Abstract
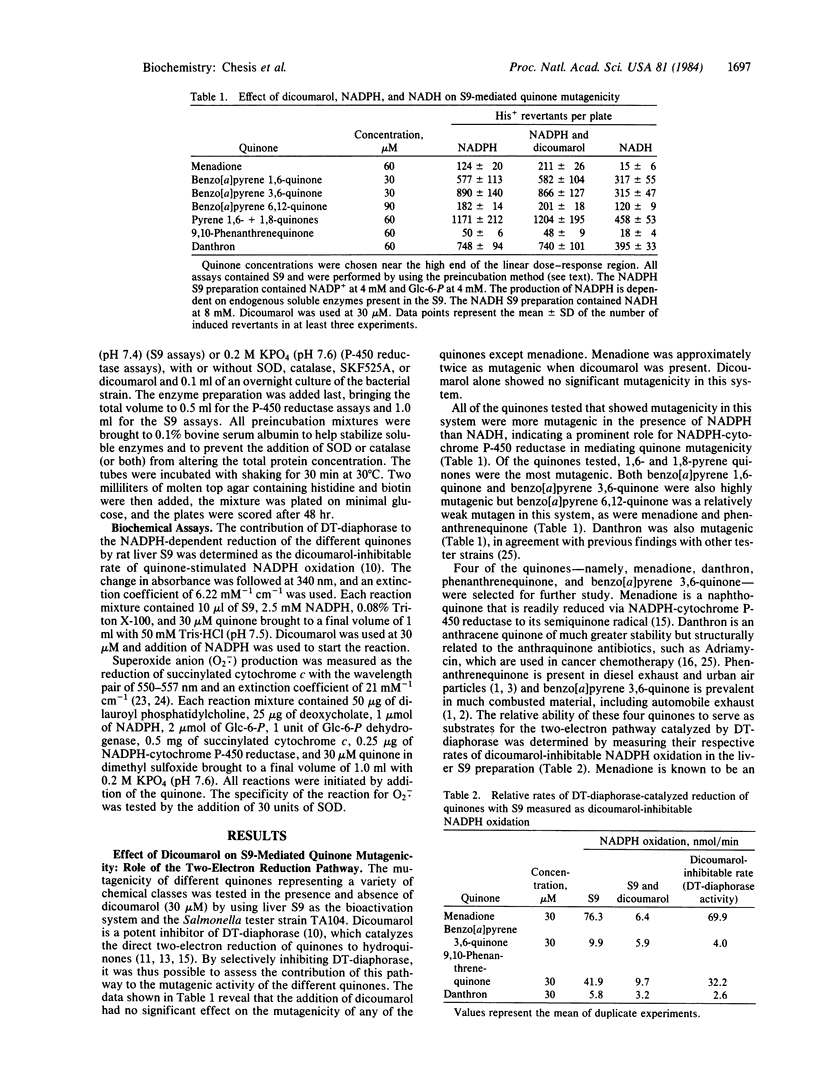
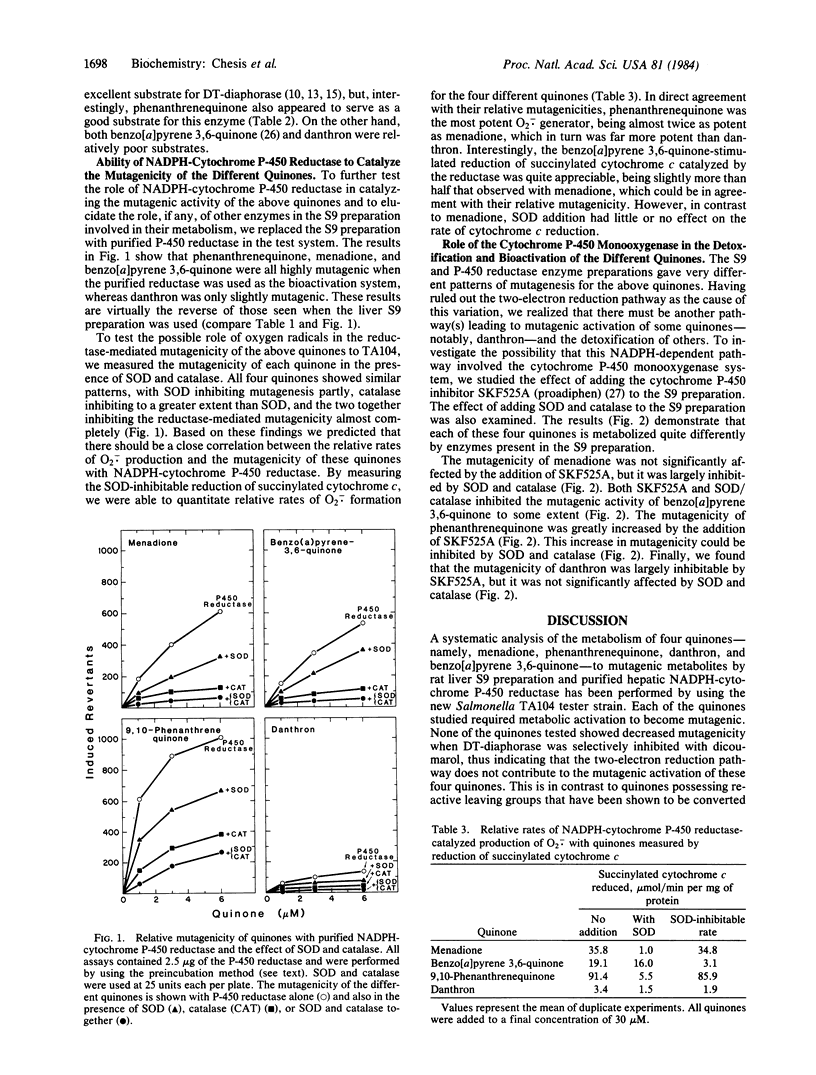
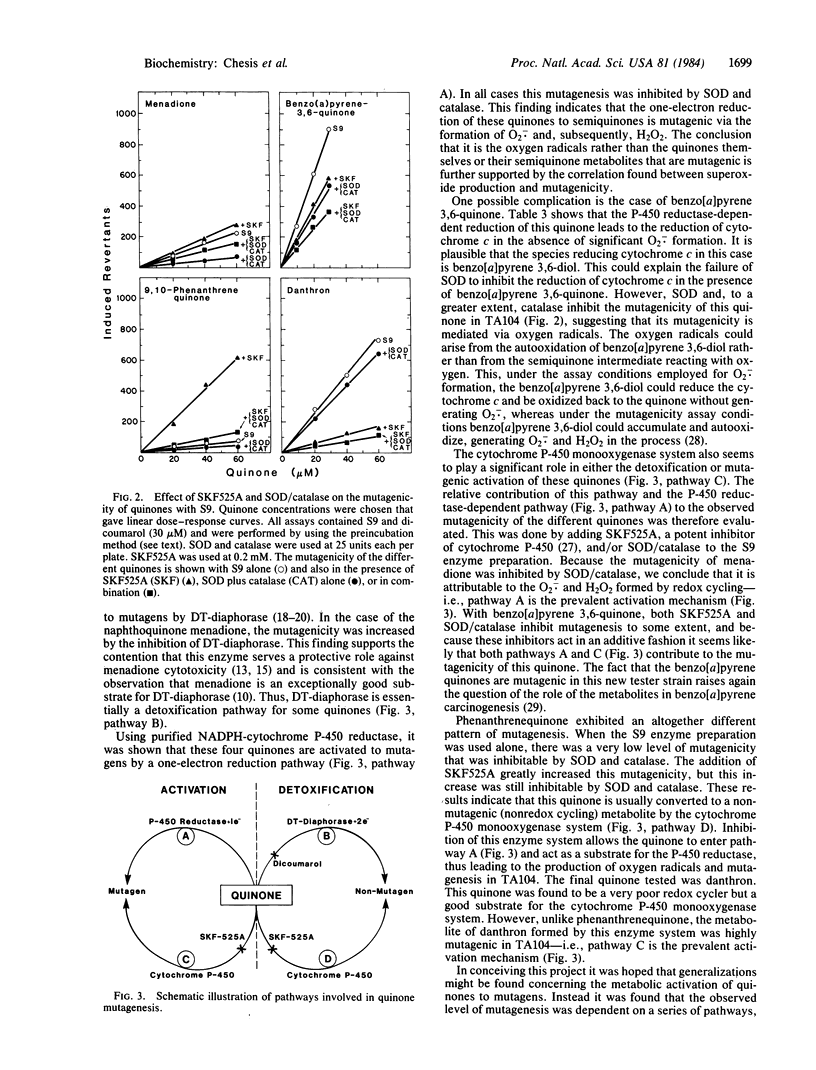
The mutagenicity of various quinones, a class of compounds widely distributed in nature, is demonstrated in the Salmonella TA104 tester strain. The metabolic pathways by which four quinones, menadione, benzo[a]pyrene 3,6-quinone, 9,10-phenanthrenequinone, and danthron, caused mutagenicity in this test system were investigated in detail as were the detoxification pathways. The two-electron reduction of these quinones by NAD(P)H-quinone oxidoreductase (DT-diaphorase) was not mutagenic, whereas the one-electron reduction, catalyzed by NADPH-cytochrome P-450 reductase, was mutagenic, except for danthron, which was only slightly mutagenic. The mutagenicity of the quinones via this pathway was found to be attributable to the generation of oxygen radicals. The cytochrome P-450 monooxygenase also played a significant role in the detoxification and bioactivation of these quinones. For example, phenanthrenequinone was converted to a nonmutagenic metabolite in a cytochrome P-450-dependent reaction, whereas danthron was converted to a highly mutagenic metabolite. These studies show the complexity of metabolic pathways involved in the mutagenicity of quinones.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V. A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 1978 Jun;38(6):1745–1750. [PubMed] [Google Scholar]
- Begleiter A. Cytocidal action of the quinone group and its relationship to antitumor activity. Cancer Res. 1983 Feb;43(2):481–484. [PubMed] [Google Scholar]
- Brown J. P. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res. 1980 May;75(3):243–277. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Buening M. K., Franklin M. R. SKF 525-A inhibition, induction, and 452-nm complex formation. Drug Metab Dispos. 1976 May-Jun;4(3):244–255. [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977 Apr 30;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Dierickx P. J. Interaction of benzo- and naphthoquinones with soluble glutathione S-transferases from rat liver. Pharmacol Res Commun. 1983 Jun;15(6):581–591. doi: 10.1016/s0031-6989(83)80029-0. [DOI] [PubMed] [Google Scholar]
- Driscoll J. S., Hazard G. F., Jr, Wood H. B., Jr, Goldin A. Structure-antitumor activity relationships among quinone derivatives. Cancer Chemother Rep 2. 1974 Apr;4(2):1–362. [PubMed] [Google Scholar]
- Iyanagi T., Yamazaki I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase). Biochim Biophys Acta. 1970 Sep 1;216(2):282–294. doi: 10.1016/0005-2728(70)90220-3. [DOI] [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Kuthan H., Ullrich V., Estabrook R. W. A quantitative test for superoxide radicals produced in biological systems. Biochem J. 1982 Jun 1;203(3):551–558. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Hollstein M., Christman M. F., Schwiers E. A., Ames B. N. A new Salmonella tester strain (TA102) with A X T base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7445–7449. doi: 10.1073/pnas.79.23.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman D. F., Fink R. C., Schaefer F. L., Mulcahy R. J., Stark A. A. Mutagenicity of anthraquinone and hydroxylated anthraquinones in the Ames/Salmonella microsome system. Appl Environ Microbiol. 1982 Jun;43(6):1354–1359. doi: 10.1128/aem.43.6.1354-1359.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. J., Pardini R. S., Cosby L. A., Lillis B. J., Shansky C. W., Sartorelli A. C. Potential bioreductive alkylating agents. 2. Antitumor effect and biochemical studies of naphthoquinone derivatives. J Med Chem. 1973 Nov;16(11):1268–1271. doi: 10.1021/jm00269a010. [DOI] [PubMed] [Google Scholar]
- Lind C., Hochstein P., Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch Biochem Biophys. 1982 Jun;216(1):178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- Lind C., Vadi H., Ernster L. Metabolism of benzo(a)pyrene-3,6-quinone and 3-hydroxybenzo(a)pyrene in liver microsomes from 3-methylcholanthrene-treated rats. A possible role of DT-diaphorase in the formation of glucuronyl conjugates. Arch Biochem Biophys. 1978 Sep;190(1):97–108. doi: 10.1016/0003-9861(78)90256-4. [DOI] [PubMed] [Google Scholar]
- Lorentzen R. J., Ts'o P. O. Benzo[a]yrenedione/benzo[a]pyrenediol oxidation-reduction couples and the generation of reactive reduced molecular oxygen. Biochemistry. 1977 Apr 5;16(7):1467–1473. doi: 10.1021/bi00626a035. [DOI] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Masters B. S., Okita R. T. The history, properties, and function of NADPH-cytochrome P-450 reductase. Pharmacol Ther. 1980;9(2):227–244. doi: 10.1016/s0163-7258(80)80020-9. [DOI] [PubMed] [Google Scholar]
- Moore H. W., Czerniak R. Naturally occurring quinones as potential bioreductive alkylating agents. Med Res Rev. 1981 Fall;1(3):249–280. doi: 10.1002/med.2610010303. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., DePierre J. W., Lind C., Guthenberg C., Mannervik B., Ernster L. Benzo(alpha)pyrene quinones can be generated by lipid peroxidation and are conjugated with glutathione by glutathione S-transferase b from rat liver. Biochem Biophys Res Commun. 1981 Mar 31;99(2):682–690. doi: 10.1016/0006-291x(81)91798-8. [DOI] [PubMed] [Google Scholar]
- Powis G., Svingen B. A., Appel P. Quinone-stimulated superoxide formation by subcellular fractions, isolated hepatocytes, and other cells. Mol Pharmacol. 1981 Sep;20(2):387–394. [PubMed] [Google Scholar]
- Schuetzle D. Sampling of vehicle emissions for chemical analysis and biological testing. Environ Health Perspect. 1983 Jan;47:65–80. doi: 10.1289/ehp.834765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. T., Thor H., Orrenius S. Detection and measurement of drug-induced oxygen radical formation. Methods Enzymol. 1984;105:505–510. doi: 10.1016/s0076-6879(84)05069-2. [DOI] [PubMed] [Google Scholar]
- Talcott R. E., Rosenblum M., Levin V. A. Possible role of DT-diaphorase in the bioactivation of antitumor quinones. Biochem Biophys Res Commun. 1983 Feb 28;111(1):346–351. doi: 10.1016/s0006-291x(83)80158-2. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Tikkanen L., Matsushima T., Natori S., Yoshihira K. Mutagenicity of natural naphthoquinones and benzoquinones in the Salmonella/microsome test. Mutat Res. 1983 Oct;124(1):25–34. doi: 10.1016/0165-1218(83)90182-9. [DOI] [PubMed] [Google Scholar]
- Wefers H., Sies H. Hepatic low-level chemiluminescence during redox cycling of menadione and the menadione-glutathione conjugate: relation to glutathione and NAD(P)H:quinone reductase (DT-diaphorase) activity. Arch Biochem Biophys. 1983 Jul 15;224(2):568–578. doi: 10.1016/0003-9861(83)90244-8. [DOI] [PubMed] [Google Scholar]



