Abstract
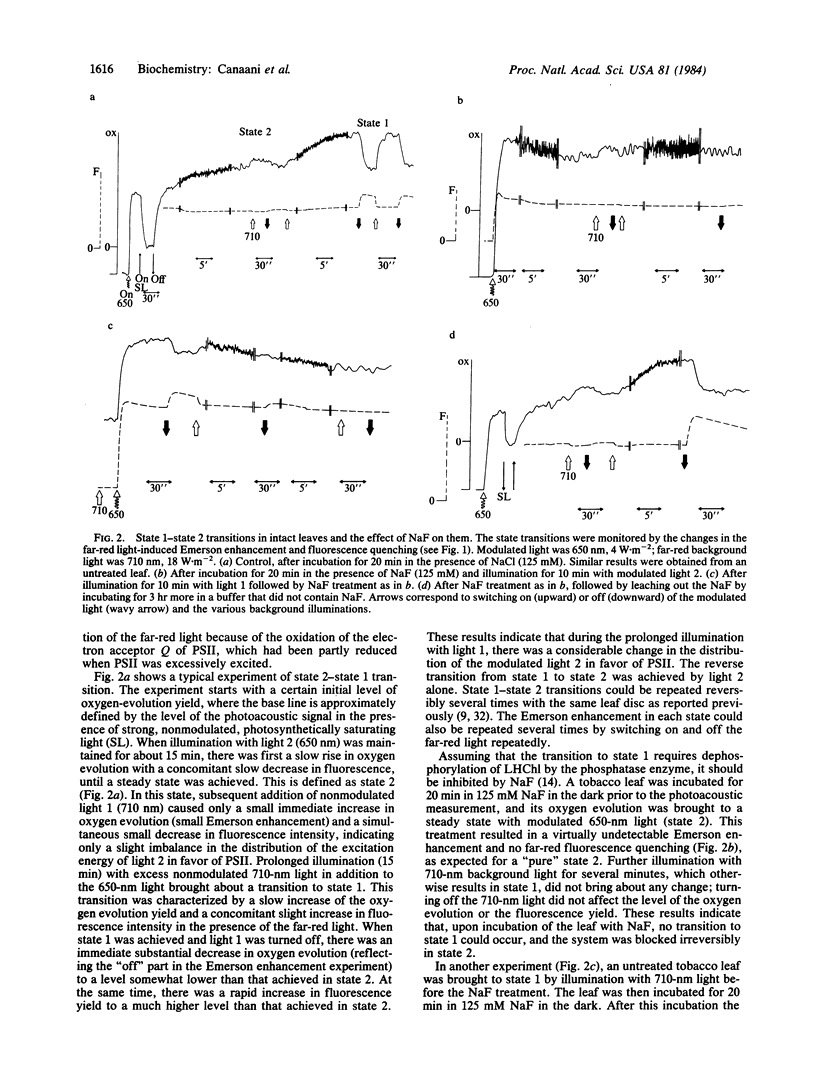
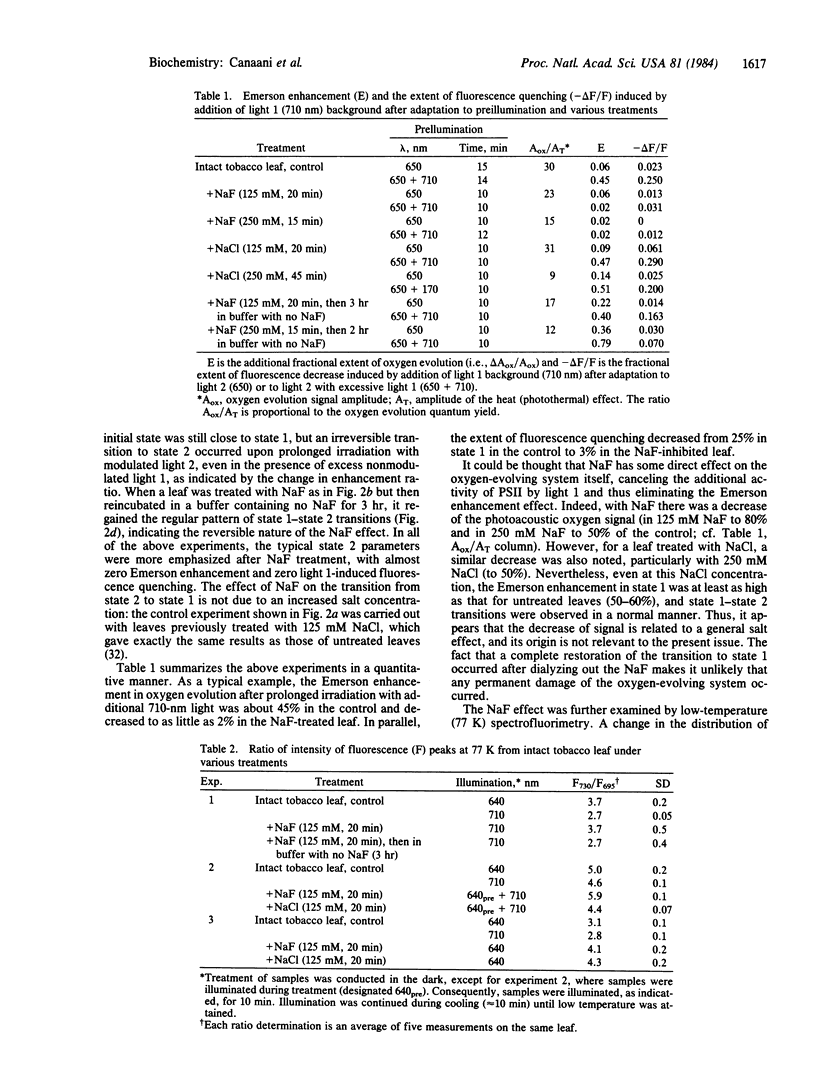
State 1-state 2 transitions in an intact tobacco leaf were monitored by the photoacoustic method. Modulated oxygen evolution yield and its enhancement by continuous far-red light (“Emerson enhancement”) were used to characterize the balance of light distribution between the two photosystems. These measurements were additionally supported by fluorimetry. Adaptation of the leaf to far-red light (λ [unk] 700 nm), mainly absorbed in photosystem I (light 1), results in state 1, where short-wavelength light (light 2) is distributed in favor of photosystem II. This is shown by a low yield of oxygen evolution, a high extent of Emerson enhancement, a concomitantly high extent of fluorescence quenching by far-red light, and a low ratio of the 77 K emission peaks at 730 and 695 nm. The magnitudes of these parameters were reversed when the leaf was adapted to light 2 (state 2), indicating a change towards a more equal distribution of the excitation between the two photosystems. Preincubation of an intact leaf with NaF, a specific phosphatase inhibitor, stimulated the extent of adaptation to light 2, shown by all the above criteria, and completely abolished adaptation to light 1. Light 1 preillumination prior to NaF treatment resulted initially in state 1, but then a transition to state 2 was irreversibly induced by any light. The NaF effect was specific because NaCl did not affect the state 1-state 2 transitions. Leaching out the NaF restored the original physiological transitions of the leaf. NaF presumably acts here in the same way as it acts in isolated thylakoids—by blocking the dephosphorylation of membranal proteins (particularly the chlorophyll a/b-protein complex) phosphorylated by a light 2-activated kinase. Our results give direct support to the suggestion [Allen, J. F., Bennett, J., Steinback, K. E. & Arntzen, C. J. (1981) Nature (London) 291, 25-29] that it is the phosphorylation level of thylakoid proteins that controls the light distribution between the two photosystems in vivo, shown previously in isolated thylakoids.
Keywords: state 1, state 2, Emerson enhancement, photosystems I and II
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfonzo R., Nelson N., Racker E. A Light-dependent Protein Kinase Activity of Chloroplasts. Plant Physiol. 1980 Apr;65(4):730–734. doi: 10.1104/pp.65.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. Evidence for a thylakoid-bound phosphoprotein phosphatase. Eur J Biochem. 1980 Feb;104(1):85–89. doi: 10.1111/j.1432-1033.1980.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. Phosphorylation of polypeptides of the light-harvesting chlorophyll protein complex. Eur J Biochem. 1979 Aug 15;99(1):133–137. doi: 10.1111/j.1432-1033.1979.tb13239.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. The protein kinase of thylakoid membranes is light-dependent. FEBS Lett. 1979 Jul 15;103(2):342–344. doi: 10.1016/0014-5793(79)81358-7. [DOI] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Farchaus J. W., Widger W. R., Cramer W. A., Dilley R. A. Kinase-induced changes in electron transport rates of spinach chloroplasts. Arch Biochem Biophys. 1982 Aug;217(1):362–367. doi: 10.1016/0003-9861(82)90512-4. [DOI] [PubMed] [Google Scholar]
- Horton P., Black M. T. Light-dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by adenosine 5'-triphosphate. Biochim Biophys Acta. 1981 Mar 12;635(1):53–62. doi: 10.1016/0005-2728(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Staehelin L. A., Arntzen C. J. Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochem Biophys. 1983 Apr 15;222(2):527–541. doi: 10.1016/0003-9861(83)90551-9. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969 Feb 25;172(2):242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Owens G. C., Ohad I. Phosphorylation of chlamydomonas reinhardi chloroplast membrane proteins in vivo and in vitro. J Cell Biol. 1982 Jun;93(3):712–718. doi: 10.1083/jcb.93.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried A., Reinhardt B. Distribution of excitation energy between photosystem I and photosystem II in red algae. III. Quantum requirements of the induction of a state 2-state 1 transition. Biochim Biophys Acta. 1980 Aug 5;592(1):76–86. doi: 10.1016/0005-2728(80)90115-2. [DOI] [PubMed] [Google Scholar]
- Yerxa E. J., Barber L. M., Diaz O., Black W., Azen S. P. Development of a hand sensitivity test for the hypersensitive hand. Am J Occup Ther. 1983 Mar;37(3):176–181. doi: 10.5014/ajot.37.3.176. [DOI] [PubMed] [Google Scholar]


