Abstract
MedImmune Vaccines has engineered a live, attenuated chimeric virus that could prevent infections caused by parainfluenza virus type 3 (PIV3) and respiratory syncytial virus (RSV), causative agents of acute respiratory diseases in infants and young children. The work here details the development of a serum-free Vero cell culture production platform for this virus vaccine candidate. Efforts to identify critical process parameters and optimize culture conditions increased infectious virus titers by approximately 2 log10 TCID50/ml over the original serum-free process. In particular, the addition of a chemically defined lipid concentrate to the pre-infection medium along with the shift to a lower post-infection cultivation temperature increased virus titers by almost 100-fold. This improved serum-free process achieved comparable virus titers to the serum-supplemented process, and demonstrated consistent results upon scale-up: Vero cultures in roller bottles, spinner flasks and bioreactors reproducibly generated maximum infectious virus titers of 8 log10 TCID50/ml.
Keywords: Bioreactors, Microcarriers, Serum-free, Vero cultures, Virus production, Roller bottles
Introduction
Human parainfluenza virus type 3 (hPIV3) and respiratory syncytial virus (RSV) are non-segmented negative-strand RNA viruses of the paramyxovirus family. Together, hPIV3 and RSV are responsible for approximately one-third of all cases of pediatric respiratory diseases leading to hospitalization (Hall 2001). Despite the prevalence of hPIV3 and RSV infections in infants and young children, vaccines against these viruses remain unavailable (Tang et al. 2004).
To create a vaccine candidate against both hPIV3 and RSV, scientists at MedImmune Vaccines first constructed a recombinant bovine PIV3 virus vector by replacing the fusion (F) and hemagglutinin–neuraminidase (HN) glycoprotein genes in bovine PIV3 with the human PIV3 F and HN genes, respectively (Haller et al. 2000). Next, they inserted the RSV F gene into the bovine/human PIV3 vector backbone so that the resulting chimeric virus—named MEDI-534—expresses the RSV F protein (Tang et al. 2003). MEDI-534 functioned as a live, attenuated, bivalent vaccine in animal studies: hamsters and non-human primates immunized with MEDI-534 produced both hPIV3 hemagglutination-inhibiting and RSV-neutralizing serum antibodies and these animals demonstrated protection from challenges with hPIV3 and RSV (Tang et al. 2003, 2004).
In light of the promising immunogenicity and protection results in pre-clinical animal models, MedImmune Vaccines decided to initiate a clinical program for MEDI-534. Clinical enrollment would require a high-yielding host cell line for propagating this virus vaccine candidate. In earlier studies, company scientists compared the replication of three viruses closely related to MEDI-534 in different permissive mammalian cell lines (Haller et al. 2003). In all instances, Vero cells—originating from a continuous African green monkey kidney cell line—generated the highest virus titers. Subsequent experiments showed that the MEDI-534 virus maintained the RSV F gene inserts stably for up to 10 serial passages in Vero cells (Tang et al. 2003). In addition to its ability to support MEDI-534 propagation, the Vero cell line also presents other advantages. For instance, Vero cells have already passed regulatory hurdles and are currently used for the commercial manufacture of human polio and rabies vaccines (Montagnon 1989). Furthermore, regulatory authorities have provided guidelines on the use of Vero cells for viral vaccine production (WHO 1987a, b). Based on these advantages, MedImmune Vaccines selected the Vero cell line for propagating MEDI-534 (Tang et al. 2003, 2004).
Although Vero cells can grow in suspension as cell aggregates (Litwin 1992), they are typically propagated in static cultures or on microcarriers (Yokomizo et al. 2004; Wu et al. 2004; Berry et al. 1999) because they are usually considered to be anchorage-dependent. Since adherent cultures of Vero cells are well-characterized, have demonstrated an absence of tumorigenicity (Vincent-Falquet et al. 1989) and also have an excellent safety record (Montagnon and Vincent-Falquet 1998), no efforts were made in this work to adapt the anchorage-dependent Vero cells to grow in suspension culture.
This report describes efforts to develop a scalable, robust, and high-yielding Vero cell culture process for manufacturing MEDI-534. By identifying critical process parameters and optimizing culture conditions in small-scale experiments, the Vero cell culture process was improved. The new serum-free process increased infectious virus titers by approximately 100-fold over the original serum-free process and it demonstrated reproducible MEDI-534 titers of approximately 8 log10 TCID50/ml in roller bottles, spinner flasks, and bioreactors. The insights gained in developing this serum-free Vero production platform might benefit future endeavors to propagate viruses in mammalian cells.
Materials and methods
Cell line and culture maintenance
A vial of Vero cells (ATCC CCL-81, passage 121) was thawed in DMEM + 5% (v/v) fetal bovine serum (FBS) and passaged four times in this FBS-supplemented medium prior to direct adaptation to serum-free growth in OptiPRO SFM, a commercially available serum-free medium (SFM) that contains no animal-derived components. These anchorage-dependent cells were then banked after 10–15 passages in OptiPRO SFM. All Vero cells used in the experiments originated from these OptiPRO SFM banks.
Vero cells were routinely seeded at 5 × 104 cells/ml—in corresponding culture volumes of 35 ml per T-75 flask, 100 ml per T-225 flask, and 350 ml per 850 cm2 roller bottle (RB)—and passaged 3–5 days later. For cultures passaged 4–5 days post-seeding, a complete medium exchange was performed on each culture 3 days post-seeding. In preparation for subculturing, the spent media were aspirated, and the cells were rinsed twice with DPBS. To detach the Vero cells from the flasks, the cultures were incubated at 37°C with a 0.05% solution of trypsin–EDTA (3 ml per T-75 flask, 6 ml per T-225 flask, and 10 ml per RB). After the cells had detached, an equal volume of lima bean trypsin inhibitor (Worthington Biochemical Corporation, Lakewood, NJ) was added to quench trypsin activity. For all uninfected Vero cells, T-flask cultures were maintained in 37°C/5% CO2/95% Rh incubators and RB cultures were placed on a roller bottle apparatus operated at 0.3 rpm in a 37°C incubator.
In general, cells were pre-adapted to the media tested in each experiment for at least one passage prior to initiating the experiment, and cultures were discarded when the cell passage number exceeded 165. Glutamine-free culture media were always supplemented with 4 mM l-glutamine before use. Cell culture reagents and supplies were sourced from GIBCO/Invitrogen (Carlsbad, CA) and tissue culture wares were purchased from Corning (Corning, NY), unless specified otherwise.
Virus constructs and seed stock preparation
Construction of the MEDI-534 virus has been previously detailed (Tang et al. 2003). To generate the virus seed stock used for infection in all experiments, MEDI-534 obtained by plasmid rescue (Tang et al. 2003) was added at multiplicity of infection (MOI) of 0.001 to T-225 flask cultures of Vero cells that had grown for 3 days in OptiPRO SFM. The culture media were collected 4 days post-infection and stabilized with 10% (v/v) sucrose phosphate prior to aliquoting into multiple 1 ml cryovials. The virus seed stocks were stored at −80°C and thawed only immediately before use.
T-flask experiments
T-75 flasks were seeded with 1.75 × 106 Vero cells in 35 ml OptiPRO SFM (unless specified otherwise) and maintained in a 37°C/5% CO2/95% Rh incubator. At the time of infection, the spent medium was removed from each flask and the cells were rinsed with 2 × 10 ml DPBS. One flask in each medium condition was trypsinized and the cells were counted. To infect the cultures, DMEM (unless stated otherwise) containing MEDI-534 at the appropriate MOI was added to the remaining flasks. Post-infection, the T-flasks were maintained in humidified incubators with 5% CO2 overlay.
RB experiments
Each 850 cm2 RB was seeded with 1.5 × 107 Vero cells in the growth medium of choice and maintained at 37°C with constant rotation at 0.3 rpm. The basal growth medium was either OptiPRO SFM or virus production serum-free medium (VP-SFM), another commercially available animal-derived component free SFM from GIBCO/Invitrogen (Carlsbad, CA). In the instances specified, the basal growth medium was supplemented with FBS sourced from JRH Biosciences, Inc. (Lenexa, KS) or with a chemically defined lipid concentrate (CDLC) purchased from GIBCO/Invitrogen. To generate growth curves in OptiPRO + 0.5% FBS and VP-SFM + 1% CDLC, duplicate RB cultures in each medium were trypsinized and counted daily. Three days post-seeding, spent medium was removed from each RB and the cells were rinsed with 300 ml DPBS prior to infection. At least one flask in each medium condition was trypsinized and the cells were counted to determine the cell yield per flask and to calculate the appropriate amount of virus to add for infection at MOI 0.001. Unless stated otherwise, 500 ml of Williams’ Medium E (WME)—a chemically defined animal-derived component free basal medium—containing MEDI-534 at MOI 0.001 was added to each of the remaining flasks at the time of infection. Post-infection, all RBs were incubated at 33°C with constant rotation at 0.3 rpm.
Spinner flask experiments
The growth medium used in spinner experiments was either OptiPRO supplemented with 0.5% (v/v) FBS or VP-SFM supplemented with 1% (v/v) CDLC. Vero cells can grow on Cytodex 1, a polycationic dextran-based polymer, in both of these media. Cytodex 1 microcarriers were rehydrated and sterilized according to the manufacturer’s recommendations (Amersham Biosciences AB, Uppsala, Sweden). The microcarrier beads were then rinsed once with the appropriate growth medium before use. Two hours pre-seeding, each 250 ml glass spinner flask (Bellco Biotechnology, Inc., Vineland, NJ) was filled with 200 ml of the chosen growth medium containing 2 g/l Cytodex 1 and incubated at 37°C/5% CO2/95% Rh with 60 rpm agitation. To inoculate the spinner flasks, 2 × 107 Vero cells were added per vessel. All cultures were incubated pre-infection at 37°C/5% CO2/95% Rh with agitation maintained at 60 rpm. To generate growth curves, well-mixed samples were taken from the spinner flasks daily for nuclei counting. Prior to infection, a sample was removed from each spinner flask to determine the nuclei count and to calculate the amount of virus to use for infection at MOI 0.001. In preparation for infection, agitation was stopped for all the spinner flasks. After the microcarrier beads had settled, approximately 90% of the spent medium was replaced by WME containing MEDI-534 at MOI 0.001. Post-infection, the cultures were incubated at 33°C/5% CO2/95% Rh with constant agitation at 60 rpm.
Bioreactor experiments
Bioreactor experiments were conducted in 3 l stirred tank bioreactors (Applikon, Foster City, CA) with dissolved oxygen maintained at 50% of air saturation. Each bioreactor was equipped with an ADI 1030 Bio Controller (Applikon) and an ADI 1035 Bio Console (Applikon). Cytodex 1 microcarriers were prepared for use following the manufacturer’s instructions. Three hours pre-seeding, three bioreactors were each filled with 2 l VP-SFM supplemented with 1% (v/v) CDLC and 2 g/l Cytodex 1. Bioreactor contents were warmed to 37°C with heating blankets and agitated at 60 rpm with single marine impellers. The pH setpoints in the three bioreactors were 7.0, 7.2, and 7.4, respectively. Culture pH was controlled at the designated levels by the CO2 percentage in the inlet gas and by the addition of 1 N NaOH solution after the CO2 percentage in the inlet gas was reduced to 0%. To ensure that all cells used within each experiment had the same passage history, all bioreactors used within an experiment were inoculated with cells pooled from multiple RBs. Bioreactor contents were sampled daily for nuclei counting to generate growth curves. To prepare for infection, agitation was stopped in the bioreactors. After the microcarrier beads had settled, approximately 90% of the spent medium was replaced by WME containing MEDI-534 at MOI 0.001. Post-infection, the temperature setpoint was lowered to 33°C and agitation was increased to 100 rpm, while retaining the same pH and dissolved oxygen setpoints.
Collection of infected culture samples for virus quantification
To stabilize the virus, 10% (v/v) sucrose phosphate was added to samples taken from infected T-flask and RB cultures. After sampling from infected spinner flasks and bioreactors, microcarrier beads in the samples were allowed to settle and the culture supernatants collected were mixed with 10% (v/v) sucrose phosphate. All sucrose phosphate stabilized virus samples were immediately stored at −80°C until analyses.
Analytical methods
Cells from T-flasks and RBs were enumerated either using a hemocytometer or the Cedex cell counting and viability testing system (Innovatis Inc., Malvern, PA) operated according to the manufacturer’s directions. Cell densities in microcarrier cultures were determined by counting nuclei released by 0.1% crystal violet in 0.1 M citric acid solution (Hu and Wang 1987). Infectious virus titers were measured with an in-house 50% tissue culture infective dose (TCID50) assay and results were quantified in log10 TCID50/ml. To minimize inter- and intra-assay variabilities inherent to the TCID50 assay, samples generated from the same experiment were tested on the same day when possible, and replicates of four were used for each virus sample.
Results and discussion
T-flask experiments
Effect of MOI on virus production
Initial experiments to identify critical process parameters and optimize culture conditions were performed in T-75 flasks. The first small-scale experiment examined the effect of multiplicity of infection (MOI) on MEDI-534 production (Fig. 1). A repeat of this experiment gave the same findings. In brief, the three highest MOIs—0.1, 0.01, and 0.001—yielded comparable maximum virus titers (∼6 log10 TCID50/ml), whereas the two lowest MOIs—0.0001 and 0.00001—yielded maximum virus titers that were lower by at least 1 log10 TCID50/ml. A low MOI is preferred for manufacturing viruses because it would require less virus inoculum per infection and should, therefore, extend the lifespan of master virus banks. Hence, to conserve virus seed stocks while maximizing virus yields, infections in subsequent experiments used MOIs ranging from 0.01 to 0.001.
Fig. 1.
Virus production profiles at different MOIs. Duplicate T-75 flasks of serum-free Vero cells were infected 3 days post-seeding with MEDI-534 at MOI of 0.1, 0.01, 0.001, 0.0001, or 0.00001. Cultures were incubated at 37°C pre- and post-infection
Effects of time of infection and post-infection temperature on virus production
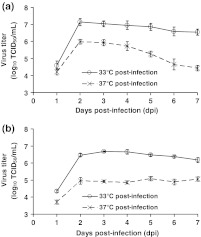
The second T-flask experiment investigated the combined effects of time of infection and post-infection cultivation temperature on MEDI-534 production (Fig. 2). By delaying the time of infection from 3 days post-seeding to 5 days post-seeding, the number of Vero cells at infection almost tripled from 0.6 × 107 cells/flask to 1.7 × 107 cells/flask. The peak virus titers measured in cultures infected at 3 days post-seeding (Fig. 2a) were slightly higher than that measured in cultures infected at 5 days post-seeding (Fig. 2b). Likewise, attempts to increase virus titers in spinner cultures by increasing the Vero cell concentration at infection failed. This “cell density effect” has been observed for adenovirus production in batch cultures: increasing the cell density at the time of infection did not increase maximum virus titers because specific virus productivity decreased (Henry et al. 2004; Kamen and Henry 2004; Nadeau and Kamen 2003). The successes in using perfusion cultures to overcome the “cell density effect” in adenovirus production (Henry et al. 2004; Yuk et al. 2004), compared with the failures encountered using conventional fed-batch strategies (Nadeau et al. 2002; Yuk et al. 2004), allude to the presence of one or more unidentified inhibitors that accumulate under batch and fed-batch conditions. Hence, cultures were typically infected 3 days post-seeding in subsequent experiments.
Fig. 2.
Effects of time of infection and post-infection temperature on virus titers. Serum-free Vero cultures were infected with MEDI-534 at MOI 0.01 either (a) 3 days post-seeding (0.6 × 107 cells/flask) or (b) 5 days post-seeding (1.7 × 107 cells/flask). Duplicate T-75 flasks were incubated at either 33°C or 37°C post-infection
In this T-flask experiment, although shifting the time of infection did not noticeably impact peak virus titers, shifting to a lower post-infection cultivation temperature (from 37 to 33°C) markedly elevated peak virus titers (by at least 1 log10 TCID50/ml). In follow-up studies, post-infection cultivation temperatures of 29, 31, and 35°C yielded peak virus titers that were intermediate between the results obtained with post-infection temperatures of 33 and 37°C (data not shown). Therefore, all ensuing MEDI-534 infections used 33°C as the post-infection incubation temperature.
This boost in virus titers from lowering cultivation temperatures is unexpected because MEDI-534 has not been cold-adapted (Tang et al. 2003). Although the specific reasons for this thermal sensitivity are not identified, amino acid changes in viral polymerases have produced PIV3 viruses that exhibit temperature-sensitive phenotypes (Feller et al. 2000; Skiadopoulos et al. 1999). Other virus systems have demonstrated similar thermal sensitivity: the cultivation of retrovirus packaging cells at 32°C instead of 37°C increased the yield of infectious virus particles by a maximum of 2- to 15-fold (Kaptein et al. 1997; Kotani et al. 1994; Lee et al. 1996).
Effect of pre-infection culture medium on virus production
The third T-flask experiment assessed the impact of pre-infection culture media on virus production (Table 1). To enhance the pre-infection growth media, Vero cultures were supplemented with FBS at the time of seeding. By adding FBS to OptiPRO SFM, cell yields at infection increased almost 2-fold, and maximum virus titers increased at least 5-fold. FBS supplementation at the 0.5, 2, and 5% (v/v) levels produced comparable cell yields (1.0–1.1 × 107 cells/flask) and virus titers (7.6–7.8 log10 TCID50/ml), suggesting that increasing the FBS concentration will not further improve cell growth or virus production. In an analogous experiment conducted using a retrovirus packaging cell line, Lee et al (1996) obtained similar results: retroviral vector titers doubled upon serum addition, but virus production was dose-independent in the serum supplementation range tested (1–20%).
Table 1.
Comparison of cell yields and virus titers in cultures titrated with FBS pre-infection
| Pre-infection culture medium | Cell yield at infection (cells/flask) | Maximum virus titer (log10 TCID50/ml) |
|---|---|---|
| DMEM + 5% FBS | 1.1 × 107 | 7.8 ± 0.2 |
| OptiPRO + 2% FBS | 1.0 × 107 | 7.6 ± 0.2 |
| OptiPRO + 0.5% FBS | 1.1 × 107 | 7.7 ± 0.2 |
| OptiPRO SFM | 0.6 × 107 | 6.9 ± 0.3 |
Vero cells were seeded in media supplemented with FBS at different concentrations. Three days post-seeding, duplicate T-75 flasks were infected with MEDI-534 at MOI 0.001 and incubated at 33°C. TCID50 data represent mean of duplicate cultures ± standard deviation
Roller bottle (RB) experiments
Effect of serum supplementation on cell growth and virus production
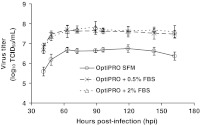
To determine the reproducibility and scalability of the previous FBS titration experiment, cell yields at the time of infection and MEDI-534 titers were measured in RB cultures supplemented with 0.5% and 2% FBS (Fig. 3). Three days post-seeding, the cell yield for the OptiPRO SFM control culture was only 1.9 × 107 cells/RB, even though all cultures were inoculated at 1.5 × 107 cells/RB. In contrast, T-flask cultures of Vero cells, seeded at 1.75 × 106 cells/flask in OptiPRO SFM, more than tripled in cell numbers after 3 days of culture (Table 1 and Fig. 2). These results indicate that OptiPRO SFM preferentially supports cell growth and attachment in static cultures. When OptiPRO SFM was supplemented with 0.5% (v/v) and 2% (v/v) FBS, cell growth in the RB cultures correlated with that observed in the T-flask cultures (Table 1)—cell numbers increased about 6-fold after 3 days in both cases. In accord with the T-flask findings (Table 1), the serum-supplemented RB cultures yielded significantly higher virus titers than the OptiPRO SFM cultures.
Fig. 3.
Virus production profiles in RB cultures titrated with FBS pre-infection. Vero cells were seeded in one of the following media in triplicate RBs: OptiPRO SFM, OptiPRO SFM + 0.5% (v/v) FBS, and OptiPRO + 2% (v/v) FBS. Three days post-seeding, one RB in each condition was trypsinized for cell counting: OptiPRO SFM (1.9 × 107 cells/flask), OptiPRO SFM + 0.5% (v/v) FBS (9.3 × 107 cells/flask), and OptiPRO + 2% (v/v) FBS (10.4 × 107 cells/flask). The remaining 2 × 3 RBs were infected with MEDI-534 at MOI 0.001
Media optimization efforts
Regulatory authorities in the United States (Food and Drug Administration) and in Europe (European Medicine Evaluation Agency) have encouraged biologics manufacturers to reduce or eliminate the use of substances of animal origin in their production processes (Castle and Robertson 1999) because of growing concerns about human exposure to infectious agents such as transmissible spongiform encephalopathies (Asher 1999; Galbraith 2002) and adventitious viruses (Erickson et al. 1989).
To minimize potential regulatory concerns, numerous RB experiments were conducted to identify a serum-free production process that can support MEDI-534 titers comparable to that achieved with the serum-containing process. Some of these experiments focused on optimizing the post-infection culture medium. In head-to-head comparisons with several media that were free of animal-derived components, WME—a chemically defined SFM of known composition (Williams and Gunn 1974)—yielded the highest virus titers (data not shown). Consequently, ensuing infections with MEDI-534 employed WME exclusively as the virus production medium. Other RB experiments focused on optimizing the pre-infection culture medium. In testing several commercially available as well as two in-house animal-derived component free media designed for Vero cultures (data not shown), the best Vero growth and MEDI-534 titers were achieved with VP-SFM developed by the same manufacturer (GIBCO/Invitrogen) as OptiPRO SFM. However, the maximum titers obtained with VP-SFM alone were at least 0.5 log10 TCID50/ml lower than those obtained with OptiPRO + 0.5% (v/v) FBS.
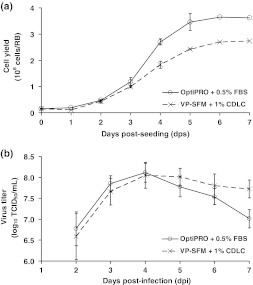
The remaining experiments in RBs focused on finding a replacement for FBS in the pre-infection medium. In screening several plant hydrolysates and lipid concentrates from different commercial suppliers, the highest titers were obtained by supplementing VP-SFM with a CDLC purchased from GIBCO/Invitrogen. Figure 4 shows the typical cell growth and virus production profiles in the two top-performing pre-infection growth media (OptiPRO + 0.5% (v/v) FBS and VP-SFM + 1% (v/v) CDLC). The maximum cell yields differed by about 30% in the two media, but peak virus titers were identical (8.1 log10 TCID50/ml). Since titrating VP-SFM with different concentrations of CDLC did not further improve culture performance (data not shown), all remaining experiments used VP-SFM + 1% (v/v) CDLC as the pre-infection serum-free Vero growth medium.
Fig. 4.
Comparison of (a) cell growth and (b) virus production in RBs using different pre-infection media. Vero cells were seeded in either OptiPRO + 0.5% (v/v) FBS or VP-SFM + 1% (v/v) CDLC. To generate the growth curves (a), duplicate RBs in each condition were counted daily. To generate the virus production profiles (b), duplicate RB cultures in the two different pre-infection media were infected with MEDI-534 3 days post-seeding. The infected cultures were sampled daily from 2 to 7 days post-infection
To our knowledge, this is the first report to demonstrate the successful use of a chemically defined lipid supplement to improve cell growth and virus production in Vero cultures. The underlying mechanisms for these observed process improvements are not known. However, accumulated evidence indicates that several viruses, including RSV, utilize plasma membrane microdomains—called lipid rafts—for virus assembly and budding (Bender et al. 2003; Brown et al. 2005). Since lipid rafts are rich in cholesterol and sphingolipids, CDLC supplementation may enhance virus replication by promoting lipid raft formation. If this hypothesis is true, the addition of CDLC to serum-free media may be effective in facilitating cell attachment and in increasing titers of enveloped viruses in other mammalian culture systems.
Spinner flask experiments
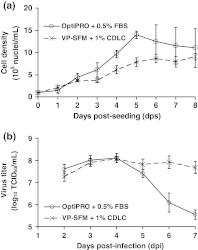
To prepare for culturing Vero cells on microcarriers in bioreactors, multiple microcarrier process parameters—including seeding density, serum-free growth medium, time of infection, agitation rate, microcarrier type and bead concentration—were tested in spinner flasks (data not shown). After the optimum microcarrier process conditions were defined, the following spinner flask experiment was conducted. This experiment compared Vero cell growth (Fig. 5a) and MEDI-534 titers (Fig. 5b) in serum-supplemented (OptiPRO + 0.5% (v/v) FBS) and serum-free (VP-SFM + 1% (v/v) CDLC) pre-infection media. Despite differences in the observed cell growth, both pre-infection media yielded identical peak virus titers of 8.1 log10 TCID50/ml. However, for unknown reasons, the infectious virus titer decreased by an average of 2.6 log10TCID50/ml between days 4 and 7 post-infection in the serum-supplemented cultures, whereas the infectious virus titer only decreased by an average of 0.4 log10 TCID50/ml over the same time period in the serum-free cultures. In contrast, serum-supplemented cultures in RBs did not exhibit such a substantial loss in infectious virus titer (Fig. 4b). As a result of these spinner flask findings, all subsequent microcarrier cultures used VP-SFM + 1% (v/v) CDLC for cell growth prior to MEDI-534 infection.
Fig. 5.
Comparison of (a) cell growth and (b) virus production in microcarrier cultures using different pre-infection media. Vero cells were seeded in spinner flasks containing 2 g/l Cytodex 1 in either OptiPRO + 0.5% (v/v) FBS or VP-SFM + 1% (v/v) CDLC. To generate the growth curves (a), samples were taken daily from uninfected duplicate flasks for nuclei counts. To generate the virus production profiles (b), duplicate flasks in each pre-infection media were infected 5 days post-seeding with MEDI-534
Bioreactor experiments
The bioreactor experiments assessed the scalability of this serum-free MEDI-534 production process and also evaluated the dependence of cell growth and virus production on culture pH. All three parallel bioreactor cultures maintained at pH setpoints of 7.0, 7.2, and 7.4 showed comparable growth and identical maximum virus titers (8.0 log10 TCID50/ml). Pre-infection cell growth and virus production profiles in the bioreactor cultures were similar that observed in the VP-SFM + 1% (v/v) CDLC spinner flask cultures (Fig. 5). A repeat of this bioreactor experiment generated the same results, indicating that cell growth and virus production are relatively pH-independent within the pH 7.0–7.4 range in bioreactors. These results demonstrate the success in scaling up the microcarrier process from spinner flasks to bioreactors.
Conclusions
The scalable serum-free production platform developed in this work supported reproducible Vero cell growth and MEDI-534 virus production. By combining pre-infection medium supplementation with post-infection temperature shift, the resulting serum-free production process increased infectious virus titers by almost 100-fold. Although cell yields were higher in the serum-supplemented cultures, virus titers were comparable in the serum-containing cultures and lipid-supplemented serum-free cultures. Vero cultures in T-flasks, RBs, spinner flasks, and bioreactors all achieved maximum infectious virus titers of ∼8 log10 TCID50/ml. The serum-free Vero process described here may potentially be used as a generic platform for the production of other PIV/RSV vaccine candidates.
Acknowledgements
We thank Richard R. Spaete, Roderick S. Tang, Mia MacPhail, Jeanne H. Schickli, Jeanne M. Guzzetta, Jasmine Kaur, Elizabeth Stillman, and Aurelia Haller for generating the original MEDI-534 vectors by plasmid rescue.
Glossary
- CDLC
Chemically defined lipid concentrate
- F
Fusion
- FBS
Fetal bovine serum
- HN
Hemagglutinin–neuraminidase
- hPIV3
Human parainfluenza virus type 3
- MEDI-534
Chimeric human parainfluenza virus type 3/respiratory syncytial virus
- MOI
Multiplicity of infection
- PIV3
Parainfluenza virus type 3
- RB
Roller bottle
- RSV
Respiratory syncytial virus
- SFM
Serum-free medium
- TCID50
50% Tissue culture infective dose
- Vero
African green monkey kidney
- VP-SFM
Virus production serum-free medium
- WME
Williams’ Medium E
References
- Asher DM. The transmissible spongiform encephalopathy agents: concerns and responses of United States regulatory agencies in maintaining the safety of biologics. Dev Biol Stand. 1999;100:103–118. [PubMed] [Google Scholar]
- Bender FC, Whitbeck JC, Leon MP, Lou H, Eisenbery RJ, Cohen GH. Specific association of glycoprotein B with lipid rafts during Herpes Simplex Virus entry. J Virol. 2003;77:9542–9552. doi: 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JM, Barnabe N, Coombe KM, Butler M. Production of reovirus type-1 and type-3 from Vero cells grown on solid and macroporous microcarriers. Biotechnol Bioeng. 1999;62:12–19. doi: 10.1002/(SICI)1097-0290(19990105)62:1<12::AID-BIT2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Brown G, Rixon HWM, Steel J, McDonald TP, Pitt AR, Graham S, Sugrue RJ. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology. 2005;338:69–80. doi: 10.1016/j.virol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Castle P, Robertson JS. Animal sera, animal sera derivatives and substitutes used in the manufacture of pharmaceuticals: viral safety and regulatory aspects. Dev Biol Stand. 1999;99:191–196. [PubMed] [Google Scholar]
- Erickson GA, Landgraf JG, Wessman SJ, Koski TA, Moss LM. Detection and elimination of adventitious agents in continuous cell lines. Dev Biol Stand. 1989;70:59–66. [PubMed] [Google Scholar]
- Feller JA, Smallwood S, Skiadopoulos MH, Murphy BR, Moyer SA. Comparison of identical temperature-sensitive mutations in the l polymerase proteins of Sendai and Parainfluenza 3 viruses. Virology. 2000;275:190–201. doi: 10.1006/viro.2000.0535. [DOI] [PubMed] [Google Scholar]
- Galbraith DN. Transmissible spongiform encephalopathies and tissue cell culture. Cytotechnology. 2002;39:117–124. doi: 10.1023/A:1022935117274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CD. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1927. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- Haller AA, Miller T, Mitiku M, Coelingh K. Expression of the surface glycoproteins of human parainfluenza virus type 3 by bovine parainfluenza virus type 3, a novel attenuated virus vaccine vector. J Virol. 2000;74:11626–11635. doi: 10.1128/JVI.74.24.11626-11635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller AA, Mitiku M, MacPhail M. Bovine parainfluenza virus type 3 (PIV3) expressing the respiratory syncytial virus (RSV) attachment and fusion proteins protects hamsters from challenge with human PIV3 and PSV. J Gen Virol. 2003;84:2153–2162. doi: 10.1099/vir.0.19079-0. [DOI] [PubMed] [Google Scholar]
- Henry O, Dormond E, Perrier M, Kamen A. Insights into adenoviral vector production kinetics in acoustic filter-based perfusion cultures. Biotechnol Bioeng. 2004;86:765–774. doi: 10.1002/bit.20074. [DOI] [PubMed] [Google Scholar]
- Hu WS, Wang DIC. Selection of microcarrier diameter for the cultivation of mammalian cells on microcarriers. Biotechnol Bioeng. 1987;30:548–557. doi: 10.1002/bit.260300412. [DOI] [PubMed] [Google Scholar]
- Kaptein LCM, Greijer AE, Valerio D, Beusechem VW. Optimized conditions for the production of recombinant amphotropic retroviral vector preparations. Gene Ther. 1997;4:172–176. doi: 10.1038/sj.gt.3300373. [DOI] [PubMed] [Google Scholar]
- Kamen A, Henry O. Development and optimization of an adenovirus production process. J Gene Med. 2004;6:S184–S192. doi: 10.1002/jgm.503. [DOI] [PubMed] [Google Scholar]
- Kotani H, Newton PB, Zhang S, Chiang YL, Otto E, Weaver L, Blaese RM, Anderson WF, McGarrity GJ. Improved methods of retroviral vector transduction and production for gene therapy. Hum Gene Ther. 1994;5:19–28. doi: 10.1089/hum.1994.5.1-19. [DOI] [PubMed] [Google Scholar]
- Lee SG, Kim S, Robbins PD, Kim BG. Optimization of environmental factors for the production and handling of recombinant retrovirus. Appl Microbiol Biotechnol. 1996;45:477–483. doi: 10.1007/BF00578459. [DOI] [PubMed] [Google Scholar]
- Litwin J. The growth of Vero cells in suspension as cell-aggregates in serum-free media. Cytotechnology. 1992;10:169–174. doi: 10.1007/BF00570893. [DOI] [PubMed] [Google Scholar]
- Montagnon BJ. Polio and rabies vaccines produced in continuous cell lines: a reality for Vero cell line. Dev Biol Stand. 1989;70:27–47. [PubMed] [Google Scholar]
- Montagnon BJ, Vincent-Falquet JC. Experience with the Vero cell line. Dev Biol Stand. 1998;93:119–123. [PubMed] [Google Scholar]
- Nadeau I, Gilbert PA, Jacob D, Perrier M, Kamen A. Low-protein medium affects the 293SF central metabolism during growth and infection with adenovirus. Biotechnol Bioeng. 2002;77:91–104. doi: 10.1002/bit.10128. [DOI] [PubMed] [Google Scholar]
- Nadeau I, Kamen A. Production of adenovirus vector for gene therapy. Biotechnol Adv. 2003;20:475–489. doi: 10.1016/S0734-9750(02)00030-7. [DOI] [PubMed] [Google Scholar]
- Skiadopoulos MH, Surman S, Tatem JM, Paschalis M, Wu SL, Udem SA, Durbin P, Collins PL, Murphy BR. Identification of mutations contributing to the temperature-sensitive, cold-adapted, and attenuation phenotypes of the live-attenuated, cold-passaged 45 (cp45) human parainfluenza virus 3 candidate vaccine. J Virol. 1999;73:1374–1381. doi: 10.1128/jvi.73.2.1374-1381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RS, Schickli JH, MacPhail M, Fernandes F, Bicha L, Spaete J, Fouchier RAM, Osterhaus ADME, Spaete R, Haller AA. Effects of human metapneumovirus and respiratory syncytial virus antigen insertion in two 3′ proximal genome positions of bovine/human parainfluenza virus type 3 on virus replication and immunogenicity. J Virol. 2003;77:10819–10828. doi: 10.1128/JVI.77.20.10819-10828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RS, MacPhail M, Schickli JH, Kaur J, Robinson CL, Lawlor HA, Guzzetta JM, Spaete RR, Haller AA. Parainfluenza virus type 3 expressing the native or soluble fusion (F) protein of respiratory syncytial virus (RSV) confers protection from RSV infection in African green monkeys. J Virol. 2004;78:11198–11207. doi: 10.1128/JVI.78.20.11198-11207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent-Falquet JC, Peyron L, Souvras M, Moulin JC, Tektoff J, Patet J. Qualification of working cell banks for the Vero cell line to produce licensed human vaccines. Dev Biol Stand. 1989;70:153–156. [PubMed] [Google Scholar]
- Williams GM, Gunn JM. Long-term cell culture of adult rat liver epithelial cells. Exp Cell Res. 1974;89:139–142. doi: 10.1016/0014-4827(74)90196-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization Requirements for continuous cell lines used for biological substances. WHO Tech Rep Ser. 1987a;745:99–115. [Google Scholar]
- World Health Organization Requirements for rabies vaccine (inactivated) for human use produced in continuous cell lines. WHO Tech Rep Ser. 1987b;760:167–189. [Google Scholar]
- Wu SC, Liu CC, Lian WC. Optimization of microcarrier cell culture process for the inactivated enterovirus type 71 vaccine development. Vaccine. 2004;22:3858–3864. doi: 10.1016/j.vaccine.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Yokomizo AY, Antoniazzi MM, Galdino PL, Azambunja N, Jr, Jorge SAC, Pereira CA. Rabies virus production in high Vero cell density cultures on macroporous microcarriers. Biotechnol Bioeng. 2004;85:506–515. doi: 10.1002/bit.10917. [DOI] [PubMed] [Google Scholar]
- Yuk IH, Olsen MM, Geyer S, Forestell SP. Perfusion cultures of human tumor cells: a scalable production platform for oncolytic adenoviral vectors. Biotechnol Bioeng. 2004;86:637–642. doi: 10.1002/bit.20158. [DOI] [PubMed] [Google Scholar]