Abstract
Several lines of human tumor cells in monolayer and soft agar cultures allow permeation of low levels of adenine nucleotides through their plasma membranes, while, in general, untransformed cells do not incorporate adenine nucleotides into their cellular pools without prior degradation of the nucleotides to adenosine. This study determined the uptake of 99mTc-radiolabeled chelated forms of adenine nucleotides, 99mTc-Ap4A (diadenosine 5',5"',P1,P4-tetraphosphate) and 99mTc-ATP chelates as radiodiagnostic agents suitable for the in vivo detection of tumors by radionuclide imaging. Biodistribution studies revealed that Ap4A accumulated preferentially in RT-24 tumors implanted in rats and that V2 carcinoma implanted in rabbits could be readily visualized by in vivo imaging. The biodistribution at various time points showed increased tumor-to-muscle ratios after 99mTc-Ap4A or 99mTc-ATP injections when compared with a nonspecific marker of the extracellular fluid space, 99mTc-labeled diethylenetriaminepentaacetic acid and with an agent known to localize in some tumors, 67Ga-labeled citrate. Studies of ectoenzymatic activities of virus-transformed animal cells and their untransformed counterparts in monolayer cultures showed marked decreases in the ectoenzymatic activities that degrade Ap4A in the transformed cells. Incorporation of en bloc [3H, 32P]Ap4A into cellular acid-soluble nucleotide pools of certain transformed cells was observed. Normal untransformed cells incorporated the radioactive label only by prior degradation to [3H]adenosine and 32Pi.
Full text
PDF

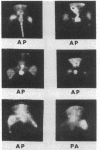
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar Waqar M., Taber R. L., Huberman J. A. Studies on the penetration of mammalian cells by deoxyribonucleoside-5'-phosphates. J Cell Physiol. 1979 Nov;101(2):251–259. doi: 10.1002/jcp.1041010207. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H. Does ATP cross the cell plasma membrane. Yale J Biol Med. 1982 Jan-Feb;55(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Eckelman W. C., Volkert W. A. In vivo chemistry of 99mTc-chelates. Int J Appl Radiat Isot. 1982 Oct;33(10):945–951. doi: 10.1016/0020-708x(82)90140-5. [DOI] [PubMed] [Google Scholar]
- Evans W. H. Nucleotide pyrophosphatase, a sialoglycoprotein located on the hepatocyte surface. Nature. 1974 Aug 2;250(465):391–394. doi: 10.1038/250391a0. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C., SYVERTON J. T. The mammalian cell-virus relationship. IV. Infection of naturally insusceptible cells with enterovirus ribonucleic acid. J Exp Med. 1959 Jul 1;110(1):65–80. doi: 10.1084/jem.110.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R. L. The medical use of gallium radionuclides: a brief history with some comments. Semin Nucl Med. 1978 Jul;8(3):183–191. doi: 10.1016/s0001-2998(78)80027-0. [DOI] [PubMed] [Google Scholar]
- Ho D. H., Frei E., 3rd Pharmacological studies of the antitumor agent 6-methylthiopurine ribonucleoside. Cancer Res. 1970 Dec;30(12):2852–2857. [PubMed] [Google Scholar]
- Larson S. M. Mechanisms of localization of gallium-67 in tumors. Semin Nucl Med. 1978 Jul;8(3):193–203. doi: 10.1016/s0001-2998(78)80028-2. [DOI] [PubMed] [Google Scholar]
- LePage G. A., Lin Y. T., Orth R. E., Gottlieb J. A. 5'-Nucleotides as potential formulations for administering nucleoside analogs in man. Cancer Res. 1972 Nov;32(11):2441–2444. [PubMed] [Google Scholar]
- Miller P. S., Braiterman L. T., Ts'o P. O. Effects of a trinucleotide ethyl phosphotriester, Gmp(Et)Gmp(Et)U, on mammalian cells in culture. Biochemistry. 1977 May 3;16(9):1988–1996. doi: 10.1021/bi00628a036. [DOI] [PubMed] [Google Scholar]
- Plunkett W., Cohen S. S. Penetration of mouse fibroblasts by 2'-deoxyadenosine 5'-phosphate and incorporation of the nucleotide into DNA. J Cell Physiol. 1977 May;91(2):261–270. doi: 10.1002/jcp.1040910211. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Fishman R. F., Gercel C. Growth inhibition of human tumor cells in soft-agar cultures by treatment with low levels of adenosine 5'-triphosphate. Cancer Res. 1983 Sep;43(9):4402–4406. [PubMed] [Google Scholar]
- Rapaport E. Treatment of human tumor cells with ADP or ATP yields arrest of growth in the S phase of the cell cycle. J Cell Physiol. 1983 Mar;114(3):279–283. doi: 10.1002/jcp.1041140305. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C., Baril E. F. HeLa cell DNA polymerase alpha is tightly associated with tryptophanyl-tRNA synthetase and diadenosine 5',5"'-P1,P4-tetraphosphate binding activities. Proc Natl Acad Sci U S A. 1981 Feb;78(2):838–842. doi: 10.1073/pnas.78.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C. Increased incorporation of adenosine into adenine nucleotide pools in serum-deprived mammalian cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1145–1147. doi: 10.1073/pnas.75.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974 Apr 29;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]