Abstract
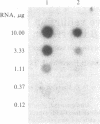
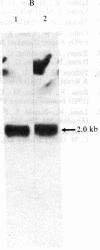
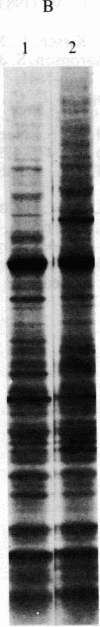
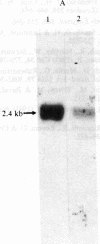
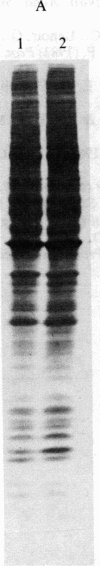
Under normal growth conditions, the human lymphoblastoid cell line Daudi expresses high levels of c-myc mRNA. These cells are also sensitive to growth inhibition by interferons. We have compared the levels of mRNA for the c-myc in untreated and human beta interferon (IFN-beta)-treated Daudi cells by RNA dot-blot and blot-hybridization analysis methods. Using a synthetic oligonucleotide complementary to the human c-myc mRNA as the probe, we detected a more than 75% reduction in the c-myc hybridizable poly(A)+ RNA in the IFN-beta-treated cells. This reduction in the c-myc mRNA appears to be selective because the level of actin mRNA is not significantly affected by the IFN-beta treatment. In addition, neither in vitro translation of mRNA extracted from IFN-beta-treated cells nor in vivo synthesis of cellular proteins in IFN-beta-treated cells are quantitatively affected. We surmise that the selective reduction in the amount of c-myc mRNA in IFN-beta-treated Daudi cells may be related to the IFN-induced inhibition of the Daudi tumor cell growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Strander H., Cantell K. Sensitivity of the Epstein-Barr virus transformed human lymphoid cell lines to interferon. J Gen Virol. 1975 Aug;28(2):207–217. doi: 10.1099/0022-1317-28-2-207. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Chen E. Y., Smith D. H., Levinson A. D. Identification and nucleotide sequence of a human locus homologous to the v-myc oncogene of avian myelocytomatosis virus MC29. Nature. 1983 Feb 24;301(5902):722–725. doi: 10.1038/301722a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., ar-Rushdi A., Drwinga H. L., Nowell P. C., Croce C. M. Transcriptional activation of the translocated c-myc oncogene in burkitt lymphoma. Proc Natl Acad Sci U S A. 1983 Feb;80(3):820–824. doi: 10.1073/pnas.80.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallongo A., Appella E., Ricciardi R., Rovera G., Croce C. M. Identification of the c-myc oncogene product in normal and malignant B cells. Science. 1983 Oct 28;222(4622):430–432. doi: 10.1126/science.6604943. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus J., Damm H., Karges H. E., Manthey K. F. Growth inhibition of human lymphoblastoid Daudi cells in vitro by interferon preparations. Arch Virol. 1976;51(1-2):87–97. doi: 10.1007/BF01317837. [DOI] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Knight E., Jr, Fahey D. Human fibroblast interferon. An improved purification. J Biol Chem. 1981 Apr 25;256(8):3609–3611. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- PAUCKER K., CANTELL K., HENLE W. Quantitative studies on viral interference in suspended L cells. III. Effect of interfering viruses and interferon on the growth rate of cells. Virology. 1962 Jun;17:324–334. doi: 10.1016/0042-6822(62)90123-x. [DOI] [PubMed] [Google Scholar]
- Rosa F., Fellous M., Dron M., Tovey M., Revel M. Presence of an abnormal beta 2-microglobulin mRNA in Daudi cells: induction by interferon. Immunogenetics. 1983;17(2):125–131. doi: 10.1007/BF00364752. [DOI] [PubMed] [Google Scholar]
- Sen G. C. Mechanism of interferon action: progress toward its understanding. Prog Nucleic Acid Res Mol Biol. 1982;27:105–156. doi: 10.1016/s0079-6603(08)60599-1. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]