Abstract
The improvement of gene therapy protocols is intimately related to the establishment of efficient gene transfer methods. Electroporation has been increasingly employed in in vitro and in vivo protocols, and much attention has been given to increasing its transfection potential. The method is based on the application of an electric field of short duration and high voltage to the cells, forming reversible pores through which molecules can enter the cell. In this work, we describe the optimization of a protocol for the electroporation of K562 cells involving the combination of electric field, resistance and capacitance values. Using RPMI 1640 as pulsing buffer and 30 μg of pEGFP-N1 plasmid, 875 V cm−1, 500 μF and infinite resistance, we achieved transfection rates of 82.41 ± 3.03%, with 62.89 ± 2.93% cell viability, values higher than those reported in the literature. Analyzing cell cycle after electroporation, with three different electric field conditions, we observed that in a heterogeneous population of cells, viability of G1 cells is less affected by electroporation than that of cells in late S and G2/M phases. We also observed that efficiency of electroporation can be improved using the DNAse inhibitor Zn, immediately after the pulse. These results can represent a significant improvement of current methods of electroporation of animal and plant cells.
Keywords: Cell cycle, DNAse inhibitors, Electroporation, Gene transfer, K562 cells
Introduction
The improvement of gene therapy protocols is intimately related to the establishment of efficient gene transfer methods. Viral vectors have been shown to present great efficiency. Non-viral vectors, however, are safer so that great effort is dedicated to the development of methods to improve their efficiency.
Electroporation, or electropermeabilization, is one of the techniques employed for the transfer of non-viral vectors, as well as for the introduction of other molecules (drugs, RNA or protein) into the target cells. The method is based on the application of an electric field of short duration and high voltage to the cells. When the electric pulse surpasses the membrane capacitance, its stability is affected and transient, reversible pores are formed through which molecules can enter the cell (reviewed in Gehl 2003).
Although the method is being largely employed in vitro and in vivo, much attention has been given to increasing its transfection potential. Different parameters are analyzed, such as the conductivity and osmolarity of the electroporation buffer (Pucihar et al. 2001), the cell cycle stage at which target cells are during electroporation (Takahashi et al. 1991; Golzio et al. 2002), or the effects of centrifugation immediately after the electric pulse is applied (Li et al. 1999). Particular care must be taken with the viability of the transfected cells, since parameters, which increase transfection efficiency generally result in higher cell death rates.
The K562 cell line was established in 1975, from a patient with chronic myeloid leukemia (CML) in acute phase (Lozzio and Lozzio 1975). This cell line is very useful for the study of the pathways involved in CML development as well as of erithroid differentiation and the patterns of globin gene expression, due to its capacity to produce embryo and fetal hemoglobin. It has been largely employed in the investigation of alternative treatments for talassemias and sickle cell disease, as well as the pathways involved in the development of CML (Park et al. 2001; Rodrigue et al. 2001; Haynes et al. 2004).
Most of the studies in which K562 cells are electroporated do not aim at the improvement of the method itself, so that the protocols employed present great variation. The highest transfection efficiency described in the literature, with conventional electroporation, is 40% with 49% cell viability (Van Tendeloo et al. 2001). A new methodology, called nucleofection, which results in high levels of transfection and low cell death indices (74.7 ± 8.0% and 5.4 ± 0.2%, respectively), was recently described (Schakowski et al. 2004). These results, however, are not reproducible using conventional approaches, since electric pulse conditions are not informed by the company which markets the technology.
In this work, we describe the optimization of a protocol for the electroporation of K562 cells with better results than those previously described. The results are even superior to those obtained with nucleoporation, although with slightly lower cell viability. Our results also show that the cells most affected by electroporation are those in advanced cell cycle phase, and that Zn2+ can be used as an inhibitor of intracellular nucleases, increasing the efficiency of electroporation.
Materials and methods
Plasmid
The plasmid pEGFP-N1 (Clontech Lab, Palo Alto, CA, USA) contains the sequence coding for the EGFP protein under the control of the human cytomegalovirus (CMV) promoter. It also presents a gene for kanamicin/neomicin resistance (kanR/neoR), regulated by the early promoter and enhancer from SV40 virus. The plasmid was extracted by alkaline lysis from competent Escherichia coli, DH5-α strain, selected with kanamycin, and purified with the Plasmid Maxi-Prep Kit (Qiagen, Valencia, CA, USA). Plasmid preparations were quantified by spectrophotometry.
Cells
The K562 cell line (ATTC CCL 243) was maintained in logarithmic growth in RPMI 1640 medium (Cultilab, Campinas, Brazil) complemented with 10% fetal calf serum (FCS) (Cultilab) and 60 μg/ml gentamycin (Schering-Plough S/A, Rio de Janeiro, Brazil). The cells were cultured at 37°C in a humidified chamber with 5% CO2 in air, and passaged 1:10 twice a week. For some of the experiments, medium was complemented with 80 μM ZnSO4 (Reagen, Rio de Janeiro, Brazil), and 1 mM EDTA or EGTA (Sigma, St. Louis, MO, USA).
Electroporation
The cells were suspended in RPMI 1640 without FCS or antibiotics, at a concentration of 107 cells ml−1. A volume of 0.3 ml was transferred to a sterile electroporation cuvette (Bio-Rad Gene Pulser cuvette, 0.4 cm), and kept at room temperature for 15 min in presence of different concentrations of pEGFP-N1 to analyze the efficiency of transfection, or 15 μg ml−1 propidium iodide (PI) (Calbiochem, La Jolla, CA, USA) to analyze the efficiency of electroporation. Electroporation was performed with a Bio-Rad Gene Pulser® Transfection Apparatus, in different conditions. Other types of pulsing buffer were also analyzed, with different osmolarity (O1, O2 and O3, Golzio et al. 1998) and conductivity (M1, M2 and M3, Pucihar et al. 2001), as well as combinations of phosphate buffered saline (PBS) with or without HEPES (Sigma) and/or sucrose (Reagen).
After receiving the electric pulse, the cells were treated according to the protocol suggested by Li et al. (1999). Briefly, cells were centrifuged at 13,000g for 30 s and maintained pelleted for 20 min. The electroporation medium was then removed and the pellet was suspended in 3 ml of RPMI 1640 with 10% FCS and gentamycin, transferred to 12-well culture plates (Corning Star, Acton, MA, USA) and incubated for 1 h at 37°C in a humidified atmosphere of 5% CO2. Viability was determined by Trypan blue exclusion and viable cells were isolated by centrifugation through Ficol-Paque™ PLUS (Amersham Pharmacia Biotech AB, Uppsala, Switzerland). Live cells were suspended in RPMI 1640 with 10% FCS at 5–7 × 105 cells ml−1, and maintained at 37°C in a humidified atmosphere of 5% CO2 until analysis.
Efficiency of electroporation and transfection
Fluorescence of the cells was analyzed within the viable cell fraction, 24 and 48 h after electroporation, by examination in an inverted microscope (Axiovert 25 CFL, Carl Zeiss, Germany), with filter set 9 (excitation: BP 450–490, beamslitter: Ft 510, emission: LP515) and by flow cytometry, using a FACScalibur (Becton Dickinson, San Jose, CA, USA) and the CellQuest software (Becton Dickinson). For analysis of transfection efficiency (expression of the EGFP gene), data were collected with the fluorescence peak of 20,000 pulses in FL1 (FL1H), and logarithmically amplified, within a gate established in a FSC × SSC plot. Electroporation efficiency (presence of membrane pores, allowing entry of PI) was analyzed by similar procedures but using the red-orange (FL2H) fluorescence detector. Negative control was represented by non-electroporated K562 cells.
Cell cycle analysis
The method described by Overton and McCoy (1994) for analysis of cell cycle was used, with slight modification. One hour after electroporation, around 5 × 105 live cells were pelleted and suspended in a solution containing 50 μg PI, 10 mM Trizma base (Sigma), 10 mM NaCl, 0.7 U RNAse (Invitrogen Carlsbad, CA, USA), and 0.01% NP−40 (USB, Switzerland). After 15–30 min, the cells were analyzed by flow cytometry with collection of data representing width (FL2W) and area (FL2A) of 20,000 FL2 (red-orange) pulses, that were analyzed with the ModFit software (Verity Software House, Topsham, ME, USA). A sample of cells submitted to all steps except electroporation was used as negative control.
Statistics
All experiments were repeated at least three times in different days, and results are expressed as mean ± standard deviation of the results obtained in independent experiments. Statistical analysis was done with the Student’s t-test, and results were considered significantly different when p < 0.01.
Results
Optimization of electroporation conditions
Different electroporation conditions were tested, including variations in electric field (500, 625, 750, 875, 1000 and 1125 V cm−1), resistance (100, 200, 400 ohms and infinite) and capacitance (250, 500 and 960 μF). In each reaction, 37.5 μg ml−1 of the pEGFP-N1 plasmid was used. The best results, represented by 71.39 ± 4.61% EGFP-positive cells, were obtained in the combination 875 V cm−1, 500 μF and infinite resistance. Cell viability under these conditions was 65.22 ± 3.89%, and the time constant was between 21.0 and 22.5 ms.
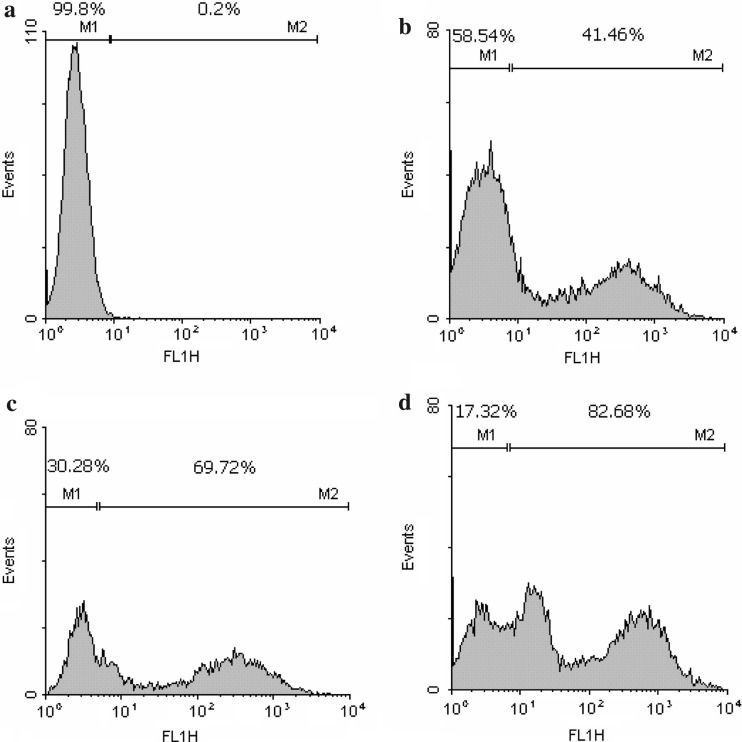
Having defined these conditions, we further tested different amounts of the plasmid. Electroporation was thus performed with 875 V cm−1, 500 μF, ∞ ohms, and with 25, 50 or 75 μg ml−1 of the pEGFP-N1 plasmid. As shown in Table 1, transfection efficiency was directly proportional to plasmid concentration. Viability was not significantly different, but a tendency for a decrease in cell viability with higher plasmid concentrations was observed. This analysis showed also two positive cell subpopulations, according to the fluorescence level, more easily seen when treatment was done with 75 μg ml−1 plasmid (Fig. 1).
Table 1.
Effect of different plasmid concentrations on electroporation efficiency
| Plasmid concentration (μg/ml) | Cell viability (%) | Electroporation efficiency (%) |
|---|---|---|
| Control | >99 | – |
| 25 | 66.23 ± 2.51 | 45.25 ± 3.81 |
| 50 | 64.06 ± 3.82 | 71.08 ± 2.16 |
| 75 | 62.89 ± 2.93 | 82.41 ± 3.03 |
Cell viability (% viable cells) was assessed by Trypan blue exclusion and transfection efficiency (% EGFP-positive cells) by flow cytometry
Fig. 1.
Electroporation of K562 cells with increasing plasmid concentration results in an increase in the frequency of EGFP-positive cells. (a) negative control—electroporation without plasmid; (b) 25 μg μl−1; (c) 50 μg ml−1; (d) 75 μg ml−1 of plasmid pEGFP-N1
The use of other types of pulsing buffer did not result in higher transfection efficiency. Cell viability was greatly reduced in media O1, O2, O3, M1, M2 or M3 (<20%), hampering the recovery of viable cells through Ficoll-Paque centrifugation and posterior analysis by flow cytometry. The use of PBS resulted in high cell viability (85.67%) but low transfection efficiency (15.78%), a result not significantly affected by the addition of HEPES and/or sucrose (data not shown).
Analysis of cell cycle after electroporation
To evaluate the effect of electroporation on cells in different phases of the cell cycle, K562 cells were electroporated in RPMI 1640 medium without FCS or antibiotics (107 cells/ml), with 15 μg PI/ml, using three different electric field conditions (875, 1000 or 1125 V cm−1). Resistance was set on infinite and capacitance on 500 μF. One hour later, cell viability was assessed by Trypan blue exclusion, viable cells were isolated as previously described and analyzed for PI staining and cell cycle. As shown in Table 2, whereas the electroporation efficiency was directly proportional to the electric field, cell viability was inversely proportional. Compared to the control samples, electroporated K562 cells presented higher frequency of cells in the G1 phase, and lower frequency of S/ G2/M cells.
Table 2.
Effect of electroporation on cells in different phases of the cell cycle
| Electric field | Cell viability (%) | Cell cycle phase (%) | Electroporation efficiency (%) | ||
|---|---|---|---|---|---|
| G1 | S | G2/M | |||
| Control | >99 | 36.4 | 55.5 | 8.0 | – |
| 875 V | 65.22 ± 1.89 | 42.9 ± 0.62% | 53.0 ± 1.04 | 4.1 ± 0.88 | 71.39 ± 4.61 |
| 1000 V | 40.51 ± 0.83 | 45.0 ± 0.89% | 50.1 ± 0.46 | 4.9 ± 1.21 | 78.09 ± 3.77 |
| 1125 V | 27.08 ± 1.46 | 48.4 ± 1.21% | 46.0 ± 0.96 | 5.6 ± 2.53 | 89.30 ± 2.56 |
Cell viability (% viable cells) was assessed by Trypan blue exclusion; cell cycle phase (% cells in each stage) and transfection efficiency (% EGFP-positive cells) were assessed by flow cytometry
Effect of DNAse inhibitors in the electroporation process
To test the effect of nuclease inhibitors on the electroporation process, K562 cells (107 cells ml−1) were electroporated with 37.5 μg ml−1 plasmid at 875 V cm−1, 500 μF and infinite resistance, in the presence of 80 μM ZnSO4 and 1 mM EDTA or EGTA. The treatment was done in four different conditions: 15 min before electroporation and until analysis of cell viability (pre), 15 min before electroporation and continued until analysis of transfection efficiency 48 h later (pre/cont), immediately after electroporation and until analysis of cell viability (post) and immediately after electroporation with maintenance of the reagents until analysis of transfection efficiency 48 h later (post/cont). Transfection efficiency was evaluated 48 h after electroporation, by analysis of the expression of EGFP by fluorescence microscopy and flow cytometry.
The results (Table 3) showed that transfection was more efficient when ZnSO4 was added after electroporation, independent of its maintenance until the moment of analysis, but not when ZnSO4 was added at the moment of electroporation. The Ca2+ chelator EGTA, on the other hand, did not affect the electroporation process, but cell viability decreased with the treatment. Treatment with EDTA, a Ca2+ and Mg2+ chelator, resulted in lower transfection efficiency and cell viability.
Table 3.
Cell viability (% viable cells) assessed by Trypan blue exclusion 1 h after medium change, and transfection efficiency (% EGFP-positive cells) analyzed by flow cytometry after electroporation done in presence of ZnSO4, EGTA or EDTA
| Treatment | Cell viability (%) | Transfection efficiency (%) |
|---|---|---|
| None | 69.62 ± 1.89 | 63.36 ± 2.19 |
| ZnSO4 pre | 46.29 ± 3.89 | 66.30 ± 1.25 |
| ZnSO4 pre/cont | 66.42 ± 1.89 | |
| ZnSO4 post | 62.79 ± 2.03 | 75.97 ± 2.36 |
| ZnSO4 post/cont | 74.70 ± 1.90 | |
| EGTA pre | 41.70 ± 2.28 | 60.21 ± 1.71 |
| EGTA pre/cont | 62.45 ± 0.86 | |
| EGTA post | 59.39 ± 1.25 | 62.90 ± 3.22 |
| EGTA post/cont | 61.27 ± 0.73 | |
| EDTA pre | 21.65 ± 3.02 | 16.75 ± 3.08 |
| EDTA pre/cont | 17.29 ± 2.44 | |
| EDTA post | 27.97 ± 2.92 | 38.21 ± 2.18 |
| EDTA post/cont | 36.89 ± 1.50 |
Discussion
This work describes the optimization of an electroporation protocol for K562 cells, involving the combination of electric field, resistance and capacitance values, combining standard electroporation processes with the post-pulse manipulation described by Li et al. (1999). The egfp reporter gene has been widely used to analyze the efficiency of gene transfer protocols, and the pEGFP-N1 plasmid was previously shown to transfect K562 cells with use of the polycationic compound SuperFect (Qiagen) (Teixeira et al. 2001). We obtained transfection rates of 82.41 ± 3.03%, with 62.89 ± 2.93% cell viability. These values are higher than those reported with standard electroporation protocols, of which the most efficient was described by Van Tendeloo et al. (2001) and resulted in 40% efficiency with 49% cell viability. The main differences presented by our protocol are the culture medium employed (RPMI 1640 versus X-vivo 15 medium) and the electroporation conditions (which in that study were electric field of 625 V cm−1 and capacitance of 1500 μF). The transfection efficiency obtained in the present study is even higher than that reported with the new electrotransfer technology known as nucleoporation (Schakowski et al. 2004), which resulted in 74.7 ± 8% positive cells. Cell viability in the present study is also higher than that in the report by Van Tendeloo et al. (2001), but lower than the viability reported by Schakowski et al. (2004), which resulted in 5.4 ± 0.2% dead cells. A comparison of our protocol with the nucleoporation method is, however, difficult because the conditions of electric field, resistance and capacitance, and the pulsing buffer composition are not described in that report.
The increase in transfection efficiency with lower cell viability, observed in higher electric field condition during the optimization experiments, has also been observed in other studies. In addition, our results show that among viable cells, isolated one hour after electroporation, viability of G1 cells is less affected by electroporation than that of cells in late S and G2/M phases. Similar results were reported by Golzio et al. (2002), but in that study purified cell populations were analyzed in G1, S or G2/M. This correlation between increased mortality among cells in the late phases of the cycle can be explained by the same formula, which explains the correlation between increase in the electric field intensity and electroporation efficiency. According to this formula, ΔVm = fEextr cosΦ electroporation begins when ΔVm exceeds the resting membrane potential (Vm), which has similar magnitude (around 1 V) in several cell types. In the formula, f is a factor which most authors describe as 1.5, Eext is the electric field used during electroporation, r represents the cell radius and Φ is the polar angle with respect to the external electric field; for spherical cells this value is zero (Pucihar et al. 2001; Gehl 2003). The voltage which will exceed the resting membrane potential of a spherical cell will thus be: Eext > 1 V (1.5r cos 0)−1 = 1 V × 1.5r−1.
For K562 cells, which have a 20 μm radius (Koeffler and Golde 1980), Eext is thus 1 V (1.5 × 20 μm)−1 or 330 V cm−1. As the cell doubles its volume at late S/G2 phases, its radius becomes 26% larger (according to the trigonometrical properties of spheres), so that for efficient electroporation the electric field must be higher than 1 V (1.5 × 25.2 μm)−1 = 260 V cm−1. This represents a 70 V cm−1 difference in the electric field necessary to exceed the membrane resting potential in G1 or G2 cells. In other words, submitted to the electric field necessary for generating a change in the potential of a G1 cell, G2 cells would have a ΔVm=330 V cm−1 × 1.5 × 0.00252 cm = 1.247 V, which is almost 25% higher. Treatment of G2 cells with the electroporation conditions which represent the optimum for G1 cells would thus produce irreversible membrane lesions, and as a consequence the number of live G1 cells after electroporation would be proportionally higher. This situation emphasizes the importance of using homogeneous populations for transfection, and suggests that the increased efficiency observed when cells are electroporated in the S (Takahashi et al. 1991) or G2/M (Goldstein et al. 1989; Golzio et al. 2002) phases is due to the adjustment of the electric field intensity which is applied, rather than to an intrinsic advantage of the phase in which the cells are.
Although G1 cells seem to have been less affected, the results suggest that they are efficiently transfected, since only 3–5 days after electroporation normal cell proliferation was observed (unpublished results). Although the study by Golzio et al. (2002) focused on the increased efficiency of transfection of G2/M cells, transfection of G1 cells was also observed. Dean (1997) showed that G1 cells can be transfected, as long as the plasmid presents a nuclear localization sequence (NLS). Plasmids containing the region of SV40 virus genome which includes the replication origin, early-late promoter and enhancer, were shown to be able to enter the nucleus of G1 cells; the most potent NLS in the sequence is the 72 bp repetition which forms the enhancer (Dean et al. 1999). This particular sequence is present in the plasmid used in the present work, as well as in the plasmid pEGFP-C1 used by Golzio et al. (2002). These observations suggest that the presence of this sequence is important in any vector used for gene transfer, particularly when the target cells of the transfection process are in G0/1 as happens in in vivo electroporation processes, as proposed by Blomberg et al. (2002).
The low proliferation rates observed after electroporation could be due to degradation of the plasmids by cytoplasmic nucleases. In this case, nucleotides derived from plasmid degradation would be incorporated in the cell dNTP pool (Lechardeur et al. 1999; Bureau et al. 2004). Depending on the plasmid sequence, this incorporation can cause an imbalance in the dNTP pool, which results in blockage of the cell cycle and/or cell death due to errors in DNA replication or DNA repair (Kunz and Kohalmi 1991).
Nucleases are responsible for plasmid degradation before their entry into the nucleus (Lechardeur et al. 1999; Bureau et al. 2004). To test the impact of this phenomenon on the results of transfection, we tested the effect of three reagents able to inhibit nucleases. EDTA, a chelator of Ca2+ and Mg2+ (necessary for the enzymatic activity of DNases) decreased electroporation efficiency and resulted in lower cell viability. Neumann et al. (1996) suggested that Ca2+ and Mg2+ are important for electroporation due to their interaction with the plasmid DNA, decreasing its negative charge and facilitating its interaction with the cell membrane. The results of post-pulse treatment in the present work, similar to those of pre-pulse treatment, suggest however that in electroporation these ions are more important for intra-cellular processes such as the transport of the plasmid through the nuclear pores. Our results with EGTA, used in a concentration able to chelate double of the Ca2+ present in RPMI 1640 medium, did not affect transfection efficiency. Cell viability was also not affected, showing that Ca2+ does not influence cell viability during electroporation as already proposed (Gehl 2003). The use of ZnSO4, on the other hand, induced significant increase in transfection efficiency, so that 15 μg plasmid in the presence of ZnSO4 had results similar to those obtained with 30 μg without the compound. These results emphasize the positive role of Zn2+, which is known to directly block DNAse activity (Mastrangeli et al. 1994). The effect was more visible when ZnSO4 was used after the electric pulse, suggesting a possible interference of this reagent during the pulse.
It is possible that the use of more potent DNAse inhibitors, such as the aurintricarboxylic acid or its combination with Zn2 can further increase the efficiency of transfection through electroporation, similar to the effect described on the transfection of salivary glands when naked DNA was used (Niedzinski et al. 2003). Finally, it is important to stress that in the present study the possibility of gene transfer mediated by Zn2+ is excluded by the fact that no significant differences were observed between the post (40 min exposure) and the post/cont (48 h exposure) Zn2+ treatments.
This study showed that modifications of the protocol for electroporation of K562 cells result in higher efficiency as compared to conventional methods. The cells most affected by electroporation were those in advanced cell cycle phase, and the use of Zn2+, inhibiting intracellular nucleases, increased the efficiency of electroporation. These results can represent a significant improvement of current methods of electroporation of animal cells.
Acknowledgments
The authors would like to thank Dr. Leonardo A. Karan Teixeira and Dr. Guido Lenz for helpful discussion and Dr. Arnaldo Zaha, Dr. Henrique B. Ferreira and their staff for gently sharing with us the Gene Pulser Transfection Apparatus. This research was funded by grants from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) and Instituto Do Milênio-CNPq/Rede De Terapia Gênica, Brazil.
References
- Blomberg P, Eskandarpour M, Xia S, Sylvén C, Islam KB. Electroporation in combination with a plasmid vector containing SV40 enhancer elements results in increased and persistent gene expression in mouse muscle. Biochem Biophys Res Commun. 2002;298:505–510. doi: 10.1016/S0006-291X(02)02486-5. [DOI] [PubMed] [Google Scholar]
- Bureau MF, Naimi S, Torero Ibad R, Seguin J, Georger C, Arnould E, Maton L, Blanche F, Delaere P, Scherman D. Intramuscular plasmid DNA electrotransfer: biodistribution and degradation. Biochim Biophys Acta. 2004;1676:138–148. doi: 10.1016/j.bbaexp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear import. Exp Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Fordis CM, Howard BH. Enhanced transfection efficiency and improved cell survival after electroporation of G2/M-synchronized cells and treatment with sodium butyrate. Nucleic Acids Res. 1989;17:3959–3971. doi: 10.1093/nar/17.10.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio M, Mora MP, Raynaud C, Delteil C, Teissie J, Rols MP. Control by osmotic pressure of voltage-induced permeabilization and gene transfer in mammalian cells. Biophys J. 1998;74:3015–3022. doi: 10.1016/S0006-3495(98)78009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio M, Teissie J, Rols MP. Cell synchronization effect on mammalian cell permeabilization and gene delivery by electric field. Biochim Biophys Acta. 2002;1563:23–28. doi: 10.1016/S0005-2736(02)00369-3. [DOI] [PubMed] [Google Scholar]
- Haynes J, Jr, Baliga BS, Obiako B, Ofori-Acquah S, Pace B. Zileuton induces hemoglobin F synthesis in erythroid progenitors: role of the L-arginine-nitric oxide signaling pathway. Blood. 2004;103:3945–3950. doi: 10.1182/blood-2003-08-2969. [DOI] [PubMed] [Google Scholar]
- Koeffler HP, Golde DW. Human myeloid leukemia cell lines: a review. Blood. 1980;56:344–350. [PubMed] [Google Scholar]
- Kunz BA, Kohalmi SE. Modulation of mutagenesis by deoxyribonucleotide levels. Annu Rev Genet. 1991;25:339–359. doi: 10.1146/annurev.ge.25.120191.002011. [DOI] [PubMed] [Google Scholar]
- Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW, Beatty B, Squire J, O’Brodovich H, Lukacs GL. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- Li LH, Ross P, Hui SW. Improving electrotransfection efficiency by post-pulse centrifugation. Gene Ther. 1999;6:364–372. doi: 10.1038/sj.gt.3300828. [DOI] [PubMed] [Google Scholar]
- Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- Mastrangeli A, O’Connell B, Aladib W, Fox PC, Baum BJ, Crystal RG. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am J Physiol. 1994;266:1146–1155. doi: 10.1152/ajpgi.1994.266.6.G1146. [DOI] [PubMed] [Google Scholar]
- Neumann E, Kakorin S, Tsoneva I, Nikolova B, Tomov T. Calcium-mediated DNA adsorption to yeast cells and kinetics of cell transformation by electroporation. Biophys J. 1996;71:868–877. doi: 10.1016/S0006-3495(96)79288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzinski EJ, Chen YJ, Olson DC, Parker EA, Park H, Udove JA, Scollay R, McMahon BM, Bennett MJ. Enhanced systemic transgene expression after nonviral salivary gland transfection using a novel endonuclease inhibitor/DNA formulation. Gene Ther. 2003;10:2133–2138. doi: 10.1038/sj.gt.3302125. [DOI] [PubMed] [Google Scholar]
- Overton WR, McCoy JP., Jr Reversing the effect of formalin on the binding of propidium iodide to DNA. Cytometry. 1994;16:351–356. doi: 10.1002/cyto.990160410. [DOI] [PubMed] [Google Scholar]
- Park JI, Choi HS, Jeong JS, Han JY, Kim IH. Involvement of p38 kinase in hydroxyurea-induced differentiation of K562. Cell Growth Differ. 2001;12:481–486. [PubMed] [Google Scholar]
- Pucihar G, Kotnik T, Kanduser M, Miklavcic D. The influence of medium conductivity on electropermeabilization and survival of cells in vitro. Bioelectrochemistry. 2001;54:107–115. doi: 10.1016/S1567-5394(01)00117-7. [DOI] [PubMed] [Google Scholar]
- Rodrigue CM, Arous N, Bachir D, Smith-Ravin J, Romeo PH, Galacteros F, Garel MC. Resveratrol, a natural dietary phytoalexin, possesses similar properties to hydroxyurea towards erythroid differentiation. Br J Haematol. 2001;113:500–507. doi: 10.1046/j.1365-2141.2001.02746.x. [DOI] [PubMed] [Google Scholar]
- Schakowski F, Buttgereit P, Mazur M, Marten A, Schottker B, Gorschluter M, Schmidt-Wolf IGH. Novel non-viral method for transfection of primary leukemia cells and cell lines. Genet Vaccines Ther. 2004;2:1. doi: 10.1186/1479-0556-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Furukawa T, Nikkuni K, Aoki A, Nomoto N, Koike T, Moriyama Y, Shinada S, Shibata A. Efficient introduction of a gene into hematopoietic cells in S-phase by electroporation. Exp Hematol. 1991;19:343–346. [PubMed] [Google Scholar]
- Teixeira LAK, Fricke CH, Bonorino CB, Bogo MR, Nardi NB. An efficient gene transfer system for hematopoietic cell line using transient and stable vectors. J Biotechnol. 2001;88:159–165. doi: 10.1016/S0168-1656(01)00276-0. [DOI] [PubMed] [Google Scholar]
- Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Broeckhoven C, Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.V98.1.49. [DOI] [PubMed] [Google Scholar]