Abstract
Recombinant adenoviruses (Ad) are being explored as promising delivery systems for gene therapy and vaccination. However, there is a concern about the possibility of generating replication-competent adenoviruses (RCA) using the human embryonic kidney 293 cell line. We have constructed a new cell line named the UR cell line which can be used to produce Ad vectors free of RCA. This cell line is based on the human embryonic lung HEL 299 cell. We first constructed a shuttle plasmid which encodes the E1A/E1B sequence that is necessary for adenovirus replication. The shuttle plasmid was then transfected into HEL 299 cells. The presence of the E1A/E1B sequence and protein expression in the stably transformed UR cells was confirmed. Viruses produced in UR cells were still RCA-free after ten test passages, while adenovirus produced in 293 cells had generated RCA during the fourth passage. We conclude that the UR cell line is sufficiently stable, can effectively produce a virus yield comparable with 293 cells, and does not generate RCA formation during Ad propagation.
Keywords: Ad5 E1 sequence, Complementing cell line, Gene delivery, Replication-incompetent adenovirus, Vaccine
Introduction
Recombinant adenoviruses (Ad) are being explored as promising delivery systems for gene therapy and vaccination (Randrianarison-Jewtoukoff and Perricaudet 1995, McConnell and Imperiale 2004, Boyer et al. 2005). The human serotype 5 adenovirus (Ad5) has been adapted for the purpose of gene delivery with a deletion of the early region 1 (E1) sequence of its genome, which is necessary for its replication (Danthinne and Imperiale 2000). This deletion becomes a gene insertion point in some recombinant Ad systems. Replication-incompetent Ad5 vectors are most commonly generated using the 293 cell line which complements E1A and E1B for viral replication. This cell line was originally produced by transforming human embryonic kidney (HEK) cells with sheared Ad5 DNA (Graham et al. 1977). The researchers estimated that the 293 cell genome contained copies of about 17% of the left-hand region of the Ad5 genome. Sequencing experiments later confirmed the presence of 4,344 bp of Ad5 viral DNA within the 293 cell genome, including the E1 region and pIX gene (Louis et al. 1997).
We are investigating the use of recombinant Ad as a means of vaccine delivery (Shi et al. 2001; Zeng et al. 2006). Currently, we employ two commercial systems for the construction of replication-incompetent adenoviruses: AdEasy™ from Stratagene (La Jolla, CA, USA) and AdMax™ from Microbix (Toronto, Canada). Using the first system, a gene of interest is amplified and cloned into the plasmid pShuttle-CMV and subsequently cloned into the Ad genomic plasmid vector pAdEasy-1 through homologous recombination in Escherichia coli BJ5183 cells (He et al. 1998; Zeng et al. 2001). The recombinant Ad plasmid is then linearized and transfected into 293 cells. In the latter system, the gene of interest is cloned into the plasmid vector pDC316, which is then co-transfected with the Ad genomic vector pBHGloxΔE1,3Cre into 293 cells. The gene of interest is inserted into the Ad vector through Cre-recombination (Ng et al. 1999).
Safety concerns arise with regards to the possibility of generating replication-competent adenoviruses (RCA) through recombination of non-E1 regions of the 293 genome with homologous regions in the Ad genomic DNA. According to FDA guidelines, replication-incompetent Ad should be examined for RCA, as it may lead to inadvertent vector replication and aggravation of inflammatory responses in the host (FDA 2001).
Although some packaging cell lines for RCA-free production of Ad have been previously reported (Fallaux et al. 1998; Schiedner et al. 2000; Kim et al. 2001), we have used a different approach to construct a new E1-complementing cell line based on human embryonic lung HEL 299 cells that we call the UR cell line. The UR cell line encodes the E1A/E1B gene region which is the minimal E1 sequence required for successful adenovirus replication. It lacks any other regions of the Ad genome, thus eliminating the possibility of homologous recombination between viral and cellular genomes. The UR RCA-free cell line produces a comparable adenovirus yield as HEK 293 cells; thus, it is also safer to be used in recombinant Ad production for gene therapy and vaccination.
Materials and methods
Construction of plasmid expression vector encoding Ad5 E1A and E1B genes
A mammalian expression vector pOPI3CAT (Stratagene, La Jolla, CA, USA) containing the neomycin-resistant gene (G418) was used to construct a plasmid pZAdE1 encoding wild-type Ad5 (ATCC VR-5) E1A and E1B genes. Ad5 genomic DNA was isolated from purified Ad5 virions using DNAZol reagent (Invitrogen, CA, USA). The Ad5 E1A/E1B sequence (nucleotides 459–3,510) which included 100 bp of sequence upstream of the E1A translation start site (Chroboczek et al. 1992) was amplified through PCR using Platinum®Pfx DNA polymerase (Invitrogen, CA) with primer 5E1R (5′-CCGGGATCCCGTGTAGTGTATTTATTTACCCG-3′) containing a BamHI (GGATCC) restriction site, and reverse primer 3E1 (5′-ATAGCGGCCGCTCAATCTGTATCTTCATCGCT-3′), which contains the NotI (GCGGCCGC) restriction site. The PCR product was digested with BamHI and NotI and subsequently cloned into the pOPI3CAT vector backbone at the BglII (position 2627) and NotI (position 3884) sites. This resulted in a shuttle plasmid pZAdE1 containing the E1A and E1B expression cassette. The transcription of the E1A and E1B genes are under the control of the rous sarcoma virus long terminal repeat (RSV-LTR) promoter and the natural E1B promoter, respectively. A thymidine kinase (TK) poly-A sequence executes polyadenylation. Correct E1A/E1B orientation was verified by restriction endonuclease digestion.
UR cell line construction
HEL 299 (ATCC, VA, USA) cells were cultured in Modified Eagle’s Medium (MEM) with 10% fetal bovine serum (FBS) to 90% confluence before being transfected with pZAdE1 plasmid utilizing Lipofectamine 2000™ (Life Technologies, NY, USA). G418 sulfate was added at the range of 50–500 μg ml−1 to culture medium 24 h post-transfection for selection of transfected cells. Stable G418-resistant cells were isolated, expanded, and subjected to verification with PCR and Western Blotting analysis.
E1A/E1B gene verification through PCR analysis
The genomic DNA from UR cells, HEK 293 cells (CRL-1573, ATCC, VA, USA), and HEL 299 cells (CCL-137, ATCC, VA, USA) were isolated using a Qiagen Genomic DNA Purification Kit (Qiagen, CA, USA) and used as templates for PCR with primers 5E1R and 3E1. The plasmid pZAdE1 DNA was also used as a template in this experiment as an additional positive control. PCR products were fractionated in a 1% agarose gel, stained with ethidium bromide, and visualized with the Kodak Imaging System 440CF.
Verification of E1A/E1B protein expression by Western blotting
Approximately 1–3 × 106 UR, HEK 293, and HEL 299 cells were lysed in a sample buffer containing 0.15 M NaCl, 50 mM Tris–HCl pH 8.0, 1% NP-40, 1% deoxycholate, and 0.1% sodium dodecylsulfate (SDS). Total protein concentrations were determined using a BCA Protein Assay Kit (Pierce, IL, USA). Cellular proteins (30 μg) were resolved on SDS containing 12% polyacrylamide (PAGE) and electrotransferred to a nitrocellulose membrane (Bio-Rad, CA, USA). E1A and E1B proteins were detected using a WesternBreeze® Chemiluminescent Kit (Invitrogen, Carlsbad, CA, USA). Briefly, non-specific binding sites were blocked with 1% bovine serum albumin (BSA). The membrane was probed with mouse anti-adenovirus type 5 E1A (BD Bioscience, Mississauga, ON, Canada) or rat anti-adenovirus type 5 E1B 55 KDa (Oncogene, Boston, MA, USA) monoclonal antibody and labeled with alkaline phosphatase-conjugated anti-mouse IgG (Upstate, Lake Placid, NY, USA) or anti-rat IgG (Calbiochem, La Jolla, CA, USA) for the detection of E1A and E1B proteins, respectively.
Evaluation of growth and morphological characteristics of UR and HEL 299 cells
The UR or parental HEL299 cells were seeded in each well of 24-well cell culture clusters at a density of 4 × 104 cells per well with 1.0 ml MEM medium containing 10% FBS. Cells were incubated at 37°C with 5% CO2 and were trypsinized for cell number counting by a hemocytometer under microscope every 24 h. The growth curves were plotted using data generated from six wells each time point. The log phase, population doubling time and saturation density were determined from the growth curve.
The images of the UR and the parental HEL 299 cells were acquired with a Zeiss microscope at 40× using an AxioCam MRc camera and Axiovision 3.0 software (Carl Zeiss).
Determination of adenovirus yield
Ad yield was first determined on a small scale. 1 × 105 HEK 293 and UR cells were grown in 6 well tissue culture clusters with MEM (Modified Eagle’s Medium) or DMEM (Dulbecco’s Modified Eagle’s Medium) plus 8% serum supplement (contains antibiotic, FBS, NCS, and L-glutamine). Cells were infected with Ad vector AdmCMV/EFn encoding the N-fragment of anthrax edema factor produced by the AdMax™ system at 0.5 multiplicity of infection (MOI) (Microbix 2004, Zeng et al. 2006). Infected cells were harvested from each well 24, 48, 72, and 96 h post infection and lysed by three freezing-thawing cycles. Infectious units of cell lysates were determined through a plaque assay described previously (Mittereder et al. 1996, Sabara and Larence 2003).
To measure the yield of large-scale virus production, adenoviruses were produced by cultivation of HEK 293 and UR cells in forty 150 mm plates to a 90% confluence. Cells were infected with 0.5 MOI AdmCMV/EFn, harvested, and lysed on day 5. Adenoviruses in the cell lysates were precipitated with PEG 8000 and purified by CsCl gradient centrifugation.
Replication competent adenovirus (RCA) assay
A two-cell line bioassay was used to check for the presence of RCA (Hehir et al. 1996; Zhu et al. 1999; Roitsch et al. 2001). The same batch of AdmCMV/EFn was used to produce the first passage of adenovirus using HEK 293 and UR cells, respectively. Human cervical carcinoma HeLa-S3 (CCL-2.2, ATCC, VA, USA) cells were grown in 6-well cell culture plates with DMEM + 10% FBS (5 ml per well) to 90% confluence and subsequently infected with 20 MOI AdmCMV/EFn from the first passage AdmCMV/EFn prepared in UR or HEK 293 cells in order to amplify any RCA. Four days after virus infection, HeLa cells were harvested and lysed by three freezing-thawing cycles. For the second stage of the assay, human lung carcinoma A549 (CCL-185, ATCC, VA, USA) cells were cultured to approximately 95% confluence in 6-well plates, then one-fifth (1 ml) of HeLa cell lysate was added per well. After the virus was adsorbed at room temperature for 30 min, the medium was replaced with fresh DMEM + 2% FBS supplement. Then the plate was incubated at 37°C in 5% CO2 for a period of 14 days. Compared with the negative control, a widespread cytopathic effect (CPE) on monolayers of A549 cells in test wells was indicative of the presence of RCA. Parallel assays with the same dose of AdmCMV/EFn mixed with 5 pfu of wild type Ad5 were carried out as a control for positive RCA. If no RCA was found in the experimental groups of this round, the second passages of virus were prepared using viruses from the first passages to infect HEK293 and UR cells, respectively. The RCA assay was repeated.
Results
Construction of UR cell line
After the construction of the plasmid expression vector pZAdE1 encoding the Ad5 E1A/E1B, we successfully transfected HEL 299 cells with this plasmid using Lipofectamine 2000™ (Life Technologies, NY, USA). After gradually increasing the concentration of G418 in the culture medium from 50 to 500 μg ml−1, we selected three stably transformed clones of G418 resistant cells which grew well in the medium. One clone was then expanded. We named it UR cell line. The cells were used for further characterization.
Ad5 E1A/E1B sequence insert verification and protein expression in UR cell line
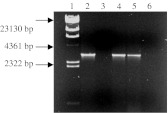
Using PCR followed by gel electrophoresis, we were able to confirm the presence of the E1A/E1B sequence in the genome of UR cells. In a gel electrophoresis (Fig. 1), E1A/E1B PCR products amplified from plasmid pZAdE1 and HEK 293 genomic DNA served as positive controls, while the PCR products from HEL 299 genomic DNA served as negative controls. The PCR products amplified from plasmid pZAdE1, HEK 293, and UR cell genomic DNA were all characterized by the presence of a band pertaining to the 3,051 bp E1A/E1B sequence (Fig. 1, lanes 2, 4, and 5).
Fig. 1.
Detection of E1A/E1B gene sequence in UR cells by PCR. Ad5 E1 sequence was amplified by PCR with genomic DNA from the UR cell line as template. Lane 1, λ/HindIII DNA size marker; Lane 2, positive control, pZAdE1; Lane 3, HEL 299 (negative control); Lane 4, positive control, HEK 293; Lane 5, UR cell line; Lane 6: PCR negative control (no DNA template)
Like the HEK 293 packaging cell line, UR cells expressed both E1A and EIB proteins as validated by Western blotting shown in Fig. 2 (lanes 4 and 5). Proteins were fractionated by size on a 12% SDS-PAGE gel and then distinguished using anti-E1A and anti-E1B probes. In contrast, HEL 299 did not show positive bands for E1A or E1B expression as expected. The UR cell line could consistently express E1A/E1B at the same level after 15 passages without addition of G418 in the medium, indicating the stable integration of the E1A/E1B expression cassette in the cell genome.
Fig. 2.
Western blotting analysis of E1A and E1B expression in UR cells. Western blotting analyses for 35–46 kDa E1A (left) and 55-KDa E1B (right) proteins expressed in the UR cells; Cells were lysed in sample buffer and the proteins were fractionated on 12% SDS-PAGE gel, transferred to a nitrocellulose membrane by electroblotting and probed with anti-Ad5 E1A or anti-E1B (55 kDa protein) monoclonal antibody, and subsequently labeled with anti-mouse alkaline phosphatase-conjugated IgG. Lane 1, marker; Lane 2, negative control HEL 299; Lane 3, positive control HEK 293; Lanes 4 and 5, UR cell line (from 2 separate samples)
Growth and morphological characteristics of UR cell line
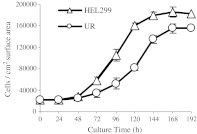
The UR cell has a smaller and less angular shape compared to the parental HEL299 cell (Fig. 3). In addition, the UR cell grows more slowly than HEL 299 cell. The log phase of the UR cells was between 72 and 144 h while the log phase of HEL299 cells was between 48 and 120 h. Population doubling times of the UR and HEL 299 cells were 80 and 58 h, respectively. The saturation density of the UR cell line was lower than that of HEL299 (Fig. 4).
Fig. 3.
Morphological comparison of the UR and parental HEL 299 cells. a HEL299 cells; b UR cells. The Images were acquired with an AxioCam MRc camera (Carl Zeiss) at 40× using Axiovision 3.0 software (Carl Zeiss)
Fig. 4.
Growth curve of the UR and parental cell line HEL 299 cells. Cells were seeded in each well of 24-well cell culture clusters at a density of 4 × 104 cells per well with 1.0 ml MEM medium containing 10% FBS. Cells were incubated at 37°C with 5% CO2 and were trypsinized for cell number counting by a hemocytometer under microscope every 24 h. Values are Means ± SD, n = 6
Virus yield
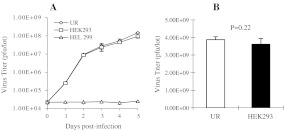
To determine whether equivalent virus yield could be produced with UR cells in comparison with HEK 293 cells, AdmCMV/EFn virus yields produced with HEK 293 and UR cells were measured by small scale and large scale virus production methods. Figure 5a traces the adenovirus production course for each cell line at a small scale using 6 well cell culture clusters, while Fig. 5b shows the final virus yields from large scale virus production using forty 150-mm cell culture dishes. The result suggests that virus production yields were similar for UR and HEK 293 cells. This confirms that the UR and HEK 293 cells are both efficient for adenovirus production.
Fig. 5.
Comparison of virus yields produced using HEK 293 and UR cells. a Time course of adenovirus production using UR and HEK293 cells: 1 × 105 cells were infected with approximately 0.5 MOI AdmCMV/EFn viral particles and harvested at 1, 2, 3, 4, and 5 days post-infection. The cell lysates were used to measure virus titers by plaque assay. The virus titers indicate the total plaque forming units (pfu) in the cell lysates. b Virus yields produced by UR and HEK 293 cell. HEK293 and UR cells were seeded onto forty 150-mm dishes, cultured to 90% confluence, and infected with 0.5 MOI AdmCMV/EFn. Five days after virus infection, cells were harvested and lysed by freezing-thawing. Viruses were purified by CsCl gradient centrifugation. The virus titers represent the total plaque forming units (pfu) in the virus preparations. The experiments were performed in triplicate, and the data of virus titers were analyzed using Student’s t test
RCA assay
We observed that CPE was generated in the virus produced in HEK 293, beginning in the 4th passage through the RCA assay. Hundred percent CPE was reached in RCA assays performed in parallel using AdmCMV/EFn mixed with wild-type Ad5 as a positive control for RCA. Although CPE generated by virus produced in HEK 293 cells was not as extensive as CPE generated by wild-type Ad5, the data show that it does increase in succeeding generations (Table 1). In contrast, the virus produced in UR cells did not generate CPE after 10 passages as determined by the two-step RCA assay, indicating that viruses generated in UR cells were RCA-free after 10 passages.
Table 1.
Summary of the results from replication competent adenovirus (RCA) assays
| Passages | AdmCMV/EFn | AdmCMV/EFn + WT Ad5 | ||
|---|---|---|---|---|
| UR | HEK293 | UR | HEK293 | |
| 1 | − | − | ++++ | ++++ |
| 2 | − | − | ++++ | ++++ |
| 3 | − | − | ++++ | ++++ |
| 4 | − | + | ++++ | ++++ |
| 5 | − | + | ++++ | ++++ |
| 6 | − | ++ | ++++ | ++++ |
| 7 | − | NA | NA | NA |
| 8 | − | NA | NA | NA |
| 9 | − | NA | NA | NA |
| 10 | − | NA | NA | NA |
The results were from three independent assays. + 20% less cells show CPE;
++++ 100% CPE; NA not analyzed; – no CPE
Discussion
In order to set aside concerns over the probability of generating RCA in Ad vector production through homologous recombination with HEK 293 genomic DNA, we constructed a new, more optimized complementing cell line (UR). The UR cell line was engineered to contain the E1 gene sequence necessary for the replication of otherwise replication-incompetent Ad5 but excludes regions that are known to be homologous with the Ad5 based vector DNA. While HEK 293 cells contain 4,344 bp of the left-hand of Ad5 genomic DNA (Louis et al. 1997), the UR cell line DNA is limited to encoding a 3,051 bp sequence consisting of E1A and E1B genes (Chroboczek et al. 1992).
From our RCA assay experiments, we can deduce that the emergence of RCA in recombinant Ad5 produced in 293 cells is highly probable, especially after multiple generations of virus. It was also observed that once RCA was established, it increased in successive generations. It therefore became increasingly important to construct a more optimized and safer complementing cell line than the 293 cell line in order to avoid inducing side effects to future recipients of this form of vaccine delivery (FDA 2001). Fortunately, the UR cell line proved that it could produce RCA-free viruses through multiple passages. Our next step would be to have the UR cells adapt to grow in suspension and serum free media so that it can meet the need for pharmaceutical industry application.
In conclusion, we have reported the construction of a HEL 299-based cell line for the RCA-free production of adenovirus that we call the UR cell line. Our experiments indicate that we have assembled a cell line meeting the requirements for safe and successful recombinant adenovirus production. The cell line is sufficiently stable, can effectively plaque adenovirus, produces adequate amounts of infectious virions, and avoids RCA formation. This cell line could be used to meet the regulatory standards in the production of Ad5 vectors for gene therapy and vaccination.
Acknowledgments
This work was supported by the US. Public Service research grants AI055946, AI059225, and DC05845 to M. Z. from the National Institutes of Health. M. E. P. was supported by US. Public Service contract VTEU N01-AI-25460 from the National Institute of Allergy and Infectious diseases. We are grateful to Natasha Girgis for the technical assistance in Ad5 E1 cloning. We thank Eric D. Hesek and Mary C. Zeng for their critical reading and insightful discussion of this manuscript. We appreciate the support from John J. Treanor, Jian-Dong Li, and Barbara H. Iglewski.
References
- Boyer JL, Kobinger G, Wilson JM, Crystal RG. Adenovirus-based genetic vaccines for biodefense. Hum Gene Ther. 2005;16:157–168. doi: 10.1089/hum.2005.16.157. [DOI] [PubMed] [Google Scholar]
- Chroboczek J, Bieber F, Jacrot B. The sequence of the genome of adenovirus type 5 and its comparison with the genome of adenovirus type 2. Virology. 1992;186:280–285. doi: 10.1016/0042-6822(92)90082-Z. [DOI] [PubMed] [Google Scholar]
- Danthinne X, Imperiale MJ. Production of first generation adenovirus vectors: a review. Gene Ther. 2000;7:1707–1714. doi: 10.1038/sj.gt.3301301. [DOI] [PubMed] [Google Scholar]
- Fallaux FJ, Bout A, Velde I, Wollenberg DJ, Hehir KM, Keegan J, Auger C, Cramer SJ, Ormondt H, Eb AJ, Valerio D, Hoeben RC. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther. 1998;9:1909–1917. doi: 10.1089/hum.1998.9.13-1909. [DOI] [PubMed] [Google Scholar]
- FDA Guidance for human somatic cell therapy and gene therapy. Hum Gene Ther. 2001;12:303–314. doi: 10.1089/10430340150218431. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehir K, Armentano D, Cardoza L, Choquette T, Berthelette P, White G, Couture L, Everton M, Keegan J, Martin J, Pratt D, Smith M, Smith A, Wadsworth S. Molecular characterization of replication-competent variants of adenovirus vectors and genome modifications to prevent their occurrence. J Virol. 1996;70:8459–8467. doi: 10.1128/jvi.70.12.8459-8467.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Lee SH, Cho YS, Park K, Kim YH, Lee JH. Development of a packaging cell line for propagation of replication-deficient adenovirus vector. Exp Mol Med. 2001;33:145–149. doi: 10.1038/emm.2001.25. [DOI] [PubMed] [Google Scholar]
- Louis N, Evelegh C, Graham FL. Cloning and sequencing of the cellular-viral junctions from the human adenovirus type 5 transformed 293 cell line. Virology. 1997;233:423–429. doi: 10.1006/viro.1997.8597. [DOI] [PubMed] [Google Scholar]
- McConnell MJ, Imperiale MJ. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004;15:1022–1033. doi: 10.1089/hum.2004.15.1022. [DOI] [PubMed] [Google Scholar]
- Microbix (2004) AdMax Adenovirus Vector Creation Kits, Microbix Biosystem, Inc., Toronto, Ontario, Canada M8Z 3A8
- Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P, Parks RJ, Cummings DT, Evelegh CM, Sankar U, Graham FL. A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther. 1999;10:2667–2672. doi: 10.1089/10430349950016708. [DOI] [PubMed] [Google Scholar]
- Randrianarison-Jewtoukoff V, Perricaudet M. Recombinant adenoviruses as vaccines. Biologicals. 1995;23:145–157. doi: 10.1006/biol.1995.0025. [DOI] [PubMed] [Google Scholar]
- Roitsch C, Achstetter T, Benchaibi M, Bonfils E, Cauet G, Gloeckler R, L’H te H, Keppi E, Nguyen M, Spehner D, Dorsselaer A, Malarme D. Characterization and quality control of recombinant adenovirus vectors for gene therapy. J Chromatogr B Biomed Sci Appl. 2001;752:263–280. doi: 10.1016/S0378-4347(00)00557-0. [DOI] [PubMed] [Google Scholar]
- Sabara MI, Larence JE. Plaque assay for avian metapneumovirus using a Japanese quail fibrosarcoma cell line (QT-35) J Virol Methods. 2003;107:9–14. doi: 10.1016/S0166-0934(02)00207-0. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Hertel S, Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum Gene Ther. 2000;11:2105–2116. doi: 10.1089/104303400750001417. [DOI] [PubMed] [Google Scholar]
- Shi Z, Zeng M, Yang G, Siegel F, Cain LJ, Kampen KR, Elmets CA, Tang DC. Protection against Tetanus by needle-free inoculation of adenovirus-vectored nasal and epicutaneous vaccines. J Virol. 2001;75:11474–11482. doi: 10.1128/JVI.75.23.11474-11482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M, Smith SK, Siegel F, Shi Z, Kampen KR, Elmets CA, Tang DC. AdEasy system made easier by selecting the viral backbone plasmid preceding homologous recombination. Biotechniques. 2001;31:260–262. doi: 10.2144/01312bm04. [DOI] [PubMed] [Google Scholar]
- Zeng M, Xu Q, Hesek ED, Pichichero ME (2006) N-fragment of edema factor as a candidate antigen for immunization against anthrax. Vaccine 24:662–670 [Epub 2005 Aug 26] [DOI] [PubMed]
- Zhu J, Grace M, Casale J, Chang AT, Musco ML, Bordens R, Greenberg R, Schaefer E, Indelicato SR. Characterization of replication-competent adenovirus isolates from large-scale production of a recombinant adenoviral vector. Hum Gene Ther. 1999;10:113–121. doi: 10.1089/10430349950019246. [DOI] [PubMed] [Google Scholar]