Abstract
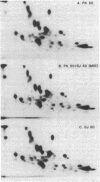
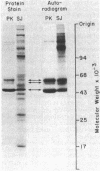
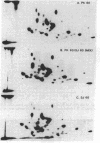
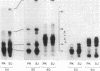
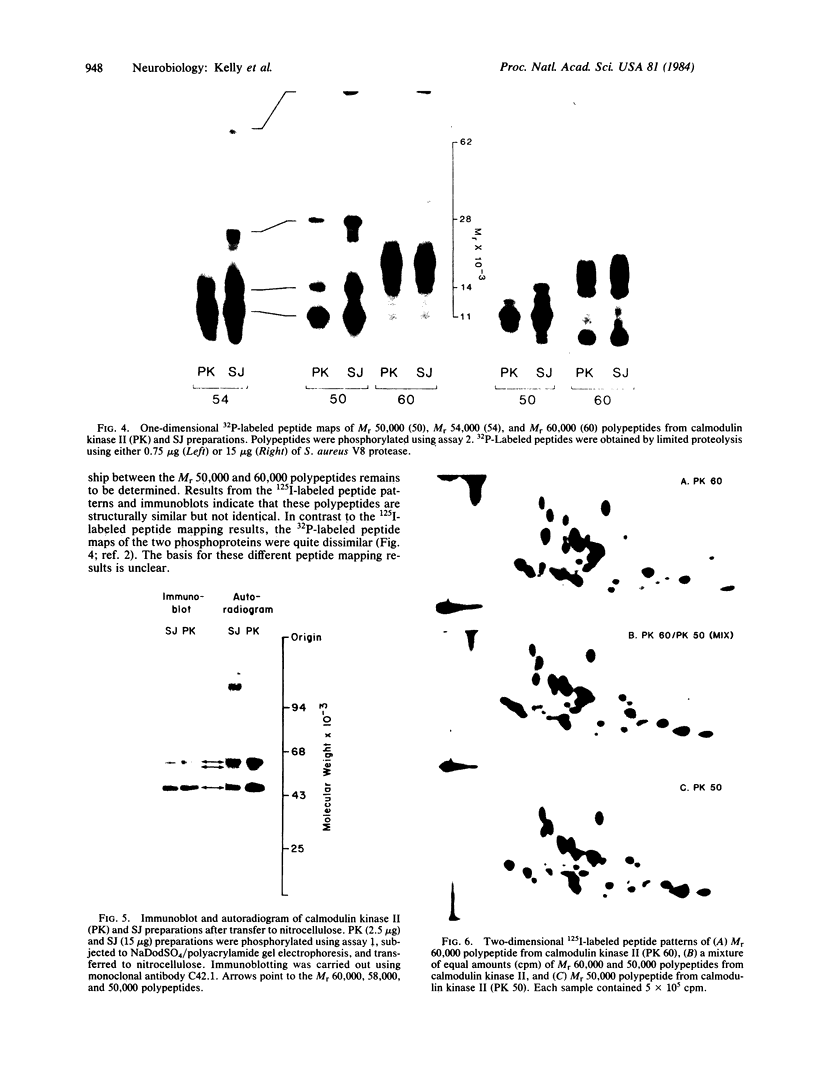
Polypeptides of Mr 50,000 and 60,000 in isolated synaptic junctions have been compared to polypeptides of corresponding molecular weight in Ca2+/calmodulin-dependent protein kinase II. The polypeptides of corresponding molecular weight from the two preparations were shown by several criteria to be indistinguishable. These criteria included 125I-labeled tryptic/chymotryptic peptide patterns, 32P-labeled proteolytic peptide maps, and crossreactivity on immunoblots using polyclonal and monoclonal antibodies. Furthermore, studies examining the phosphorylation of substrate proteins, by the endogenous synaptic junction kinase and by Ca2+/calmodulin-dependent protein kinase II, indicated that the two enzymes have similar substrate specificities. Since the Mr 50,000 polypeptide present in synaptic junctions is known to be the major postsynaptic density protein, the present results indicate that the major postsynaptic density protein is a component of Ca2+/calmodulin-dependent protein kinase II.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G., Churchill L., Cotman C. W. Proteins of the postsynaptic density. J Cell Biol. 1974 Nov;63(2 Pt 1):456–465. doi: 10.1083/jcb.63.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg F., Cohen R. S., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. J Cell Biol. 1977 Jul;74(1):204–225. doi: 10.1083/jcb.74.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. Function of a calmodulin in postsynaptic densities. III. Calmodulin-binding proteins of the postsynaptic density. J Cell Biol. 1981 Jun;89(3):449–455. doi: 10.1083/jcb.89.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Harris S. M., Jr, Huttner W. B., Greengard P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983 May;96(5):1355–1373. doi: 10.1083/jcb.96.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan S. D., Yost B., Crawford G. Putative 51,000-Mr protein marker for postsynaptic densities is virtually absent in cerebellum. J Cell Biol. 1982 Sep;94(3):743–748. doi: 10.1083/jcb.94.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D. J., Carlin R. K., Siekevitz P. Function of a calmodulin in postsynaptic densities. II. Presence of a calmodulin-activatable protein kinase activity. J Cell Biol. 1981 Jun;89(3):440–448. doi: 10.1083/jcb.89.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groswald D. E., Montgomery P. R., Kelly P. T. Synaptic junctions isolated from cerebellum and forebrain: comparisons of morphological and molecular properties. Brain Res. 1983 Nov 14;278(1-2):63–80. doi: 10.1016/0006-8993(83)90225-1. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W., Largen M. Cyclic AMP-stimulated protein kinases at brain synaptic junctions. J Biol Chem. 1979 Mar 10;254(5):1564–1575. [PubMed] [Google Scholar]
- Kelly P. T., Montgomery P. R. Subcellular localization of the 52,000 molecular weight major postsynaptic density protein. Brain Res. 1982 Feb 11;233(2):265–286. doi: 10.1016/0006-8993(82)91202-1. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Greengard P. Two calcium/calmodulin-dependent protein kinases, which are highly concentrated in brain, phosphorylate protein I at distinct sites. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1293–1297. doi: 10.1073/pnas.78.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci. 1983 Apr;3(4):818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Greengard P., Berzins K., Cohen R. S., Blomberg F., Grab D. J., Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979 Nov;83(2 Pt 1):308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]