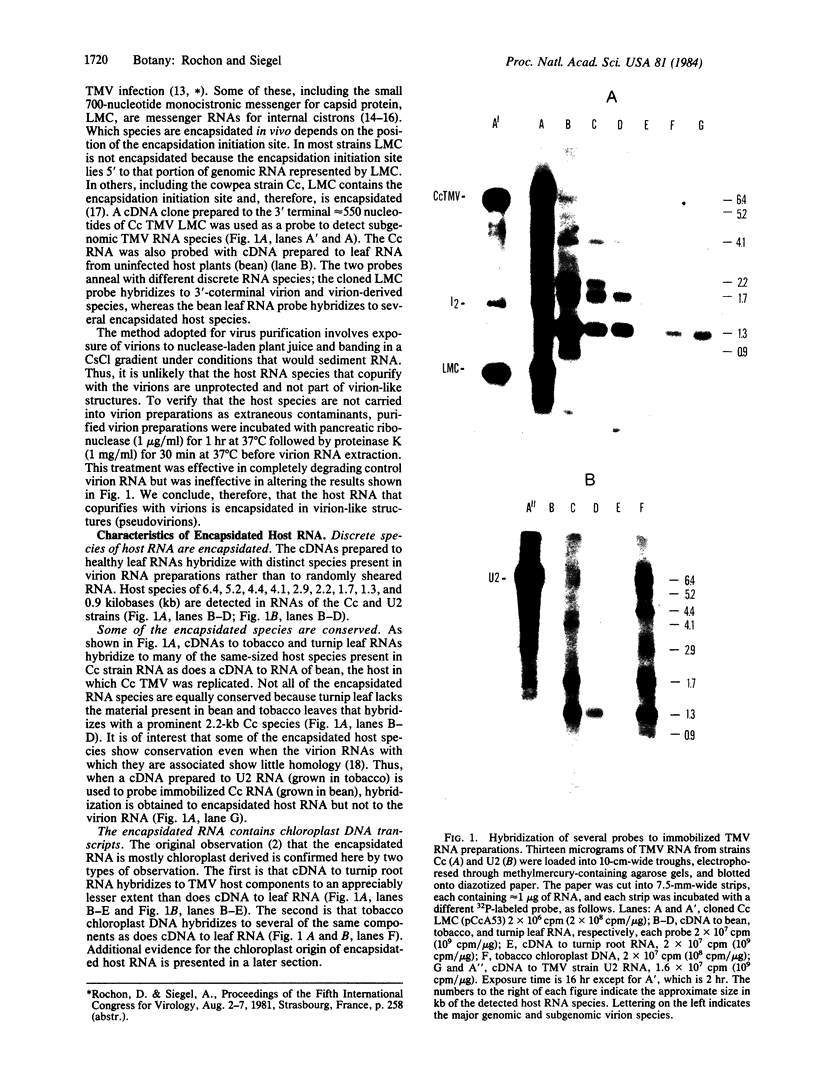
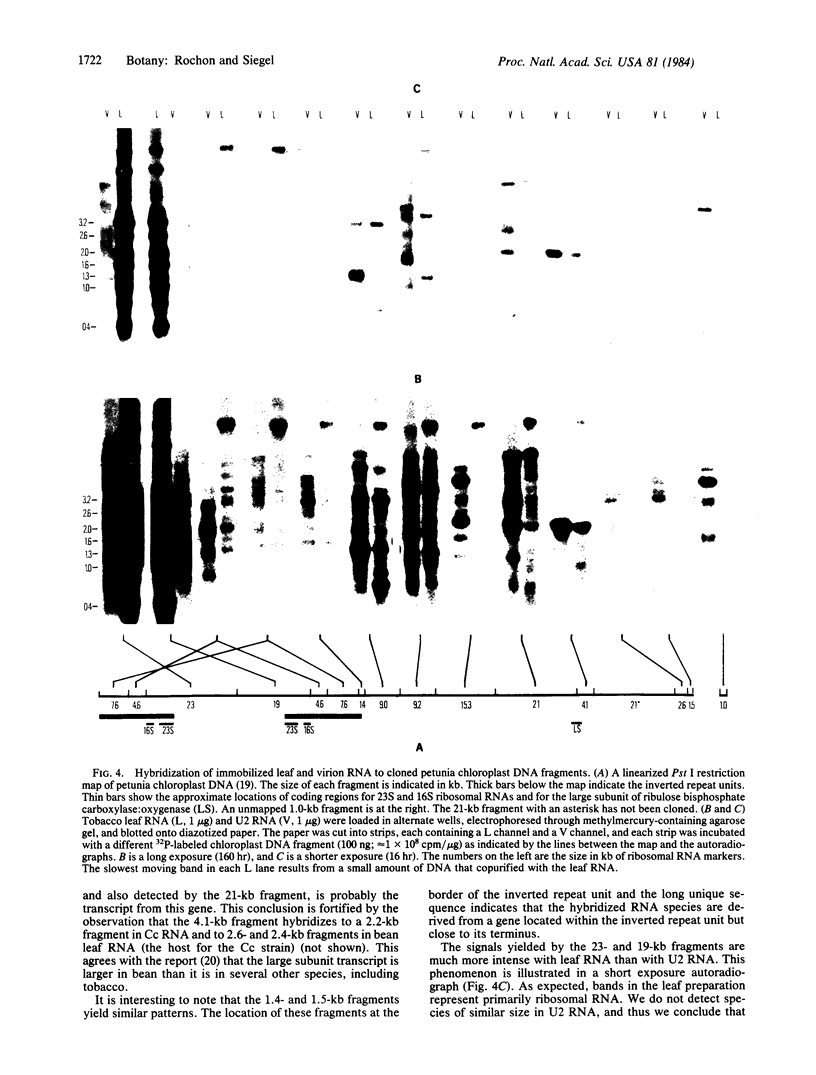
Abstract
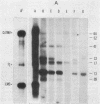
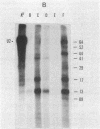
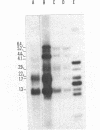
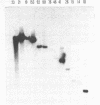
Preparations of tobacco mosaic virus contain pseudovirions, particles resembling virions but containing host rather than viral RNA. The encapsidated host RNA was found to be composed of discrete-sized species derived from a large portion of the chloroplast genome except that very little, if any, ribosomal RNA is present. Pseudovirions contain the same chloroplast DNA transcripts as those detected in extracts from uninfected leaves, although not always in the same relative amounts. Several strains of tobacco mosaic virus were tested and all were found to contain pseudovirions, with the U2 strain containing more than the others.
Keywords: pseudovirions, subgenomic components
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bisaro D., Siegel A. Sequence Homology between Chloroplast DNAs from Several Higher Plants. Plant Physiol. 1980 Feb;65(2):234–237. doi: 10.1104/pp.65.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakke M. K., Van Pelt N. Linear-log sucrose gradients for estimating sedimentation coefficients of plant viruses and nucleic acids. Anal Biochem. 1970 Nov;38(1):56–64. doi: 10.1016/0003-2697(70)90155-7. [DOI] [PubMed] [Google Scholar]
- Bruening G., Beachy R. N., Scalla R., Zaitlin M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology. 1976 Jun;71(2):498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- Goelet P., Karn J. Tobacco mosaic virus induces the synthesis of a family of 3' coterminal messenger RNAs and their complements. J Mol Biol. 1982 Jan 25;154(3):541–550. doi: 10.1016/s0022-2836(82)80013-2. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Higgins T. J., Goodwin P. B., Whitfeld P. R. Occurrence of short particles in beans infected with the cowpea strain of TMV. II. Evidence that short particles contain the cistron for coat-protein. Virology. 1976 Jun;71(2):486–497. doi: 10.1016/0042-6822(76)90376-7. [DOI] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A. The assembly of tobacco mosaic virus: structure and specificity. Harvey Lect. 1980;74:141–172. [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ohno T., Iba H., Okada Y. Nucleotide sequence of a cloned cDNA copy of TMV (cowpea strain) RNA, including the assembly origin, the coat protein cistron, and the 3' non-coding region. Mol Gen Genet. 1981;184(1):20–25. doi: 10.1007/BF00271189. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder A., Leder P. Unusual alpha-globin-like gene that has cleanly lost both globin intervening sequences. Proc Natl Acad Sci U S A. 1980 May;77(5):2806–2809. doi: 10.1073/pnas.77.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Edwards H., Jorgensen R. A., Thompson W. F. Novel evolutionary variation in transcription and location of two chloroplast genes. Nucleic Acids Res. 1982 Nov 11;10(21):6819–6832. doi: 10.1093/nar/10.21.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shalla T. A., Petersen L. J., Giunchedi L. Partial characterization of virus-like particles in chloroplasts of plants infected with the U5 strain of TMV. Virology. 1975 Jul;66(1):94–105. doi: 10.1016/0042-6822(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Siegel A., Hari V., Montgomery I., Kolacz K. A messenger RNA for capsid protein isolated from tobacco mosaic virus-infected tissue. Virology. 1976 Sep;73(2):363–371. doi: 10.1016/0042-6822(76)90397-4. [DOI] [PubMed] [Google Scholar]
- Siegel A. Pseudovirions of tobacco mosaic virus. Virology. 1971 Oct;46(1):50–59. doi: 10.1016/0042-6822(71)90005-5. [DOI] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]