Abstract
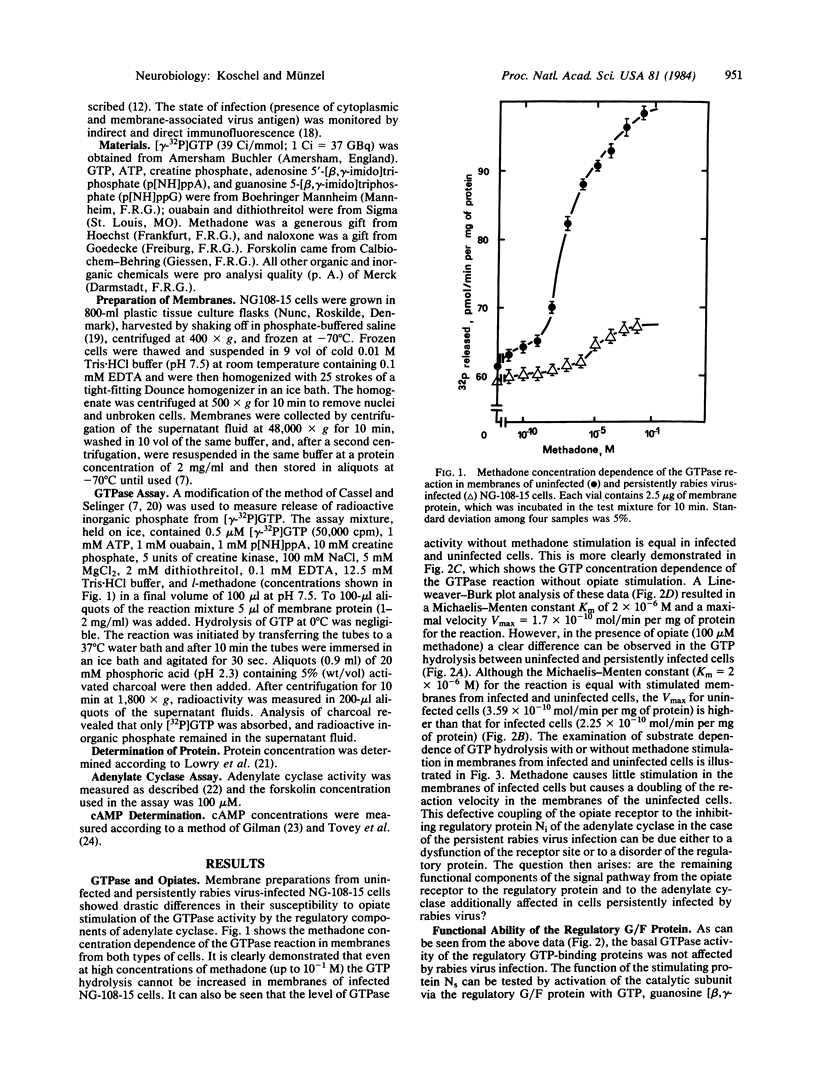
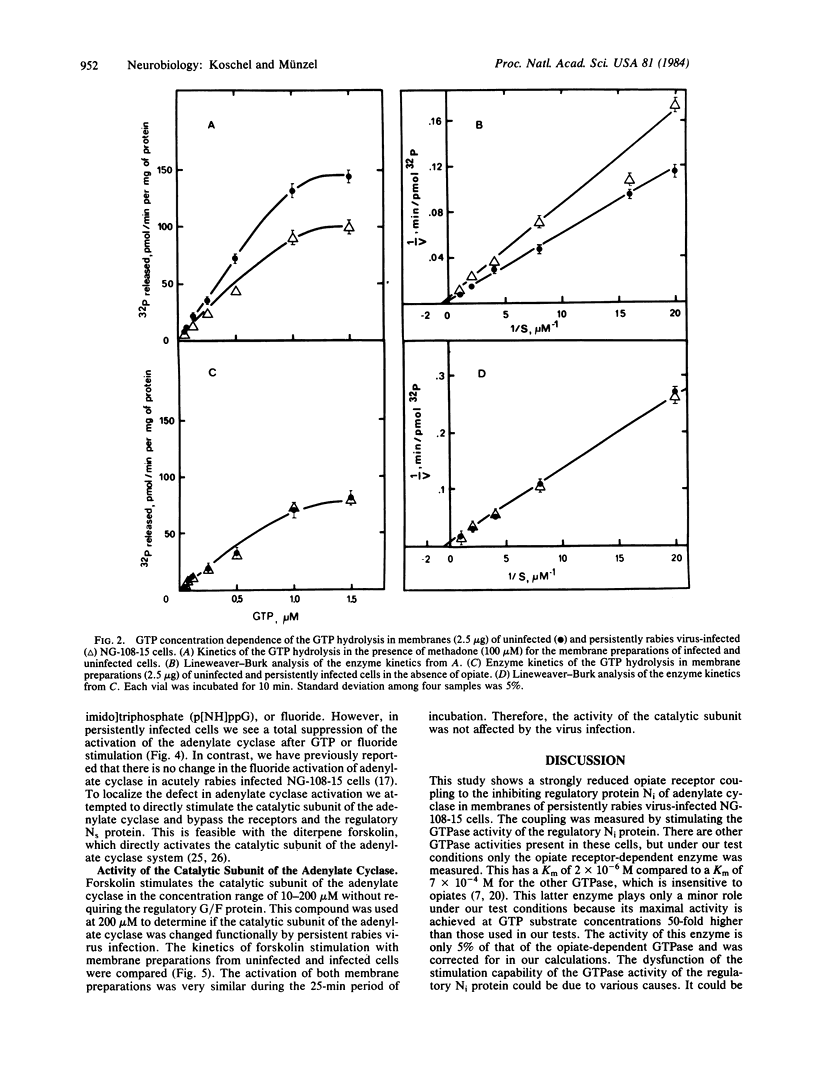
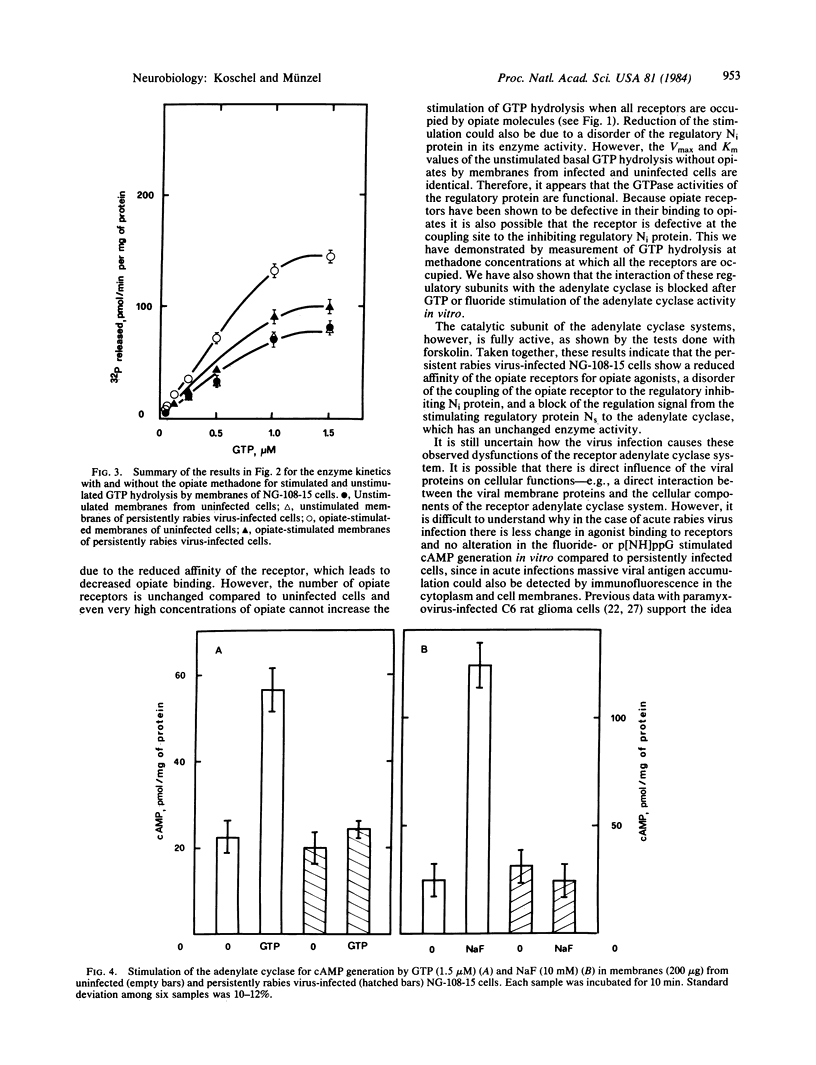
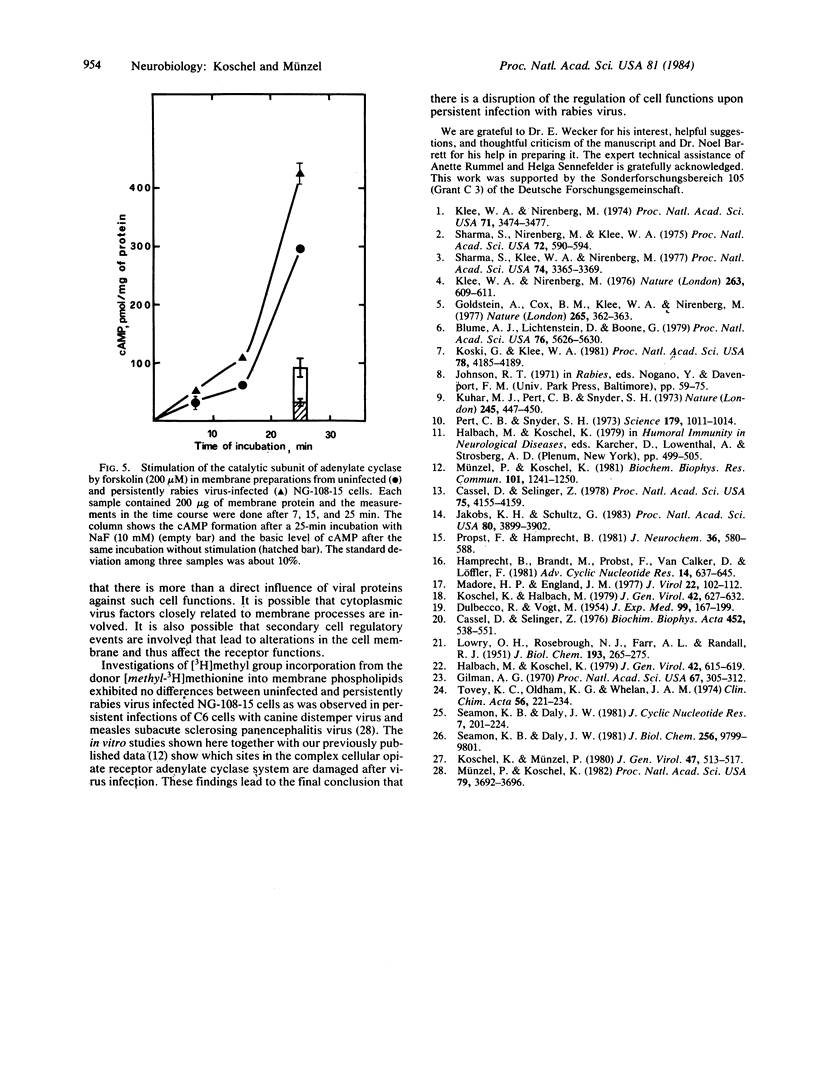
Acute and persistent rabies virus infection of mouse neuroblastoma-rat glioma hybrid cells (NG-108-15) results in a loss of the normal inhibiting function of opiates via the opiate receptor on hormone-stimulated adenylate cyclase activity. Previous studies of these persistently infected cells have shown a decrease in the affinity of the opiate receptors for agonists without any change in the number of these receptors. We now demonstrate that persistently infected cells are unable to couple the opiate receptors to the inhibitory regulatory protein Ni of the adenylate cyclase, as measured by the loss of stimulation of the GTPase activity of this protein. However, the unstimulated basal GTPase activities of the regulatory components Ni and Ns are unchanged in the persistently infected cells. These studies also reveal a disorder of the stimulation of the adenylate cyclase by GTP or fluoride via the stimulating regulatory G/F protein (Ns) in persistently infected cells, whereas direct stimulation of the catalytic subunit of the adenylate cyclase by forskolin remains unchanged. Therefore, there are different points of dysfunction caused by the persistent rabies infection in the signal pathway from the opiate receptor to the adenylate cyclase and from the stimulating Ns protein to the enzyme: (i) opiate receptor binding is reduced by a decrease of agonist affinity (previously published data), (ii) the stimulation of GTPase activity of the inhibiting regulatory component Ni of the adenylate cyclase system is inhibited, and (iii) the signal pathway from the stimulating regulatory component of the adenylate cyclase system to the unchanged activity of the catalytic subunit is defective.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A. J., Lichtshtein D., Boone G. Coupling of opiate receptors to adenylate cyclase: requirement for Na+ and GTP. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5626–5630. doi: 10.1073/pnas.76.11.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A., Cox B. M., Klee W. A., Nirenberg M. Endorphin from pituitary inhibits cyclic AMP formation in homogenates of neuroblastoma X glioma hybrid cells. Nature. 1977 Jan 27;265(5592):362–363. doi: 10.1038/265362a0. [DOI] [PubMed] [Google Scholar]
- Halbach M., Koschel K. Impairment of hormone dependent signal transfer by chronic SSPE virus infection. J Gen Virol. 1979 Mar;42(3):615–619. doi: 10.1099/0022-1317-42-3-615. [DOI] [PubMed] [Google Scholar]
- Hamprecht B., Brandt M., Propst F., van Calker D., Löffler F. Regulation by neurohormones of cyclic AMP concentration in cells derived from the nervous system. Adv Cyclic Nucleotide Res. 1981;14:637–645. [PubMed] [Google Scholar]
- Jakobs K. H., Schultz G. Occurrence of a hormone-sensitive inhibitory coupling component of the adenylate cyclase in S49 lymphoma cyc- variants. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3899–3902. doi: 10.1073/pnas.80.13.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. Mode of action of endogenous opiate peptides. Nature. 1976 Oct 14;263(5578):609–612. doi: 10.1038/263609a0. [DOI] [PubMed] [Google Scholar]
- Koschel K., Halbach M. Rabies virus infection selectively impairs membrane receptor functions in neuronal model cells. J Gen Virol. 1979 Mar;42(3):627–632. doi: 10.1099/0022-1317-42-3-627. [DOI] [PubMed] [Google Scholar]
- Koschel K., Muenzel P. Persistent paramyxovirus infections and behaviour of beta-adrenergic receptors in C-6 rat glioma cells. J Gen Virol. 1980 Apr;47(2):513–517. doi: 10.1099/0022-1317-47-2-513. [DOI] [PubMed] [Google Scholar]
- Koski G., Klee W. A. Opiates inhibit adenylate cyclase by stimulating GTP hydrolysis. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4185–4189. doi: 10.1073/pnas.78.7.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar M. J., Pert C. B., Snyder S. H. Regional distribution of opiate receptor binding in monkey and human brain. Nature. 1973 Oct 26;245(5426):447–450. doi: 10.1038/245447a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madore H. P., England J. M. Rabies virus protein synthesis in infected BHK-21 cells. J Virol. 1977 Apr;22(1):102–112. doi: 10.1128/jvi.22.1.102-112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel P., Koschel K. Alteration in phospholipid methylation and impairment of signal transmission in persistently paramyxovirus-infected C6 rat glioma cells. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3692–3696. doi: 10.1073/pnas.79.12.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel P., Koschel K. Rabies virus decreases agonist binding to opiate receptors of mouse neuroblastoma-rat glioma hybrid cells 108-CC-15. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1241–1250. doi: 10.1016/0006-291x(81)91581-3. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Snyder S. H. Opiate receptor: demonstration in nervous tissue. Science. 1973 Mar 9;179(4077):1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Propst F., Hamprecht B. Opioids, noradrenaline and GTP analogs inhibit cholera toxin activated adenylate cyclase in neuroblastoma x glioma hybrid cells. J Neurochem. 1981 Feb;36(2):580–588. doi: 10.1111/j.1471-4159.1981.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Sharma S. K., Klee W. A., Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey K. C., Oldham K. G., Whelan J. A. A simple direct assay for cyclic AMP in plasma and other biological samples using an improved competitive protein binding technique. Clin Chim Acta. 1974 Nov 8;56(3):221–234. doi: 10.1016/0009-8981(74)90133-8. [DOI] [PubMed] [Google Scholar]



