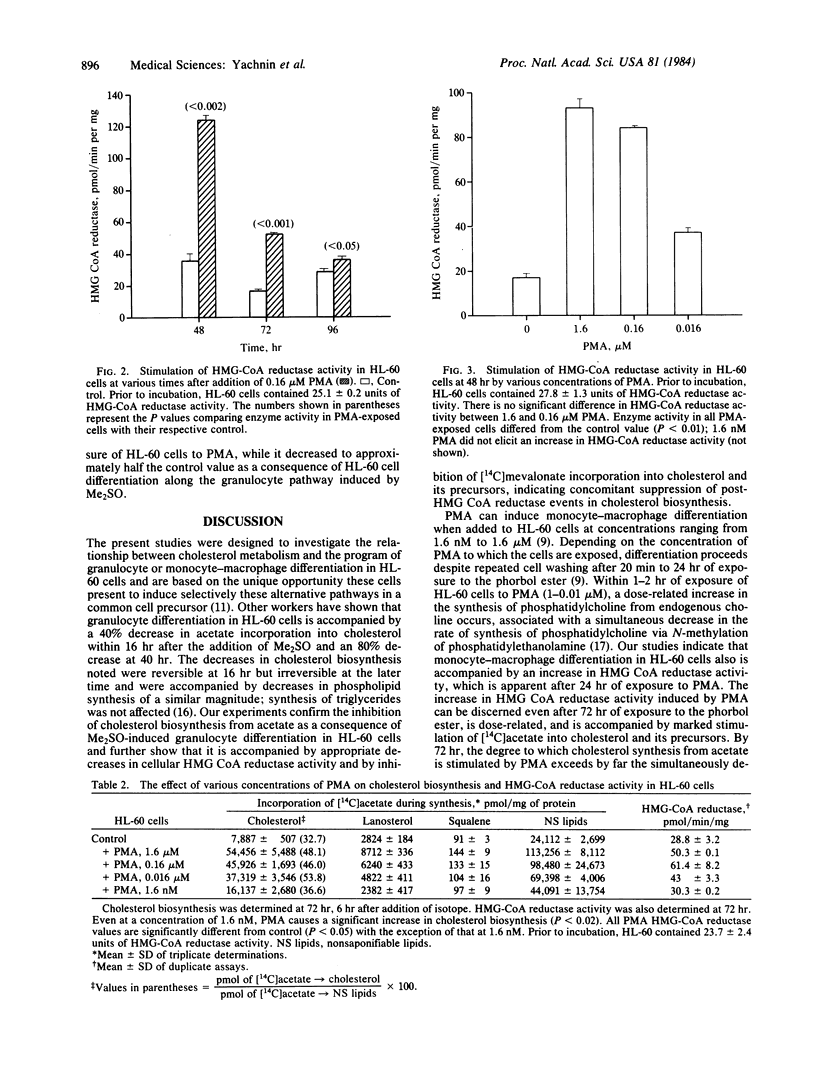
Addition of dimethyl sulfoxide or phorbol myristate acetate (PMA) to HL-60 cell cultures induces granulocytic or monocyte-macrophage differentiation, respectively, in HL-60 cells. Dimethyl sulfoxide-induced granulocyte differentiation in HL-60 cells is associated with a decrease in cellular 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase activity and with a decrease in the incorporation of [14C]acetate and mevalonate into products of the cholesterol biosynthetic pathway. PMA-induced monocyte-macrophage differentiation in HL-60 cells is associated with a rapid and profound fall in cell proliferation but nonetheless is accompanied by a dose-dependent increase in cellular HMG-CoA reductase activity and [14C]acetate incorporation into the cholesterol biosynthetic pathway. In addition, PMA induces an increase in [14C]mevalonate incorporation into cholesterol and its precursors, suggesting that post-HMG-CoA reductase events in cholesterol biosynthesis are also enhanced. Mature peripheral blood human monocytes possess an active cholesterol biosynthetic pathway, whereas mature human granulocytes are almost entirely lacking in the ability to synthesize post-squalene products. Our results with HL-60 cells indicate that this divergence in sterol-synthesizing ability between two cell lineages, which normally also derive from a common stem cell, can be observed as an early event in the differentiation process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassileth P. A., Suholet D., Cooper R. A. Early changes in phosphatidylcholine metabolism in human acute promyelocytic leukemia cells stimulated to differentiate by phorbol ester. Blood. 1981 Aug;58(2):237–243. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Ip S. H., Cassileth P. A., Kuo A. L. Inhibition of sterol and phospholipid synthesis in HL-60 promyelocytic leukemia cells by inducers of myeloid differentiation. Cancer Res. 1981 May;41(5):1847–1852. [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Synthesis of ubiquinone and cholesterol in human fibroblasts: regulation of a branched pathway. Arch Biochem Biophys. 1979 Jan;192(1):86–99. doi: 10.1016/0003-9861(79)90074-2. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Fogelman A. M., Hokom M. M., Haberland M. E., Tanaka R. D., Edwards P. A. Lipoprotein regulation of cholesterol metabolism in macrophages derived from human monocytes. J Biol Chem. 1982 Dec 10;257(23):14081–14086. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Edwards P. A., Hokom M., Popják G. Cholesterol biosynthesis in human lymphocytes, monocytes, and granulocytes. Biochem Biophys Res Commun. 1977 May 9;76(1):167–173. doi: 10.1016/0006-291x(77)91682-5. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Hokom M., Edwards P. A. Separation of and cholesterol synthesis by human lymphocytes and monocytes. J Lipid Res. 1979 Mar;20(3):379–388. [PubMed] [Google Scholar]
- Fontana J. A., Colbert D. A., Deisseroth A. B. Identification of a population of bipotent stem cells in the HL60 human promyelocytic leukemia cell line. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3863–3866. doi: 10.1073/pnas.78.6.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEWITT B. R. Spectrophotometric determination of total carbohydrate. Nature. 1958 Jul 26;182(4630):246–247. [PubMed] [Google Scholar]
- Ho Y. K., Smith R. G., Brown M. S., Goldstein J. L. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood. 1978 Dec;52(6):1099–1114. [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayden H. J., Hatam L., Beratis N. G. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and the esterification of cholesterol in human long term lymphoid cell lines. Biochemistry. 1976 Feb 10;15(3):521–528. doi: 10.1021/bi00648a011. [DOI] [PubMed] [Google Scholar]
- Knight B. L., Patel D. D., Soutar A. K. The regulation of 3-hydroxy-3-methylglutaryl-CoA reductase activity, cholesterol esterification and the expression of low-density lipoprotein receptors in cultured monocyte-derived macrophages. Biochem J. 1983 Feb 15;210(2):523–532. doi: 10.1042/bj2100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter I., Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J Lipid Res. 1981 Jan;22(1):63–71. [PubMed] [Google Scholar]
- Shechter I., Fogelman A. M., Popják G. A deficiency of mixed function oxidase activities in the cholesterol biosynthetic pathway of human granulocytes. J Lipid Res. 1980 Mar;21(3):277–283. [PubMed] [Google Scholar]
- Traber M. G., Defendi V., Kayden H. J. Receptor activities for low-density lipoprotein and acetylated low-density lipoprotein in a mouse macrophage cell line (IC21) and in human monocyte-derived macrophages. J Exp Med. 1981 Dec 1;154(6):1852–1867. doi: 10.1084/jem.154.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber M. G., Kayden H. J. Low density lipoprotein receptor activity in human monocyte-derived macrophages and its relation to atheromatous lesions. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5466–5470. doi: 10.1073/pnas.77.9.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnin S., Golomb H. M., West E. J., Saffold C. Increased cholesterol biosynthesis in leukemic cells from patients with hairy cell leukemia. Blood. 1983 Jan;61(1):50–60. [PubMed] [Google Scholar]
- Yachnin S., Larson R. A., West E. J. Rates of cholesterol biosynthesis are related to early differentiation in acute non-lymphocytic leukaemia cells. Br J Haematol. 1983 Jul;54(3):459–466. doi: 10.1111/j.1365-2141.1983.tb02120.x. [DOI] [PubMed] [Google Scholar]



